Expression of Antimicrobial Peptide Genes in the Canine Amniotic Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Canine Amniotic Membrane
2.2. Sample Collection
2.3. RNA Isolation
2.4. Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Röck, T.; Bartz-Schmidt, K.U.; Landenberger, J.; Bramkamp, M.; Röck, D. Amniotic Membrane Transplantation in Reconstructive and Regenerative Ophthalmology. Ann. Transplant. 2018, 23, 160–165. [Google Scholar] [CrossRef]
- Castellanos, G.; Bernabé-García, Á.; Moraleda, J.M.; Nicolás, F.J. Amniotic Membrane Application for the Healing of Chronic Wounds and Ulcers. Placenta 2017, 59, 146–153. [Google Scholar] [CrossRef]
- Amer, M.I.; Abd-El-Maeboud, K.H. Amnion Graft Following Hysteroscopic Lysis of Intrauterine Adhesions. J. Obstet. Gynaecol. 2006, 32, 559–566. [Google Scholar] [CrossRef]
- Adamowicz, J.; van Breda, S.; Tyloch, D.; Pokrywczynska, M.; Drewa, T. Application of Amniotic Membrane in Reconstructive Urology; the Promising Biomaterial Worth Further Investigation. Expert Opin. Biol. 2019, 19, 9–24. [Google Scholar] [CrossRef]
- Francisco, J.C.; Correa Cunha, R.; Cardoso, M.A.; Baggio Simeoni, R.; Mogharbel, B.F.; Picharski, G.L.; Silva Moreira Dziedzic, D.; Guarita-Souza, L.C.; Carvalho, K.A.T. Decellularized Amniotic Membrane Scaffold as a Pericardial Substitute: An In Vivo Study. Transplant. Proc. 2016, 48, 2845–2849. [Google Scholar] [CrossRef]
- Odet, S.; Louvrier, A.; Meyer, C.; Nicolas, F.J.; Hofman, N.; Chatelain, B.; Mauprivez, C.; Laurence, S.; Kerdjoudj, H.; Zwetyenga, N.; et al. Surgical Application of Human Amniotic Membrane and Amnion-Chorion Membrane in the Oral Cavity and Efficacy Evaluation: Corollary with Ophthalmological and Wound Healing Experiences. Front. Bioeng. Biotechnol. 2021, 9, 685128. [Google Scholar] [CrossRef]
- De Rötth, A. Plastic Repair of Conjunctival Defects with Fetal Membranes. Arch. Ophthalmol. 1940, 23, 522–525. [Google Scholar] [CrossRef]
- Kim, J.C.; Tseng, S.C. Transplantation of Preserved Human Amniotic Membrane for Surface Reconstruction in Severely Damaged Rabbit Corneas. Cornea 1995, 19, 473–484. [Google Scholar] [CrossRef]
- Walkden, A. Amniotic Membrane Transplantation in Ophthalmology: An Updated Perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Siu, G.D.J.Y.; Kam, K.W.; Young, A.L. Amniotic Membrane Transplant for Bullous Keratopathy: Confocal Microscopy & Anterior Segment Optical Coherence Tomography. Semin. Ophthalmol. 2019, 34, 163–167. [Google Scholar] [CrossRef]
