Pet Owners’ Perceptions of COVID-19, Zoonotic Disease, and Veterinary Medicine: The Impact of Demographic Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Survey
2.3. Statistical Analysis
3. Results
3.1. Demographics
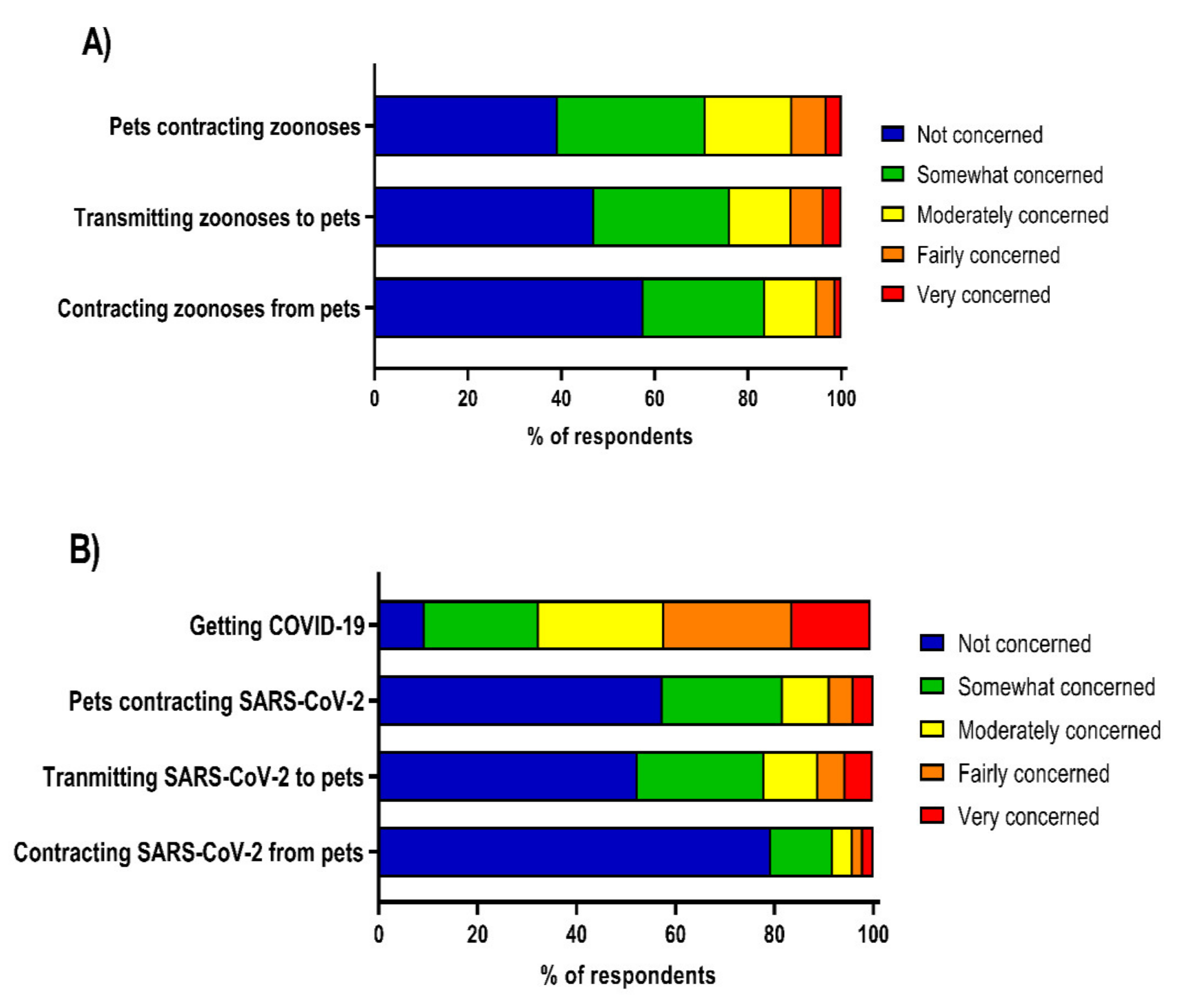
3.2. Perceptions of Zoonotic Disease and COVID-19
3.3. Demographic Characteristics and Perceptions of Zoonotic Disease and Transmission of SARS-CoV-2
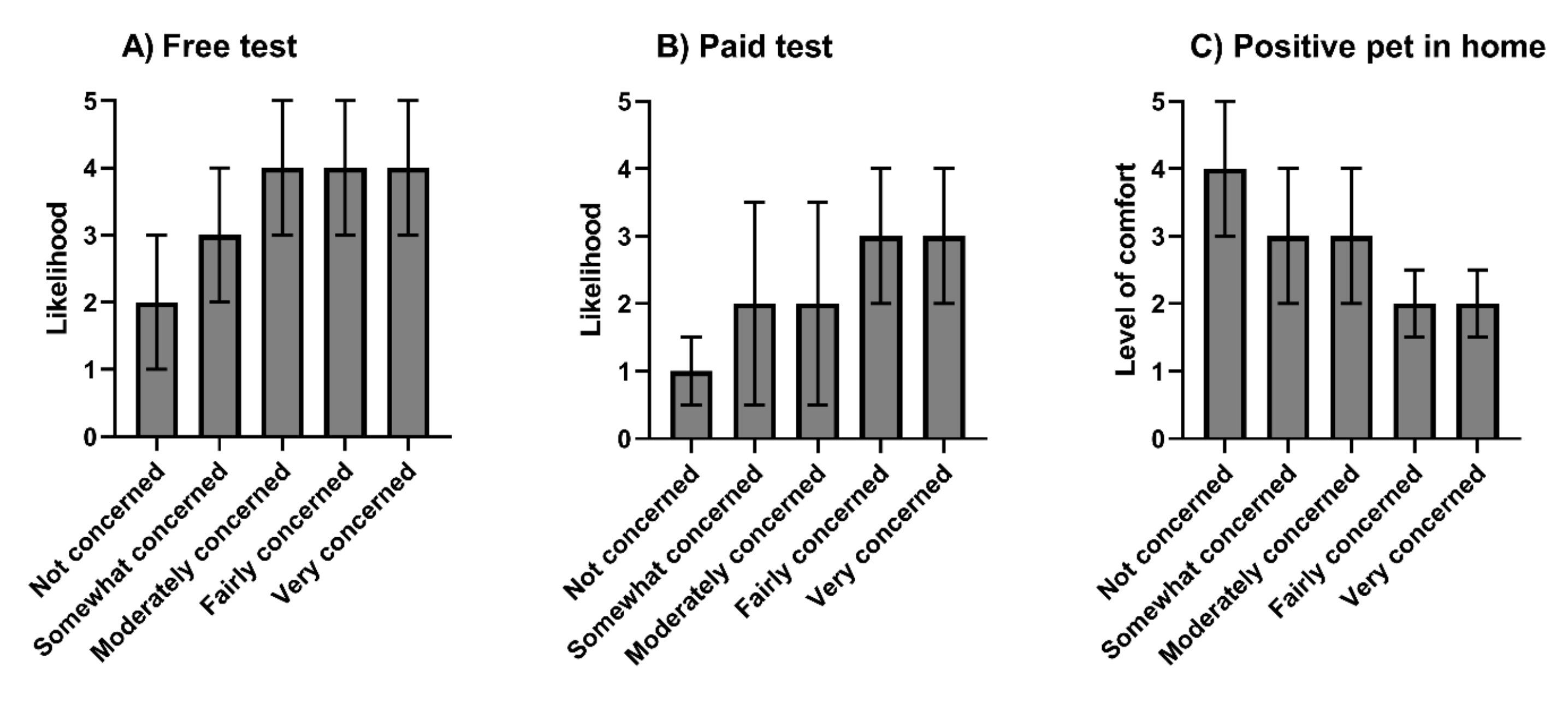
3.4. SARS-CoV-2 Testing in Pets
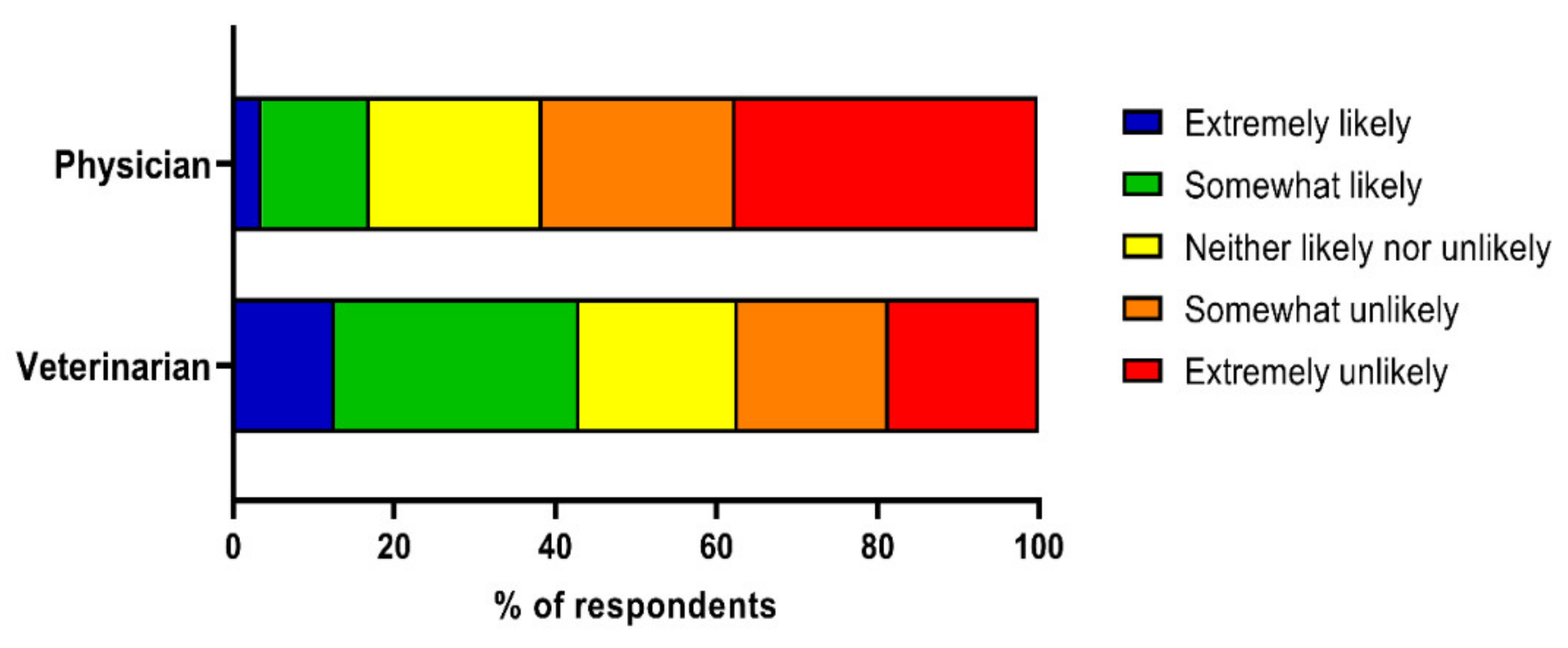
3.5. Perceptions of Physicians and Veterinarians as Resources for Zoonotic Disease Information
3.6. Access to Veterinary Care
3.7. Changes in Access to Veterinary Care during the COVID-19 Pandemic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Director—General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard 2021. Available online: https://covid19.who.int/ (accessed on 5 January 2022).
- Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Greger, M. The human/animal interface: Emergence and resurgence of zoonotic infectious diseases. Crit. Rev. Microbiol. 2007, 33, 243–299. [Google Scholar] [CrossRef] [PubMed]
- Overgaauw, P.A.; Vinke, C.M.; van Hagen, M.A.; Lipman, L.J. A one health perspective on the human–companion animal relationship with emphasis on zoonotic aspects. Int. J. Environ. Res. Public Health 2020, 17, 3789. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, E.C.; Reid, T.J. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound. Emerg. Dis. 2021, 68, 1850–1867. [Google Scholar] [CrossRef]
- Korath, A.D.; Janda, J.; Untersmayr, E.; Sokolowska, M.; Feleszko, W.; Agache, I.; Hartmann, K.; Jensen-Jarolim, E.; Pali-Schöll, I. One Health: EAACI Position Paper on coronaviruses at the human-animal interface, with a specific focus on comparative and zoonotic aspects of SARS-CoV-2. Allergy 2022, 77, 55–71. [Google Scholar] [CrossRef]
