Immune Responses Induced by Recombinant Bacillus subtilis Expressing the PEDV Spike Protein Targeted at Microfold Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. B. subtilis, Plasmids and Animals
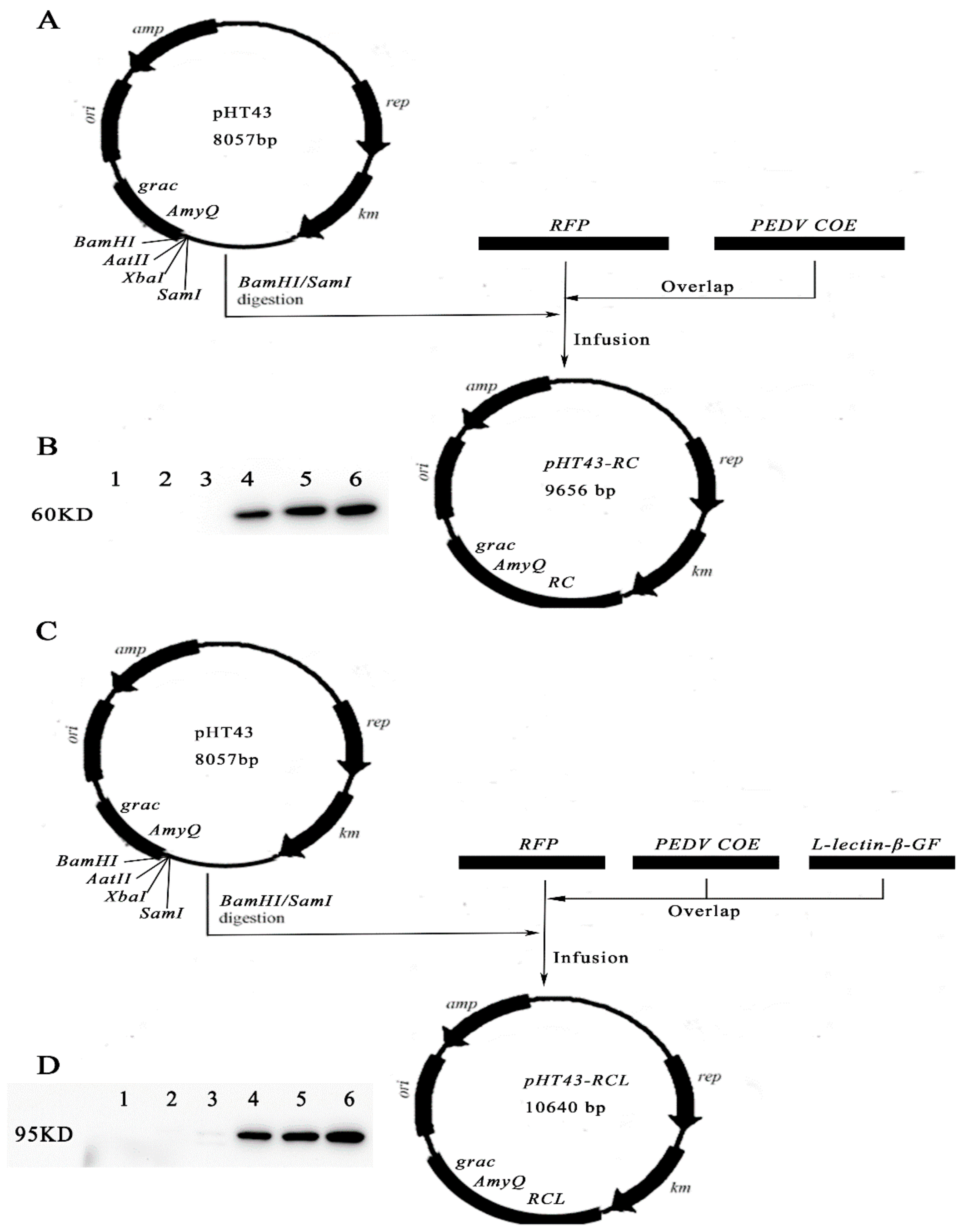
2.2. The Construction of Recombinant B.S.-RC and B.S.-RCL
2.3. Analysis of Fusion Protein
2.4. Ligated Loop Experiments
2.5. Immunisation Schedule and Samples Collection
2.6. Immunofluorescence Staining
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Statistical Analysis
3. Results
3.1. Construction of Recombinant Plasmids and Fusion Protein Expression
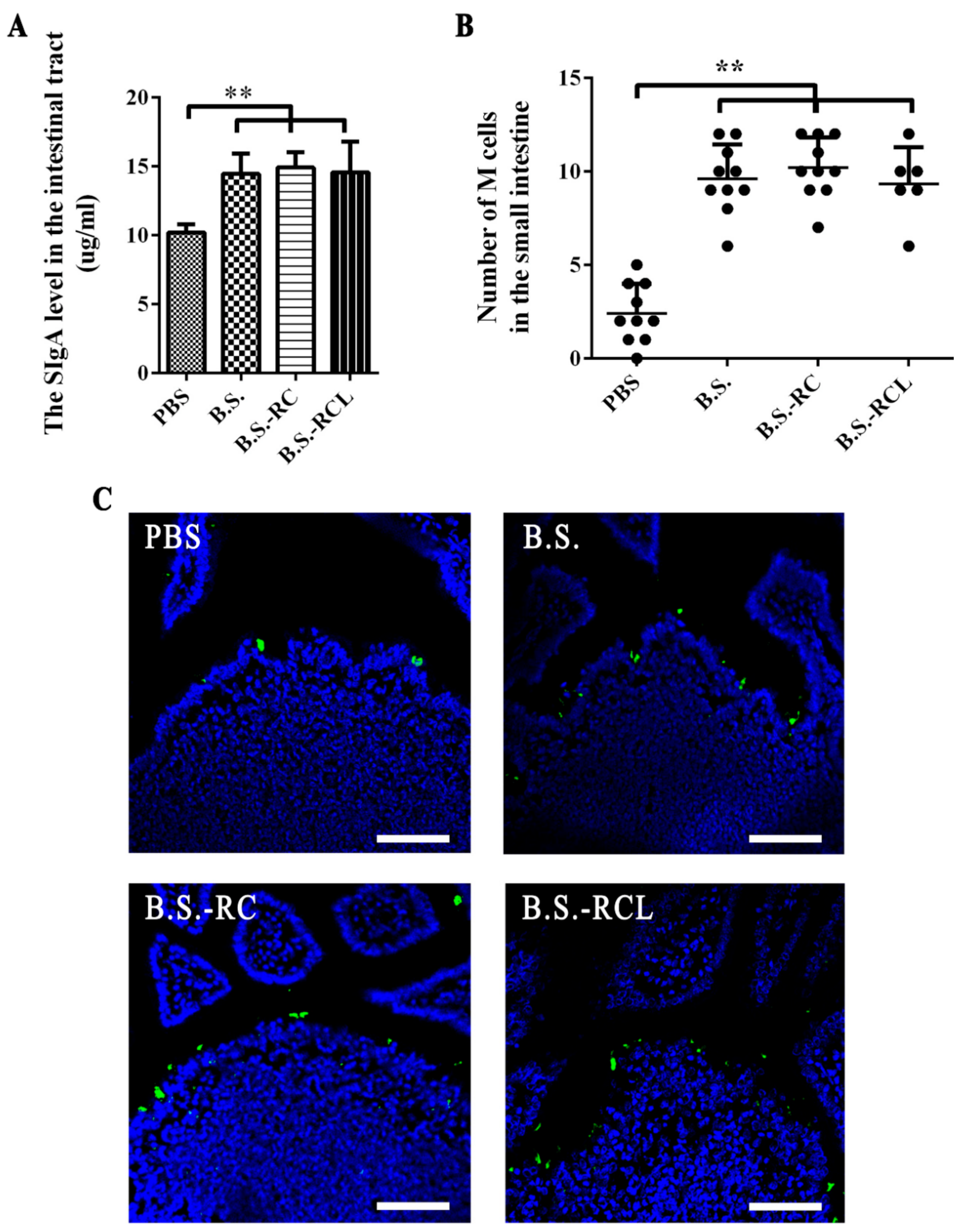
3.2. Animal Experiments Design and sIgA Levels and M Cell Numbers in Mouse Intestines
3.3. Co-Localisation of M Cells and Recombinant B. subtilis
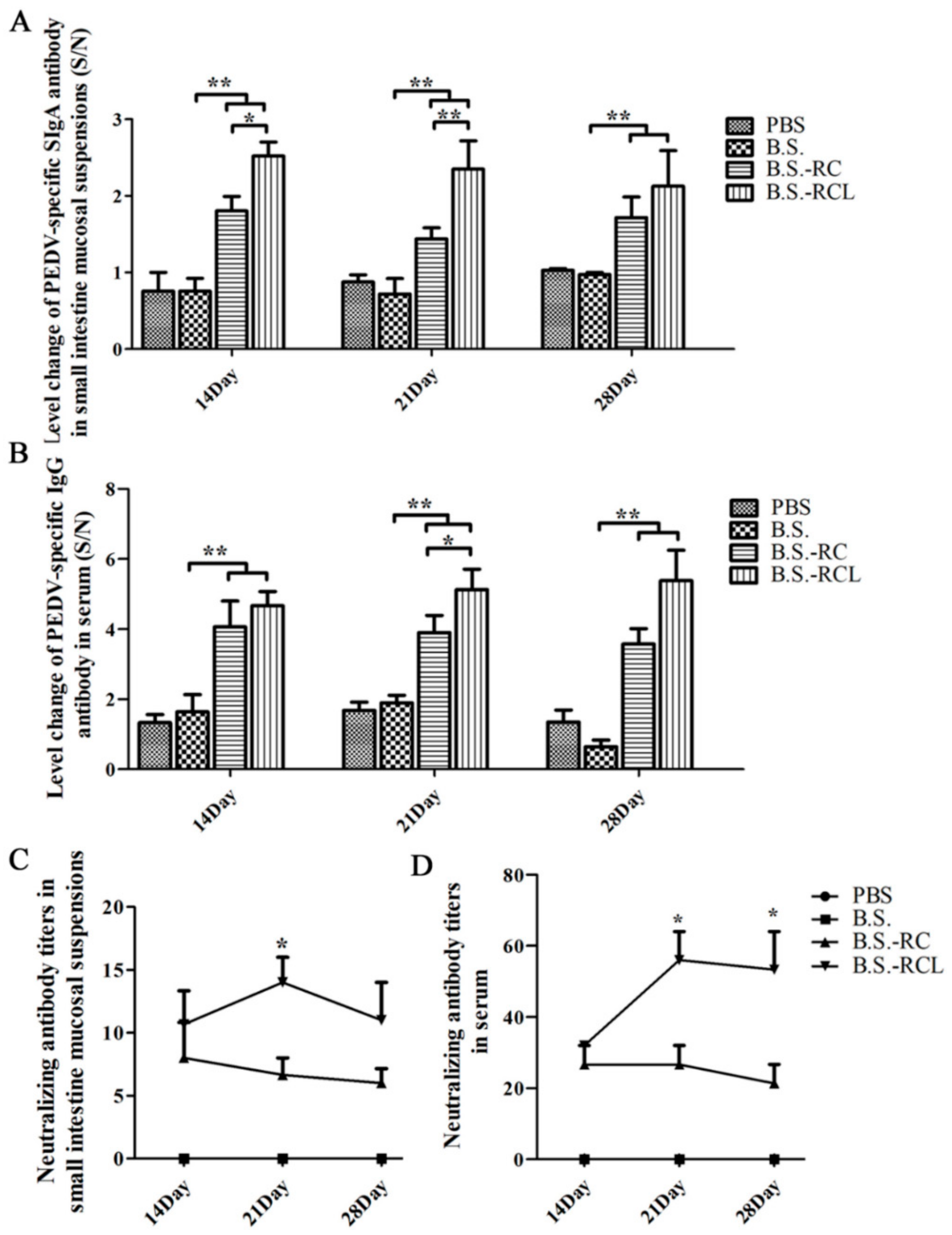
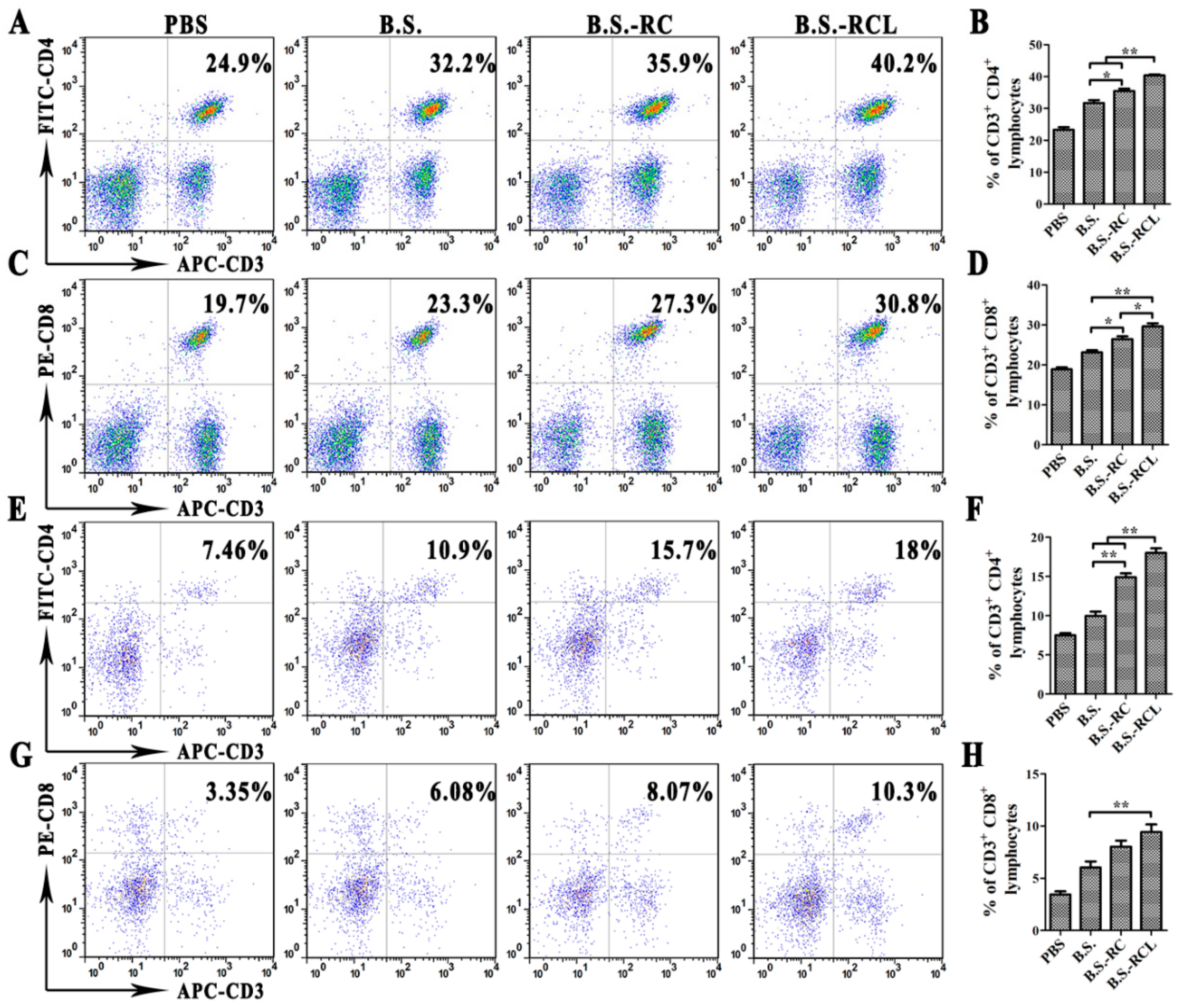
3.4. Systemic and Local Immune Responses after Oral Immunisation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, X.; Piao, X.; Ru, Y.; Zhang, H.; Peron, A.; Zhang, H. Effect of Bacillus amyloliquefaciens-based Direct-fed Microbial on Performance, Nutrient Utilization, Intestinal Morphology and Cecal Microflora in Broiler Chickens. Asian-Australas. J. Anim. Sci. 2015, 28, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Qin, T.; Yin, Y.; Gao, X.; Lin, J.; Yang, Q.; Yu, Q. Bacillus amyloliquefaciens SQR9 induces dendritic cell maturation and enhances the immune response against inactivated avian influenza virus. Sci. Rep. 2016, 6, 21363. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, L.L.; Li, Y.C.; Zhang, P.H.; Yu, Q.H.; Yang, Q. Bacillus subtilis Spore-Trained Dendritic Cells Enhance the Generation of Memory T Cells via ICAM1. Cells 2021, 10, 2267. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Wang, H.; Zhang, J.W.; Tang, X.M.; Raheem, A.; Wang, M.Y.; Lin, W.D.; Liang, L.; Qi, Y.Z.; Zhu, Y.L.; et al. Modulatory Effects of Bacillus subtilis on the Performance, Morphology, Cecal Microbiota and Gut Barrier Function of Laying Hens. Animals 2021, 11, 1523. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Ferreira, R.C.; Schumann, W. Bacillus subtilis as a tool for vaccine development: From antigen factories to delivery vectors. An. Acad. Bras. Cienc. 2005, 77, 113–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoess, R.H. Bacteriophage lambda as a vehicle for peptide and protein display. Curr. Pharm. Biotechnol. 2002, 3, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Zhu, L.; Xing, X.; Lin, J.; Yang, Q. Immune responses induced by recombinant Bacillus subtilis expressing the spike protein of transmissible gastroenteritis virus in pigs. Antivir. Res. 2016, 131, 74–84. [Google Scholar] [CrossRef]
- Spivey, M.A.; Dunn-Horrocks, S.L.; Duong, T. Epithelial cell adhesion and gastrointestinal colonization of Lactobacillus in poultry. Poult. Sci. 2014, 93, 2910–2919. [Google Scholar] [CrossRef]