- Sabater, A.L.; Perez, V.L. Amniotic Membrane Use for Management of Corneal Limbal Stem Cell Deficiency. Curr. Opin. Ophthalmol. 2017, 28, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Meller, D.; Prabhasawat, P.; John, T.; Espana, E.M.; Steuhl, K.-P.; Tseng, S.C.G. Amniotic Membrane Grafts for Nontraumatic Corneal Perforations, Descemetoceles, and Deep Ulcers. Ophthalmology 2002, 109, 694–703. [Google Scholar] [CrossRef]
- Jirsova, K.; Jones, G.L.A. Amniotic Membrane in Ophthalmology: Properties, Preparation, Storage and Indications for Grafting—A Review. Cell Tissue Bank. 2017, 18, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Inatomi, T.; Sotozono, C.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth Factor MRNA and Protein in Preserved Human Amniotic Membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef]
- Tseng, S.C.G.; Espana, E.M.; Kawakita, T.; di Pascuale, M.A.; Li, W.; He, H.; Liu, T.S.; Cho, T.H.; Gao, Y.Y.; Yeh, L.K.; et al. How Does Amniotic Membrane Work? Ocul. Surf. 2004, 2, 177–187. [Google Scholar] [CrossRef]
- Hao, Y.; Hui-Kang Ma, D.; Hwang, D.G.; Kim, W.-S.; Zhang, F. Identification of Antiangiogenic and Antiinflammatory Proteins in Human Amniotic Membrane. Cornea 2000, 19, 348–352. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, J.-C.; Hahn, T.-W.; Park, W.-C. Amniotic Membrane Transplantation in Infectious Corneal Ulcer. Cornea 2001, 20, 720–726. [Google Scholar] [CrossRef]
- Ramuta, T.Ž.; Šket, T.; Starčič Erjavec, M.; Kreft, M.E. Antimicrobial Activity of Human Fetal Membranes: From Biological Function to Clinical Use. Front. Bioeng. Biotechnol. 2021, 9, 691522. [Google Scholar] [CrossRef]
- Stock, S.J.; Kelly, R.W.; Riley, S.C.; Calder, A.A. Natural Antimicrobial Production by the Amnion. Am. J. Obstet. Gynecol. 2007, 196, 255.e1–255.e6. [Google Scholar] [CrossRef]
- King, A.E.; Paltoo, A.; Kelly, R.W.; Sallenave, J.M.; Bocking, A.D.; Challis, J.R.G. Expression of Natural Antimicrobials by Human Placenta and Fetal Membranes. Placenta 2007, 28, 161–169. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Jabr, M.; Buhimschi, C.S.; Petkova, A.P.; Weiner, C.P.; Saed, G.M. The Novel Antimicrobial Peptide Β3-Defensin Is Produced by the Amnion: A Possible Role of the Fetal Membranes in Innate Immunity of the Amniotic Cavity. Am. J. Obstet. Gynecol. 