- American Veterinary Medical Association. AVMA Pet Ownership and Demographics Sourcebook, 2017–2018 ed.; American Veterinary Medical Association: Schaumburg, IL, USA, 2018. [Google Scholar]
- Fritz, M.; Rosolen, B.; Krafft, E.; Becquart, P.; Elguero, E.; Vratskikh, O.; Denolly, S.; Boson, B.; Vanhomwegen, J.; Gouilh, M.A.; et al. High prevalence of SARS-CoV-2 antibodies in pets from COVID-19+ households. One Health 2021, 11, 100192. [Google Scholar] [CrossRef]
- Calvet, G.A.; Pereira, S.A.; Ogrzewalska, M.; Pauvolid-Corrêa, A.; Resende, P.C.; Tassinari, W.D.S.; Costa, A.D.; Keidel, L.O.; da Rocha, A.S.; da Silva, M.F.; et al. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS ONE 2021, 16, e0250853. [Google Scholar] [CrossRef]
- Bienzle, D.; Rousseau, J.; Marom, D.; Macnicol, J.; Jacobsen, L.; Sparling, S.; Weese, J.S. Seropositivity for SARS-CoV-2 in Cats and Dogs; European Congress of Clinical Microbiology & Infectious Diseases (ECCMID): Lisbon, Portugal, 2021. [Google Scholar]
- Broens, E.M.; Kannekens-Jager, M.; Groot De, Y.; Kooistra, H.S.; Egberink, H.F.; Zhao, S.; Wagenaar, J.A.; Duim, B. High Prevalence of SARS-CoV-2 in Dogs and Cats Living in COVID-19 Positive Households; European Congress of Clinical Microbiology & Infectious Diseases (ECCMID): Lisbon, Portugal, 2021. [Google Scholar]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Halfmann, P.J.; Hatta, M.; Chiba, S.; Maemura, T.; Fan, S.; Takeda, M.; Kinoshita, N.; Hattori, S.I.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; et al. Transmission of SARS-CoV-2 in domestic cats. N. Engl. J. Med. 2020, 383, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Gordy, P.W.; Nehring, M.; Byas, A.D.; VandeWoude, S.; Ragan, I.K.; Maison, R.M.; Bowen, R.A.; et al. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. USA 2020, 117, 26382–26388. [Google Scholar] [CrossRef] [PubMed]
- American Veterinary Medical Association (AVMA). Testing Animals for SARS-CoV-2 2021. Available online: https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/testing-animals-sars-cov-2 (accessed on 6 January 2022).