- Kenngott, E.E.; Kiefer, R.; Schneider-Daum, N.; Hamann, A.; Schneider, M.; Schmitt, M.J.; Breinig, F. Surface-modified yeast cells: A novel eukaryotic carrier for oral application. J. Control. Release 2016, 224, 1–7. [Google Scholar] [CrossRef]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- Woodrow, K.A.; Bennett, K.M.; Lo, D.D. Mucosal vaccine design and delivery. Annu. Rev. Biomed. Eng. 2012, 14, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Gebert, A. The role of M cells in the protection of mucosal membranes. Histochem. Cell Biol. 1997, 108, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.R.; Reddy, G.B.; Ahmad, N.; Swaminathan, C.P.; Mitra, N.; Surolia, A. Legume lectin family, the ‘natural mutants of the quaternary state’, provide insights into the relationship between protein stability and oligomerization. Biochim. Biophys. Acta 2001, 1527, 102–111. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Legume lectins—A large family of homologous proteins. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1990, 4, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Jiang, Y.L.; Zhang, J.; Wang, L.; Bai, X.H.; Zhang, S.J.; Ren, Y.M.; Li, N.; Zhang, Y.H.; Zhang, Z.; et al. Structural insights into SraP-mediated Staphylococcus aureus adhesion to host cells. PLoS Pathog. 2014, 10, e1004169. [Google Scholar] [CrossRef] [PubMed]
- Rochereau, N.; Drocourt, D.; Perouzel, E.; Pavot, V.; Redelinghuys, P.; Brown, G.D.; Tiraby, G.; Roblin, X.; Verrier, B.; Genin, C.; et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 2013, 11, e1001658. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef]