2004, 191, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.A.; Modaresifar, K.; Azizian, S.; Niknejad, H. Induction of Antimicrobial Peptides Secretion by IL-1β Enhances Human Amniotic Membrane for Regenerative Medicine. Sci. Rep. 2017, 7, 17022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruitwala, S.; El-Naccache, D.W.; Chang, T.L. Multifaceted Immune Functions of Human Defensins and Underlying Mechanisms. Semin. Cell Dev. Biol. 2019, 88, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.B.; van Sterkenburg, M.A.; Rabe, K.F.; Schalkwijk, J.; Hiemstra, P.S.; Datson, N.A. Transcriptional Response of Bronchial Epithelial Cells to Pseudomonas Aeruginosa: Identification of Early Mediators of Host Defense. Physiol. Genom. 2005, 21, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Teng, G.; Wu, T.; Tian, Y.; Wang, H. Expression and Clinical Significance of Elafin in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2134–2141. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Shaw, L.; Wiedow, O. Therapeutic Potential of Human Elafin. Biochem. Soc. Trans. 2011, 39, 1450–1454. [Google Scholar] [CrossRef]
- Ollivier, F.J.; Kallberg, M.E.; Plummer, C.E.; Barrie, K.P.; O’reilly, S.; Taylor, D.P.; Gelatt, K.N.; Brooks, D.E. Amniotic Membrane Transplantation for Corneal Surface Reconstruction after Excision of Corneolimbal Squamous Cell Carcinomas in Nine Horses. Vet. Ophthalmol. 2006, 9, 404–413. [Google Scholar] [CrossRef]
- Ion, L.; Ionascu, I.; Garcia De Joz, C.; Cerrada, I.; Birtoiu, A.; Huguet, E. Human Amniotic Membrane Transplantation in the Treatment of Feline Corneal Sequestrum: Preliminary Results. AgroLife Sci. J. 2016, 5, 91–98. [Google Scholar]
- Korittum, A.; Kassem, M.; Adel, A.; Gaith, A.; Habashi, N. Effect of Human Amniotic Membrane Transplantation in Reconstruction of Canine Corneal Wound. Alex. J. Vet. Sci. 2019, 60, 56. [Google Scholar] [CrossRef]
- Costa, D.; Leiva, M.; Sanz, F.; Espejo, V.; Esteban, J.; Vergara, J.; Díaz, C.; Huguet, E.; Cairó, M.; Ríos, J.; et al. A Multicenter Retrospective Study on Cryopreserved Amniotic Membrane Transplantation for the Treatment of Complicated Corneal Ulcers in the Dog. Vet. Ophthalmol. 2019, 22, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Barros, P.S.; Garcia, J.A.; Laus, J.L.; Ferreira, A.L.; Salles Gomes, T.L. The Use of Xenologous Amniotic Membrane to Repair Canine Corneal Perforation Created by Penetrating Keratectomy. Vet. Ophthalmol. 1998, 1, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Plummer, C.E. The Use of Amniotic Membrane Transplantation for Ocular Surface Reconstruction: A Review and Series of 58 Equine Clinical Cases (2002–2008). Vet. Ophthalmol. 2009, 12 (Suppl. 1), 17–24. [Google Scholar] [CrossRef]