- Kim, Y.-I.; Kim, S.-G.; Kim, S.-M.; Kim, E.-H.; Park, S.-J.; Yu, K.-M.; Chang, J.H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 2020, 27, 704–709.e2. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Siu, T. Hong Kong to Cull 2000 Hamsters after COIVD-19 Outbreak; Reuters: London, UK, 2022. [Google Scholar]
- Dryhurst, S.; Schneider, C.R.; Kerr, J.; Freeman, A.L.; Recchia, G.; Van Der Bles, A.M.; Spiegelhalter, D.; Van Der Linden, S. Risk perceptions of COVID-19 around the world. J. Risk Res. 2020, 23, 994–1006. [Google Scholar] [CrossRef]
- Lee, J.J.; Kang, K.-A.; Wang, M.P.; Zhao, S.Z.; Wong, J.Y.H.; O’Connor, S.; Yang, S.C.; Shin, S. Associations between COVID-19 misinformation exposure and belief with COVID-19 knowledge and preventive behaviors: Cross-sectional online study. J. Med. Internet Res. 2020, 22, e22205. [Google Scholar] [CrossRef]
- Herbert, M.; Basha, R.; Thangaraj, S. Community perception regarding rabies prevention and stray dog control in urban slums in India. J. Infect. Public Health 2012, 5, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Aenishaenslin, C.; Michel, P.; Ravel, A.; Gern, L.; Milord, F.; Waaub, J.-P.; Bélanger, D. Factors associated with preventive behaviors regarding Lyme disease in Canada and Switzerland: A comparative study. BMC Public Health 2015, 15, 185. [Google Scholar] [CrossRef] [Green Version]
- Wiethoelter, A.K.; Sawford, K.; Schembri, N.; Taylor, M.R.; Dhand, N.K.; Moloney, B.; Wright, T.; Kung, N.; Field, H.E.; Toribio, J.A.L. “We’ve learned to live with it”—A qualitative study of Australian horse owners’ attitudes, perceptions and practices in response to Hendra virus. Prev. Vet. Med. 2017, 140, 67–77. [Google Scholar] [CrossRef]