- Suda, Y.; Miyazaki, A.; Miyazawa, K.; Shibahara, T.; Ohashi, S. Systemic and intestinal porcine epidemic diarrhea virus-specific antibody response and distribution of antibody-secreting cells in experimentally infected conventional pigs. Vet. Res. 2021, 52, 2. [Google Scholar] [CrossRef]
- Madson, D.M.; Magstadt, D.R.; Arruda, P.H.; Hoang, H.; Sun, D.; Bower, L.P.; Bhandari, M.; Burrough, E.R.; Gauger, P.C.; Pillatzki, A.E.; et al. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet. Microbiol. 2014, 174, 60–68. [Google Scholar] [CrossRef]
- Tobler, K.; Bridgen, A.; Ackermann, M. Sequence analysis of the nucleocapsid protein gene of porcine epidemic diarrhoea virus. Adv. Exp. Med. Biol. 1993, 342, 49–54. [Google Scholar]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Fake, G.M.; Walker, J.H.; Saltzman, R.; Howard, J.A. Oral Administration of Coronavirus Spike Protein Provides Protection to Newborn Pigs When Challenged with PEDV. Vaccines 2021, 9, 1416. [Google Scholar] [CrossRef] [PubMed]
- Brian, D.A.; Baric, R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005, 287, 1–30. [Google Scholar] [PubMed]
- Narita, J.; Okano, K.; Kitao, T.; Ishida, S.; Sewaki, T.; Sung, M.H.; Fukuda, H.; Kondo, A. Display of alpha-amylase on the surface of Lactobacillus casei cells by use of the PgsA anchor protein, and production of lactic acid from starch. Appl. Environ. Microbiol. 2006, 72, 269–275. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Q.; Gao, J.; Yang, Q. Mucosal and systemic immune responses induced by recombinant Lactobacillus spp. expressing the hemagglutinin of the avian influenza virus H5N1. Clin. Vaccine Immunol. CVI 2012, 19, 174–179. [Google Scholar] [CrossRef]
- Mou, C.; Zhu, L.; Xing, X.; Yang, Q. Expression of major antigenic sites A and D in S gene of Transmissible gastroenteritis virus of swine (TGEV) in Escherichia coli and development of indirect ELISA for detection of the antibody against TGEV. Chin. Vet. Sci. 2015, 45, 356–360. [Google Scholar]
- Morin, M.J.; Warner, A.; Fields, B.N. A pathway for entry of reoviruses into the host through M cells of the respiratory tract. J. Exp. Med. 1994, 180, 1523–1527. [Google Scholar] [CrossRef]
- Taylor, R.T.; Patel, S.R.; Lin, E.; Butler, B.R.; Lake, J.G.; Newberry, R.D.; Williams, I.R. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J. Immunol. 2007, 178, 5659–5667. [Google Scholar] [CrossRef]
- Gat, O.; Inbar, I.; Aloni-Grinstein, R.; Zahavy, E.; Kronman, C.; Mendelson, I.; Cohen, S.; Velan, B.; Shafferman, A. Use of a promoter trap system in Bacillus anthracis and Bacillus subtilis for the development of recombinant protective antigen-based vaccines. Infect. Immun. 2003, 71, 801–813. [Google Scholar] [CrossRef][Green Version]
- Olmos-Soto, J.; Contreras-Flores, R. Genetic system constructed to overproduce and secrete proinsulin in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2003, 62, 369–373. [Google Scholar] [CrossRef]