- Arcelli, R.; Tibaldini, P.; Angeli, G.; Bellezza, E. Equine Amniotic Membrane Transplantation in Some Ocular Surface Diseases in the Dog and Cat: A Preliminary Study. Vet. Res. Commun. 2009, 33, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, Y.M.; Jeong, S.W.; Williams, D.L.; Kim, J.Y. Effect of Bovine Freeze-Dried Amniotic Membrane (Amnisite-BA TM) on Uncomplicated Canine Corneal Erosion. Vet. Ophthalmol. 2009, 12, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, K.; Yamashita, K.; Izumisawa, Y.; Kotani, T.; Tsuzuki, K. Microstructure and Glycosaminoglycan Ratio of Canine Cornea after Reconstructive Transplantation with Glycerin-Preserved Porcine Amniotic Membranes. Vet. Ophthalmol. 2008, 11, 222–227. [Google Scholar] [CrossRef]
- Barros, P.S.; Safatle, A.M.; Godoy, C.A.; Souza, M.S.; Barros, L.F.; Brooks, D.E. Amniotic Membrane Transplantation for the Reconstruction of the Ocular Surface in Three Cases. Vet. Ophthalmol. 2005, 8, 189–192. [Google Scholar] [CrossRef]
- Vongsakul, S.; Tuntivanich, P.; Sirivaidyapong, S.; Kalpravidh, M. Canine Amniotic Membrane Transplantation for Ocular Surface Reconstruction of Created Deep Corneal in Dogs. Thai J. Vet. Med. 2009, 39, 135–144. [Google Scholar]
- Kalpravidh, M.; Tuntivanich, P.; Vongsakul, S.; Sirivaidyapong, S. Canine Amniotic Membrane Transplantation for Corneal Reconstruction after the Excision of Dermoids in Dogs. Vet. Res. Commun. 2009, 33, 1003–1012. [Google Scholar] [CrossRef]
- Withavatpongtorn, N.; Tuntivanich, N. Characterization of Cryopreserved Canine Amniotic Membrane. Membranes 2021, 11, 824. [Google Scholar] [CrossRef]
- Phoomvuthisarn, P.; Suriyaphol, G.; Tuntivanich, N. Effect of Glycerol Concentrations and Temperatures on Epidermal Growth Factor Protein Expression in Preserved Canine Amniotic Membrane. Cell Tissue Bank. 2019, 20, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Pálffy, R.; Gardlík, R.; Behuliak, M.; Kadasi, L.; Turna, J.; Celec, P. On the Physiology and Pathophysiology of Antimicrobial Peptides. Mol. Med. 2009, 15, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.A.; Cai, Y.; Sang, Y.; Blecha, F.; Zhang, G. Cross-Species Analysis of the Mammalian-Defensin Gene Family: Presence of Syntenic Gene Clusters and Preferential Expression in the Male Reproductive Tract. Physiol. Genom. 2005, 23, 5–17. [Google Scholar] [CrossRef]