- Zhong, B.-L.; Luo, W.; Li, H.-M.; Zhang, Q.-Q.; Liu, X.-G.; Li, W.-T.; Li, Y. Knowledge, attitudes, and practices towards COVID-19 among Chinese residents during the rapid rise period of the COVID-19 outbreak: A quick online cross-sectional survey. Int. J. Biol. Sci. 2020, 16, 1745. [Google Scholar] [CrossRef]
- Steele, S.G.; Booy, R.; Manocha, R.; Mor, S.M.; Toribio, J.A.L. Towards One Health clinical management of zoonoses: A parallel survey of Australian general medical practitioners and veterinarians. Zoonoses Public Health 2021, 68, 88–102. [Google Scholar] [CrossRef]
- Grant, S.; Olsen, C.W. Preventing zoonotic diseases in immunocompromised persons: The role of physicians and veterinarians. Emerg. Infect. Dis. 1999, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.A.; Petty, G.C.; Erwin, P.C.; Souza, M.J. A survey of Tennessee veterinarian and physician attitudes, knowledge, and practices regarding zoonoses prevention among animal owners with HIV infection or AIDS. J. Am. Vet. Med. Assoc. 2012, 240, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.; Mor, S. Client knowledge, attitudes and practices regarding zoonoses: A metropolitan experience. Aust. Vet. J. 2015, 93, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Esch, K.J.; Petersen, C.A. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 2013, 26, 58–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stull, J.W.; Brophy, J.; Weese, J. Reducing the risk of pet-associated zoonotic infections. CMAJ 2015, 187, 736–743. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.L.; Holland, G.N. Annual burden of ocular toxoplasmosis in the United States. Am. J. Trop. Med. Hyg. 2010, 82, 464. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Improving Data on Rabies. Available online: https://www.who.int/activities/improving-data-on-rabies/rabies-epidemiology-and-burden (accessed on 24 February 2022).
- Verma, A.; Carney, K.; Taylor, M.; Amsler, K.; Morgan, J.; Gruszynski, K.; Erol, E.; Carter, C.; Locke, S.; Callipare, A.; et al. Occurrence of potentially zoonotic and cephalosporin resistant enteric bacteria among shelter dogs in the Central and South-Central Appalachia. BMC Vet. Res. 2021, 17, 313. [Google Scholar] [CrossRef]
