Serum Amyloid A Concentrations of Healthy and Clinically Diseased Japanese Black Breeding Cattle—Preliminary Measurements for Determining the Cut-Off Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. JB Breeding Herds and Cows
2.2. SAA Concentration Measurement
2.3. Data Management and Statistical Analysis
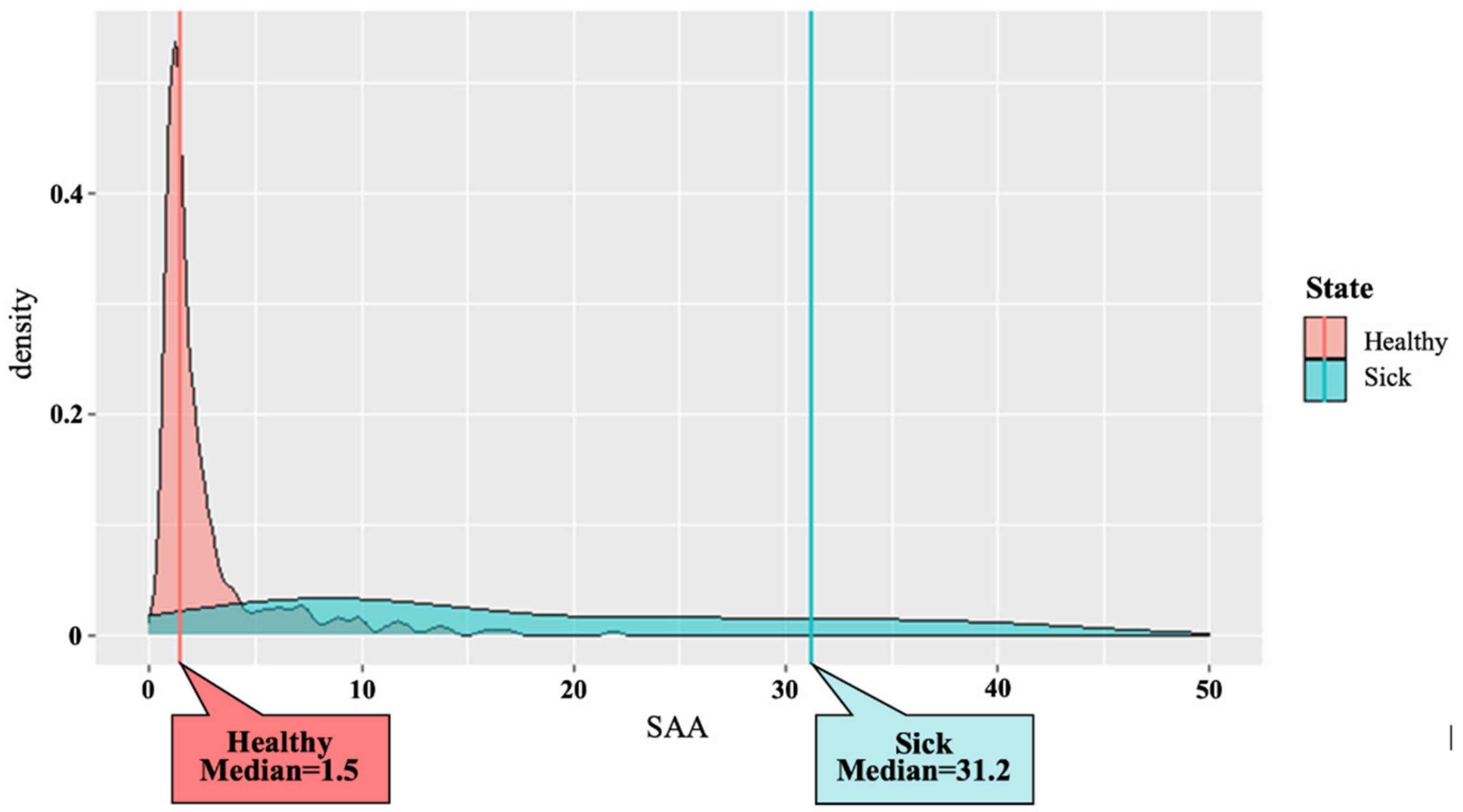
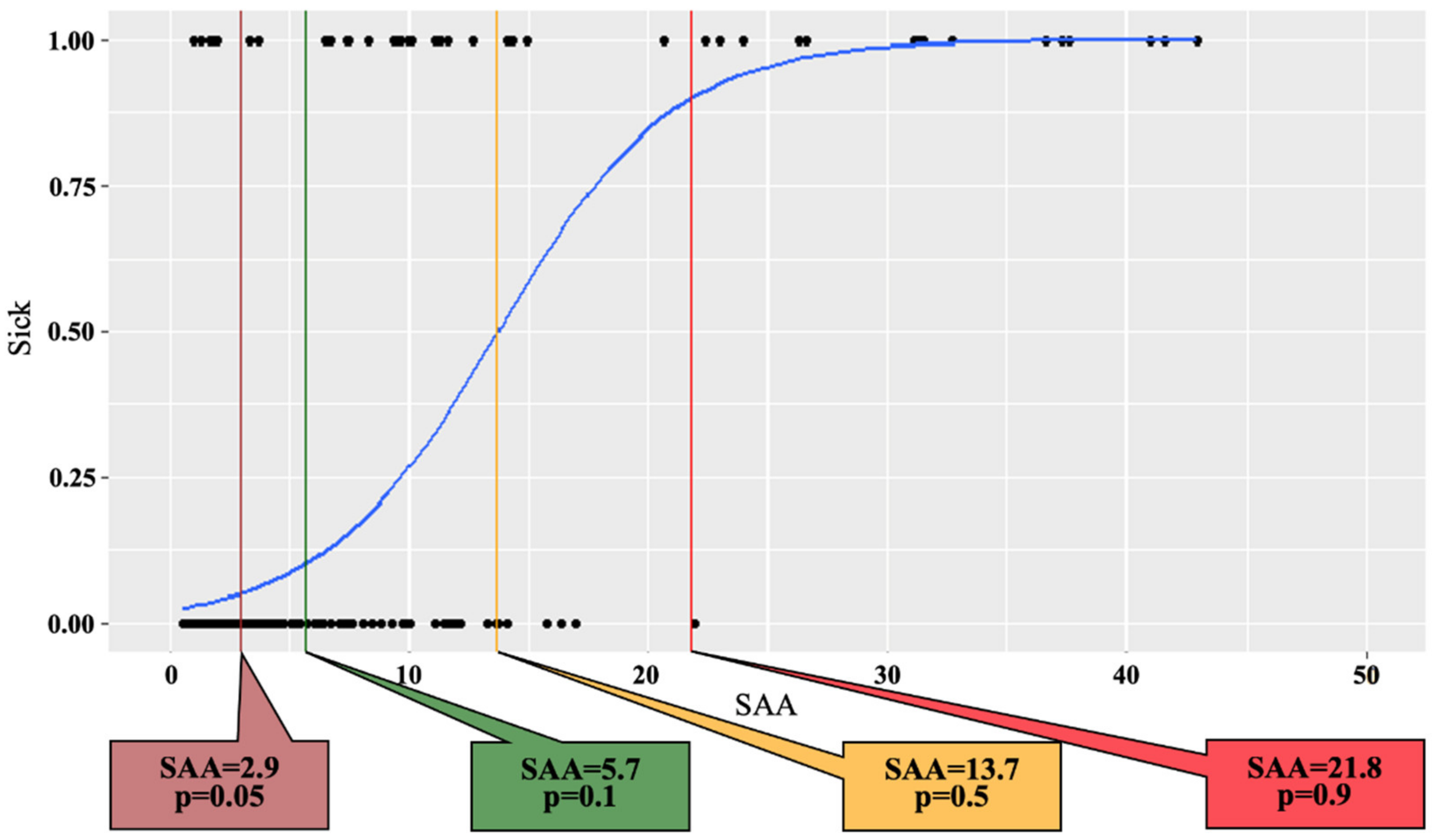
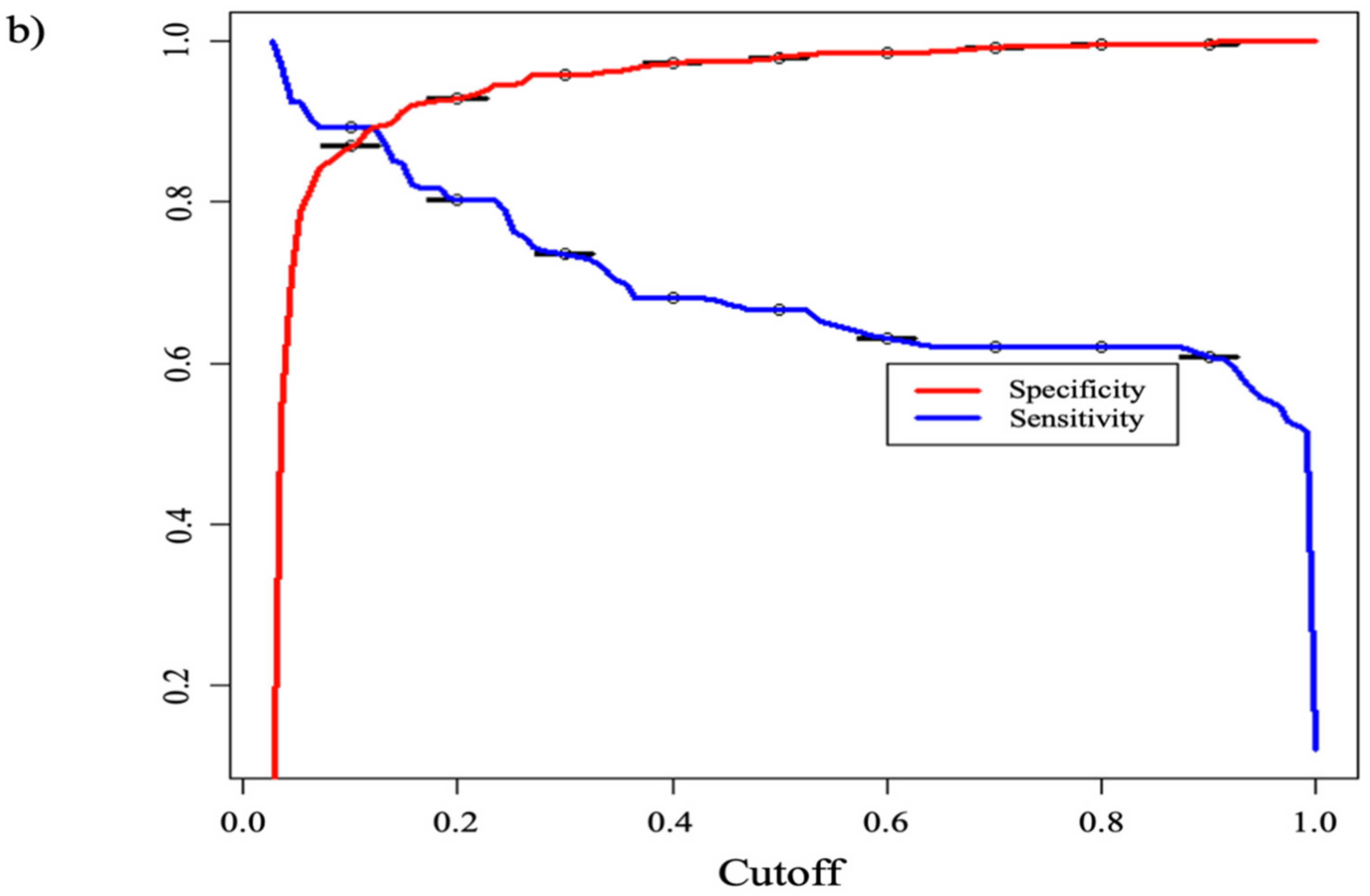
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasser, R.G.; Williams, R.J.; Bull, R.C.; Ruder, C.A.; Falk, D.G. Postpartum reproductive performance in crude protein-restricted beef cows: Return to estrus and conception. J. Anim. Sci. 1988, 66, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Short, R.E.; Adams, D.C. Nutritional and hormonal interrelationships in beef cattle reproduction. Can. J. Anim. Sci. 1988, 68, 29–39. [Google Scholar] [CrossRef]
- Stagg, K.; Spicer, L.J.; Sreenan, J.M.; Roche, J.F.; Diskin, M.G. Effect of calf isolation on follicular wave dynamics, gonadotropin and metabolic hormone changes, and interval to first ovulation in beef cows fed either of two energy levels postpartum. Biol. Reprod. 1998, 59, 777–783. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huzzey, J.M.; Mann, S.; Nydam, D.V.; Grant, R.J.; Overton, T.R. Associations of peripartum markers of stress and inflammation with milk yield and reproductive performance in Holstein dairy cows. Prev. Vet. Med. 2015, 120, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Shimada, N.; Yoshioka, M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- Abdallah, A.; Hewson, J.; Francoz, D.; Selim, H.; Buczinski, S. Systematic review