- Wingate, K.V.; Torres, S.M.; Silverstein, K.A.T.; Hendrickson, J.A.; Rutherford, M.S. Expression of Endogenous Antimicrobial Peptides in Normal Canine Skin. Vet. Dermatol. 2008, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hughes, A.L.; Ando, J.; Matsuda, Y.; Cheng, J.F.; Skinner-Noble, D.; Zhang, G. A Genome-Wide Screen Identifies a Single β-Defensin Gene Cluster in the Chicken: Implications for the Origin and Evolution of Mammalian Defensins. BMC Genom. 2004, 5, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.K.; Le, M.T.; Nguyen, D.T.; Choi, H.; Kim, W.; Kim, J.H.; Chun, J.; Hyeon, J.; Seo, K.; Park, C. Genome-Level Identification, Gene Expression, and Comparative Analysis of Porcine ß-Defensin Genes. BMC Genet. 2012, 13, 98. [Google Scholar] [CrossRef] [Green Version]
- Meade, K.G.; Cormican, P.; Narciandi, F.; Lloyd, A.; O’Farrelly, C. Bovine β-Defensin Gene Family: Opportunities to Improve Animal Health? Physiol. Genom. 2014, 46, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Leonard, B.C.; Marks, S.L.; Outerbridge, C.A.; Affolter, V.K.; Kananurak, A.; Young, A.; Moore, P.F.; Bannasch, D.L.; Bevins, C.L. Activity, Expression and Genetic Variation of Canine β-Defensin 103: A Multifunctional Antimicrobial Peptide in the Skin of Domestic Dogs. J. Innate Immun. 2012, 4, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Aono, S.; Dennis, J.C.; He, S.; Wang, W.; Tao, Y.X.; Morrison, E.E. Exploring Pleiotropic Functions of Canine β-Defensin 103: Nasal Cavity Expression, Antimicrobial Activity, and Melanocortin Receptor Activity. Anat. Rec. 2021, 304, 210–221. [Google Scholar] [CrossRef]
- Erles, K.; Brownlie, J. Expression of β-Defensins in the Canine Respiratory Tract and Antimicrobial Activity against Bordetella Bronchiseptica. Vet. Immunol. Immunopathol. 2010, 135, 12–19. [Google Scholar] [CrossRef]
- Sang, Y.; Ortega, M.T.; Blecha, F.; Prakash, O.; Melgarejo, T. Molecular Cloning and Characterization of Three β-Defensins from Canine Testes. Infect. Immun. 2005, 73, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- van Damme, C.M.M.; Willemse, T.; van Dijk, A.; Haagsman, H.P.; Veldhuizen, E.J.A. Altered Cutaneous Expression of β-Defensins in Dogs with Atopic Dermatitis. Mol. Immunol. 2009, 46, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Lancto, C.A.; Torres, S.M.F.; Hendrickson, J.A.; Martins, K.V.; Rutherford, M.S. Altered Expression of Antimicrobial Peptide Genes in the Skin of Dogs with Atopic Dermatitis and Other Inflammatory Skin Conditions. Vet. Dermatol. 2013, 24, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Chinn, R.M.; Vorachek, W.R.; Gorman, M.E.; Jewell, D.E. Aged Beagle Dogs Have Decreased Neutrophil Phagocytosis and Neutrophil-Related Gene Expression Compared to Younger Dogs. Vet. Immunol. Immunopathol. 