- Kaspar, U.; von Lützau, A.; Schlattmann, A.; Roesler, U.; Köck, R.; Becker, K. Zoonotic multidrug-resistant microorganisms among small companion animals in Germany. PLoS ONE 2018, 13, e0208364. [Google Scholar]
- Wright, J.G.; Tengelsen, L.A.; Smith, K.E.; Bender, J.; Frank, R.K.; Grendon, J.H.; Rice, D.H.; Thiessen, A.M.B.; Gilbertson, C.J.; Sivapalasingam, S.; et al. Multidrug-resistant Salmonella Typhimurium in four animal facilities. Emerg. Infect. Dis. 2005, 11, 1235. [Google Scholar] [CrossRef] [Green Version]
- Kutner, M.; Greenburg, E.; Jin, Y.; Paulsen, C. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy; National Center for Education Statistics: Washington, DC, USA, 2006.
- Brown, S.-E. Ethnic variations in pet attachment among students at an American school of veterinary medicine. Soc. Anim. 2002, 10, 249–266. [Google Scholar] [CrossRef]
- Zajacova, A.; Lawrence, E.M. The relationship between education and health: Reducing disparities through a contextual approach. Annu. Rev. Public Health 2018, 39, 273–289. [Google Scholar] [CrossRef] [Green Version]
- Dubin, R.J.; Angliss, G.; Eng, C.; Cisneros, T.; Griffon, D. Veterinarians’ perceptions of COVID-19 pandemic–related influences on veterinary telehealth and on pet owners’ attitudes toward cats and dogs. J. Am. Vet. Med. Assoc. 2021, 259, 1140–1147. [Google Scholar] [CrossRef]
- D’Angelo, D.; Chirico, A.; Sacchettino, L.; Manunta, F.; Martucci, M.; Cestaro, A.; Avallone, L.; Giordano, A.; Ciani, F. Human-Dog Relationship during the First COVID-19 Lockdown in Italy. Animals 2021, 11, 2335. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). COVID-19 Events in Animals 2022. Available online: https://www.oie.int/en/what-we-offer/emergency-and-resilience/covid-19/#ui-id-3 (accessed on 13 January 2022).