of the diagnostic accuracy of haptoglobin, serum amyloid A, and fibrinogen versus clinical reference, standards for the diagnosis of bovine respiratory disease. J. Vet. Intern. Med. 2016, 30, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Alsemgeest, S.P.M.; Kalsbeek, H.C.; Wensing, T.; Koeman, J.P.; van Ederen, A.M.; Gruys, E. Concentrations of serum amyloid-A (SAA) and haptoglobin (HP) as parameters of inflammatory diseases in cattle. Vet. Q. 1994, 16, 21–23. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase protein in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Horadagoda, N.U.; Knox, K.M.G.; Gibbs, H.A.; Reid, S.W.J.; Horadagoda, A.; Edwards, S.E.R.; Eckersall, P.D. Acute phase proteins in cattle: Discrimination between acute and chronic inflammation. Vet. Rec. 1999, 144, 437–441. [Google Scholar] [CrossRef]
- Petersen, H.H.; Nielsen, J.P.; Heegaard, P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004, 35, 163–187. [Google Scholar] [CrossRef]
- Heegaard, P.M.H.; Godson, D.L.; Toussaint, M.J.M.; Tjørnehøj, K.; Larsen, L.E.; Viuff, B.; Rønsholt, L. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 2000, 77, 151–159. [Google Scholar] [CrossRef]
- Otsuka, M.; Sugiyama, M.; Ito, S.; Tsukano, K.; Oikawa, S.; Suzuki, K. Diagnostic utility of measuring serum amyloid A with a latex agglutination turbidimetric immunoassay in bovine mastitis: Comparison with haptoglobin and alpha 1 acid glycoprotein. J. Vet. Med. Sci. 2021, 83, 329–332. [Google Scholar] [CrossRef]