2010, 137, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Day, P.E.; Ntani, G.; Crozier, S.R.; Mahon, P.A.; Inskip, H.M.; Cooper, C.; Harvey, N.C.; Godfrey, K.M.; Hanson, M.A.; Lewis, R.M.; et al. Maternal Factors Are Associated with the Expression of Placental Genes Involved in Amino Acid Metabolism and Transport. PLoS ONE 2015, 10, e0143653. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.E.; Brown, T.I.; Roghanian, A.; Sallenave, J.M. SLPI and Elafin: One Glove, Many Fingers. Clin. Sci. 2006, 110, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Bhagavan, N.V.; Ha, C.-E. Regulation of Gene Expression. In Essentials of Medical Biochemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 447–464. [Google Scholar]
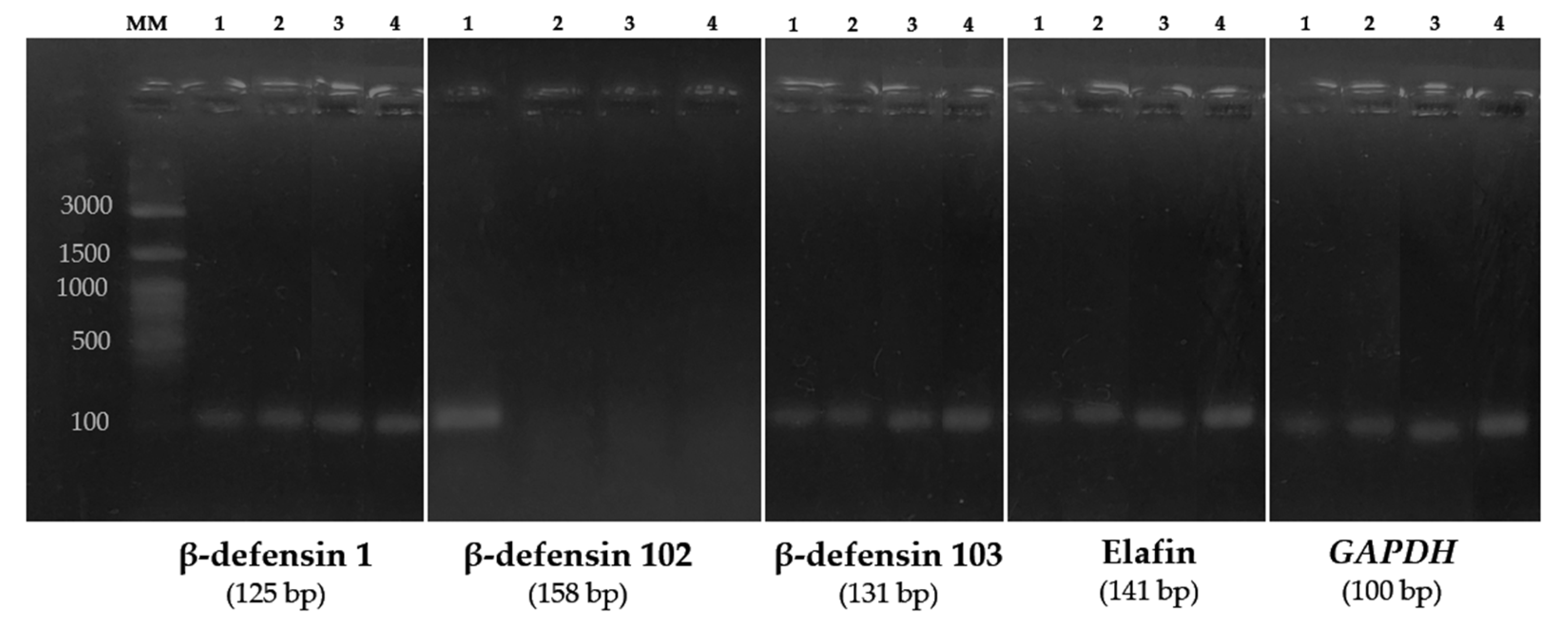
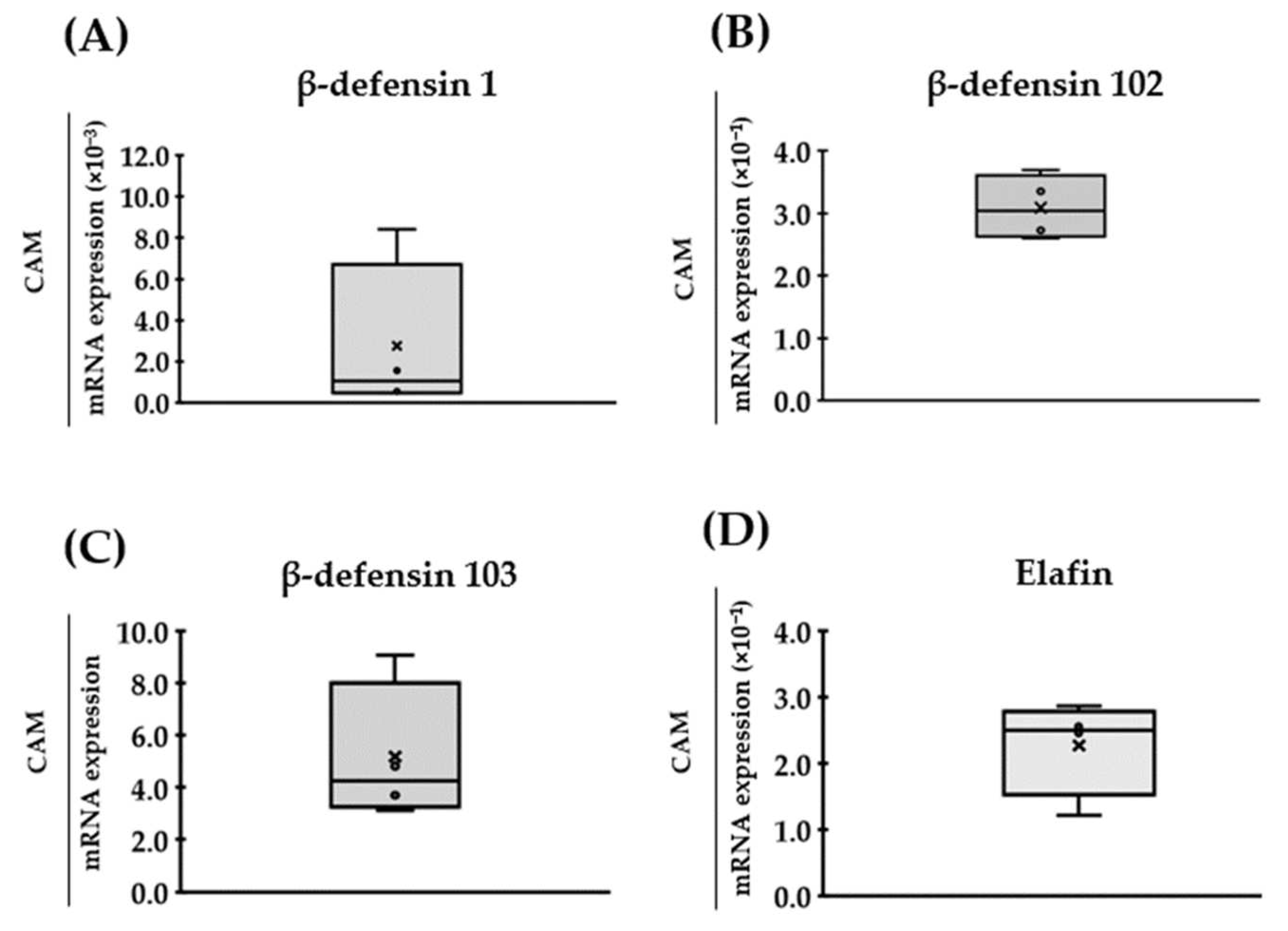
| Gene | Accession Number | Sequences | Primer Pairs | Tm (°C) | Length (bp) |
|---|---|---|---|---|---|
| cBD1 | NM_001113713 | Forward | ATGAGGCCTCTCTACTTGCTG | 59.24 | 125 |
| Reverse | CCTCCTTTCCTGGCACAGATG | 60.68 | |||
| cBD102 | NM_001113715 | Forward | CCCTGAGTTTGTCAACCATGA | 58.14 | 158 |
| Reverse | CCGGTTATGAGGGCATCTGAAT | 60.22 | |||
| cBD103 | NM_001129980 | Forward | GCCTGTTGGTCATGAGGATCT | 59.79 | 131 |
| Reverse | GCACCGACCGCTCCTTATT | 60.15 | |||
| Elafin | NM_001290099 | Forward | TTCTTGGTCCTGGCAGTGTT | 59.45 | 141 |
| Reverse | CGGATCTCGACCTCTAACCG | 59.41 | |||
| GAPDH | NM_001003142 | Forward | CCAACTGCTTGGCTCCTCTA | 59.38 | 100 |
| Reverse | GTCTTCTGGGTGGCAGTGAT | 59.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohajaroensub, R.; Sawangmake, C.; Rodkhum, C.; Tuntivanich, N. Expression of Antimicrobial Peptide Genes in the Canine Amniotic Membrane. Vet. Sci. 2022, 9, 200. https://doi.org/10.3390/vetsci9050200
Lohajaroensub R, Sawangmake C, Rodkhum C, Tuntivanich N. Expression of Antimicrobial Peptide Genes in the Canine Amniotic Membrane. Veterinary Sciences. 2022; 9(5):200. https://doi.org/10.3390/vetsci9050200
Chicago/Turabian StyleLohajaroensub, Rajit, Chenphop Sawangmake, Channarong Rodkhum, and Nalinee Tuntivanich. 2022. "Expression of Antimicrobial Peptide Genes in the Canine Amniotic Membrane" Veterinary Sciences 9, no. 5: 200. https://doi.org/10.3390/vetsci9050200
APA StyleLohajaroensub, R., Sawangmake, C., Rodkhum, C., & Tuntivanich, N. (2022). Expression of Antimicrobial Peptide Genes in the Canine Amniotic Membrane. Veterinary Sciences, 9(5), 200. https://doi.org/10.3390/vetsci9050200