- Mauriello, E.M.; Duc Le, H.; Isticato, R.; Cangiano, G.; Hong, H.A.; De Felice, M.; Ricca, E.; Cutting, S.M. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 2004, 22, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Wang, X.; Yu, Q.; Yang, Q. Co-administration of attenuated Mycoplasma hyopneumoniae 168 strain with bacterial DNA enhances the local and systemic immune response after intranasal vaccination in pigs. Vaccine 2012, 30, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Santoso, U.; Tanaka, K.; Ohtani, S. Effect of dried Bacillus subtilis culture on growth, body composition and hepatic lipogenic enzyme activity in female broiler chicks. Br. J. Nutr. 1995, 74, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.M.; Fournier, R.S.; Isberg, R.R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990, 9, 1979–1989. [Google Scholar] [CrossRef]
- Marra, A.; Isberg, R.R. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer’s patch intestinal epithelium. Infect. Immun. 1997, 65, 3412–3421. [Google Scholar] [CrossRef]
- Kerneis, S.; Bogdanova, A.; Kraehenbuhl, J.P.; Pringault, E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 1997, 277, 949–952. [Google Scholar] [CrossRef]
- Rochereau, N.; Pavot, V.; Verrier, B.; Ensinas, A.; Genin, C.; Corthesy, B.; Paul, S. Secretory IgA as a vaccine carrier for delivery of HIV antigen to M cells. Eur. J. Immunol. 2015, 45, 773–779. [Google Scholar] [CrossRef]


| Groups | Treatments | |
|---|---|---|
| Intestinal ligation | PBS-15 min | PBS (200 mL for 15 min or 1 h) |