- Okawa, H.; Monniaux, D.; Mizokami, C.; Fujikura, A.; Takano, T.; Sato, S.; Shinya, U.; Kawashima, C.; Yamato, O.; Fushimi, Y.; et al. Association between Anti-Müllerian hormone concentration and inflammation markers in serum during the peripartum period in dairy cows. Animals 2021, 11, 1241. [Google Scholar] [CrossRef]
- Sasazaki, N.; Obi, T.; Aridome, C.; Fujimoto, Y.; Furumoto, M.; Toda, K.; Hasunuma, H.; Matsumoto, D.; Sato, S.; Okawa, H.; et al. Effects of dietary feed supplementation of heat-treated Lactobacillus sakei HS-1 on the health status, blood parameters, and fecal microbes of Japanese Black calves. J. Vet. Med. Sci. 2020, 82, 1428–1435. [Google Scholar] [CrossRef]
- Hess, B.W.; Lake, S.L.; Scholljegerdes, E.J.; Weston, T.R.; Nayigihugu, V.; Molle, J.D.C.; Moss, G.E. Nutritional controls of beef cow reproduction. J. Anim. Sci. 2005, 83, E90–E106. [Google Scholar]
- Filho, B.D.O.; Toniollo, G.H.; Oliveira, A.F.D.; Viu, M.A.O.; Ferraz, H.T.; Lopes, D.T.; Gambarini, M.L. The effect of offering an energy and protein supplement to grazing Canchim beef cows either postpartum or both pre- and postpartum on lipid blood metabolites and folliculogenesis. Anim. Reprod. Sci. 2010, 121, 39–45. [Google Scholar] [CrossRef]
- Watanabe, U.; Okamoto, K.; Miyamoto, A.; Otoi, T.; Yamato, O.; Tshering, C.; Takagi, M. A Japanese Black breeding herd exhibiting low blood urea nitrogen: A metabolic profile study examining the effect on reproductive performance. Anim. Sci. J. 2013, 84, 389–394. [Google Scholar] [CrossRef]
- Watanabe, U.; Takagi, M.; Yamato, O.; Otoi, T.; Okamoto, K. Retrospective surveillance of metabolic parameters affecting reproductive performance of Japanese Black breeding multiparous cows. J. Vet. Sci. 2014, 15, 283–288. [Google Scholar] [CrossRef]