- Parry, N.M. COVID-19 and pets: When pandemic meets panic. Forensic Sci. Int. Rep. 2020, 2, 100090. [Google Scholar] [CrossRef]
- Yanez, N.D.; Weiss, N.S.; Romand, J.-A.; Treggiari, M.M. COVID-19 mortality risk for older men and women. BMC Public Health 2020, 20, 1742. [Google Scholar] [CrossRef]
- De Bruin, W.B.; Saw, H.-W.; Goldman, D.P. Political polarization in US residents’ COVID-19 risk perceptions, policy preferences, and protective behaviors. J. Risk Uncertain. 2020, 61, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Naeim, A.; Baxter-King, R.; Wenger, N.; Stanton, A.L.; Sepucha, K.; Vavreck, L. Effects of age, gender, health status, and political party on COVID-19–related concerns and prevention behaviors: Results of a large, longitudinal cross-sectional survey. JMIR Public Health Surveill. 2021, 7, e24277. [Google Scholar] [CrossRef] [PubMed]
- Valencak, T.G.; Csiszar, A.; Szalai, G.; Podlutsky, A.; Tarantini, S.; Fazekas-Pongor, V.; Papp, M.; Ungvari, Z. Animal reservoirs of SARS-CoV-2: Calculable COVID-19 risk for older adults from animal to human transmission. GeroScience 2021, 43, 2305–2320. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Sharun, K.; Pathak, M.; Tiwari, R.; Yatoo, M.I.; Malik, Y.S.; Sah, R.; Rabaan, A.A.; Panwar, P.K.; et al. SARS-CoV-2 jumping the species barrier: Zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med. Infect. Dis. 2020, 37, 101830. [Google Scholar] [CrossRef]
- Bir, C.; Ortez, M.; Olynk Widmar, N.J.; Wolf, C.A.; Hansen, C.; Ouedraogo, F.B. Familiarity and use of veterinary services by US resident dog and cat owners. Animals 2020, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Gates, M.; Walker, J.; Zito, S.; Dale, A. Cross-sectional survey of pet ownership, veterinary service utilisation, and pet-related expenditures in New Zealand. N. Z. Vet. J. 2019, 67, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Stull, J.W.; Peregrine, A.S.; Sargeant, J.M.; Weese, J.S. Household knowledge, attitudes and practices related to pet contact and associated zoonoses in Ontario, Canada. BMC Public Health 2012, 12, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingham, G.M.; Budke, C.M.; Slater, M.R. Knowledge and perceptions of dog-associated zoonoses: Brazos County, Texas, USA. Prev. Vet. Med. 2010, 93, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wiltzius, A.J.; Blackwell, M.J.; Krebsbach, S.B.; Daugherty, L.; Kreisler, R.; Forsgren, B.; Moyer, M.; Manifold, S.; Snyder, S.; Favre, D.; et al. Access to Veterinary Care: Barriers, Current Practices, and Public Policy. 2018. Available online: https://pphe.utk.edu/wp-content/uploads/2020/09/avcc-report.pdf (accessed on 15 September 2021).
- Powell, L.; Walsh, M.; Reinhard, C.L.; Jankowski, K.; Watson, B. One Health clinic promotes veterinarian-client trust among underserved pet owners and provides learning opportunities for veterinary students. J. Am. Vet. Med. Assoc. 2022, 1, 1–9. [Google Scholar] [CrossRef]
- LaVallee, E.; Mueller, M.K.; McCobb, E. A systematic review of the literature addressing veterinary care for underserved communities. J. Appl. Anim. Welf. Sci. 2017, 20, 381–394. [Google Scholar] [CrossRef]
- Park, R.M.; Gruen, M.E.; Royal, K. Association between Dog Owner Demographics and Decision to Seek Veterinary Care. Vet. Sci. 2021, 8, 7. [Google Scholar] [CrossRef]
- Lem, M. Barriers to accessible veterinary care. Can. Vet. J. 2019, 60, 891. [Google Scholar]
- U.S. Bureau of Labor Statistics. Labor Force Characteristics by Race and Ethnicity, 2019. 2020. Available online: https://www.bls.gov/opub/reports/race-and-ethnicity/2019/home.htm (accessed on 14 May 2021).