| PBS-1 h | ||
| B.S.-RC-15 min | DyLight 488-labeled recombinant B.S.-RC (200 mL for 15 min or 1 h) | |
| B.S.-RC-1 h | ||
| B.S.-RCL-15 min | DyLight 488-labeled recombinant B.S.-RCL (200 mL for 15 min or 1 h) | |
| B.S.-RCL-1 h | ||
| Oral administration | PBS | PBS |
| PBS (0.01M PBS 150 μL, n = 24) | PBS (0.01 M PBS 150 μL, n = 24) | |
| B.S. | B.S. | |
| B.S. (1010 cfu/kg) | B.S. (1010 cfu/kg) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Mou, C.; Zhang, S.; Zhu, L.; Li, Y.; Yang, Q. Immune Responses Induced by Recombinant Bacillus subtilis Expressing the PEDV Spike Protein Targeted at Microfold Cells. Vet. Sci. 2022, 9, 211. https://doi.org/10.3390/vetsci9050211
Lin J, Mou C, Zhang S, Zhu L, Li Y, Yang Q. Immune Responses Induced by Recombinant Bacillus subtilis Expressing the PEDV Spike Protein Targeted at Microfold Cells. Veterinary Sciences. 2022; 9(5):211. https://doi.org/10.3390/vetsci9050211
Chicago/Turabian StyleLin, Jian, Chunxiao Mou, Shuai Zhang, Liqi Zhu, Yuchen Li, and Qian Yang. 2022. "Immune Responses Induced by Recombinant Bacillus subtilis Expressing the PEDV Spike Protein Targeted at Microfold Cells" Veterinary Sciences 9, no. 5: 211. https://doi.org/10.3390/vetsci9050211
APA StyleLin, J., Mou, C., Zhang, S., Zhu, L., Li, Y., & Yang, Q. (2022). Immune Responses Induced by Recombinant Bacillus subtilis Expressing the PEDV Spike Protein Targeted at Microfold Cells. Veterinary Sciences, 9(5), 211. https://doi.org/10.3390/vetsci9050211