- Watanabe, U.; Takagi, M.; Yamato, O.; Otoi, T.; Tshering, C.; Okamoto, K. Metabolic profile of Japanese Black breeding cattle herds: Usefulness in selection for nutrient supplementation to enhance reproductive performance and regional differences. J. Vet. Med. Sci. 2013, 75, 481–487. [Google Scholar] [CrossRef]
- Shinya, U.; Yamato, O.; Sato, S.; Kajisa, M.; Takagi, M. Metabolic profile of Japanese Black breeding cattle herds: Its usefulness in the detection of abnormal metabolism resulting from high protein diets. Anim. Nutr. Feed Technol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Giannetto, C.; Fazio, F.; Casella, S.; Marafioti, S.; Giudice, E.; Piccione, G. Acute phase protein response during road transportation and lairage at a slaughterhouse in feedlot beef cattle. J. Vet. Med. Sci. 2011, 73, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Gånheim, C.; Alenius, S.; Waller, K.P. Acute phase proteins as indicators of calf herd health. Vet. J. 2007, 173, 645–651. [Google Scholar] [CrossRef]
- Schmitt, R.; Pieper, L.; Gonzalez-Grajales, L.A.; Swinkels, J.; Gelfert, C.C.; Staufenbiel, R. Evaluation of different acute-phase proteins for herd health diagnostics in early postpartum Holstein Friesian dairy cows. J. Dairy Res. 2021, 88, 33–37. [Google Scholar] [CrossRef]
- Karreman, H.J.; Wentink, G.H.; Wensing, T. Using serum amyloid A to screen dairy cows for sub-clinical inflammation. Vet. Q. 2000, 22, 175–178. [Google Scholar] [CrossRef]

| Clinical Diagnosis | No. of Cows |
|---|---|
| Reproductive disease | 14 |
| Fever, no appetite | 11 |
| Enteritis | 10 |
| Hepatitis | 7 |
| Lymphoma | 7 |
| Musculoskeletal disease | 7 |
| Unable to stand, downer | 6 |
| Urosis | 3 |
| Unknown | 1 |
| Total | 66 |
| Method | y | Group 1 | Group 2 | Statistic | p-Value |
|---|---|---|---|---|---|
| Mann-whitney | SAA | Healthy | Disease | 1153 | 6.68 × 10−29 |
| Student | SAA | Healthy | Disease | −11.586 | 1.59 × 10−26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinya, U.; Iwamura, Y.; Yamato, O.; Pambudi, D.; Widodo, O.S.; Taniguchi, M.; Takagi, M. Serum Amyloid A Concentrations of Healthy and Clinically Diseased Japanese Black Breeding Cattle—Preliminary Measurements for Determining the Cut-Off Concentrations. Vet. Sci. 2022, 9, 198. https://doi.org/10.3390/vetsci9050198
Shinya U, Iwamura Y, Yamato O, Pambudi D, Widodo OS, Taniguchi M, Takagi M. Serum Amyloid A Concentrations of Healthy and Clinically Diseased Japanese Black Breeding Cattle—Preliminary Measurements for Determining the Cut-Off Concentrations. Veterinary Sciences. 2022; 9(5):198. https://doi.org/10.3390/vetsci9050198
Chicago/Turabian StyleShinya, Urara, Yuka Iwamura, Osamu Yamato, Dhidhi Pambudi, Oky Setyo Widodo, Masayasu Taniguchi, and Mitsuhiro Takagi. 2022. "Serum Amyloid A Concentrations of Healthy and Clinically Diseased Japanese Black Breeding Cattle—Preliminary Measurements for Determining the Cut-Off Concentrations" Veterinary Sciences 9, no. 5: 198. https://doi.org/10.3390/vetsci9050198
APA StyleShinya, U., Iwamura, Y., Yamato, O., Pambudi, D., Widodo, O. S., Taniguchi, M., & Takagi, M. (2022). Serum Amyloid A Concentrations of Healthy and Clinically Diseased Japanese Black Breeding Cattle—Preliminary Measurements for Determining the Cut-Off Concentrations. Veterinary Sciences, 9(5), 198. https://doi.org/10.3390/vetsci9050198