- Kogan, L.R.; Erdman, P.; Bussolari, C.; Currin-McCulloch, J.; Packman, W. The initial months of COVID-19: Dog owners’ veterinary-related concerns. Front. Vet. Sci. 2021, 8, 45. [Google Scholar] [CrossRef]
- Kogan, L.R.; Accornero, V.H.; Gelb, E.; Slater, M.R. Community veterinary medicine programs: Pet owners’ perceptions and experiences. Front. Vet. Sci. 2021, 8, 587. [Google Scholar] [CrossRef]
- Morris, A.; Wu, H.; Morales, C. Barriers to Care in Veterinary Services: Lessons Learned From Low-Income Pet Guardians’ Experiences at Private Clinics and Hospitals during COVID-19. Front. Vet. Sci. 2021, 8, 764753. [Google Scholar] [CrossRef]
- Wu, H.; Bains, R.S.; Morris, A.; Morales, C. Affordability, feasibility, and accessibility: Companion Animal guardians with (dis) abilities’ access to veterinary medical and behavioral services during COVID-19. Animals 2021, 11, 2359. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, P.; Webb, T.L. The intention–behavior gap. Soc. Personal. Psychol. Compass 2016, 10, 503–518. [Google Scholar] [CrossRef]
| Demographic Characteristics | n | % |
|---|---|---|
| Gender | ||
| Male | 96 | 8.3 |
| Female | 1041 | 90.2 |
| Non-binary | 7 | 0.6 |
| Prefer not to answer | 10 | 0.9 |
| Age | ||
| 18–29 | 361 | 31.3 |
| 30–39 | 220 | 19.1 |
| 40–49 | 173 | 15.0 |
| 50–59 | 217 | 18.8 |
| 60+ | 183 | 15.9 |
| Ethnicity | ||
| Hispanic/Latino/Spanish | 46 | 4.0 |
| Not Hispanic | 1108 | 96.0 |
| Race a | ||
| American Indian/Alaskan Native | 6 | 0.5 |
| Asian | 29 | 2.5 |
| Black/African American | 39 | 3.4 |
| Native Hawaiian/Pacific Islander | 2 | 0.2 |
| White | 1055 | 91.4 |
| Other/Prefer not to answer | 36 | 3.1 |
| Annual household income b | ||
| Less than $30,000 | 132 | 11.4 |
| $30,000–$49,999 | 158 | 13.7 |
| $50,000–$99,999 | 356 | 30.8 |
| $100,000–$350,000 | 460 | 39.8 |
| More than $350,000 | 48 | 4.2 |
| Education | ||
| High school diploma or less | 156 | 13.5 |
| Associate degree/Undergraduate university degree | 584 | 50.6 |
| Postgraduate university degree | 414 | 35.9 |
| Region | ||
| Northeast | 980 | 84.9 |
| Midwest | 23 | 2.0 |
| South | 105 | 9.1 |
| West | 43 | 3.7 |
| Number of people in house | ||
| ≤2 | 715 | 62.0 |
| 3–4 | 377 | 32.7 |
| ≥5 | 62 | 5.4 |
| Heard the Term ‘Zoonotic Disease’ | Knew the Meaning of ‘Zoonotic Disease’ | |||
|---|---|---|---|---|
| Demographic Characteristics | OR (95% CI) | p | OR (95% CI) | p |
| Sex a | 1.32 (0.84–2.06) | 0.23 | 1.23 (0.79–1.91) | 0.36 |
| Age | ||||
| 18–29 | Reference | Reference | ||
| 30–39 | 0.63 (0.43–0.93) | 0.02 * | 0.69 (0.47–1.01) | 0.06 |
| 40–49 | 0.57 (0.38–0.86) | 0.01 * | 0.65 (0.43–0.98) | 0.04 * |
| 50–59 | 0.47 (0.32–0.69) | <0.001 * | 0.48 (0.32–0.70) | <0.001 * |
| 60+ | 0.47 (0.32–0.71) | <0.001 * | 0.47 (0.32–0.70) | <0.001 * |
| Race | ||||
| Caucasian | Reference | Reference | ||
| African American | 1.32 (0.54–2.39) | 0.74 | 1.26 (0.59–2.67) | 0.56 |
| Asian | 0.70 (0.30–1.67) | 0.42 | 0.89 (0.37–2.11) | 0.79 |
| Ethnicity | ||||
| Hispanic/Latino/Spanish | 0.76 (0.35–1.64) | 0.48 | 1.01 (0.47–2.18) | 0.98 |
| Education | ||||
| High school or less | Reference | Reference | ||
| Undergraduate | 2.13 (1.40–3.24) | <0.001 * | 1.75 (1.17–2.61) | 0.01 * |
| Postgraduate | 3.29 (2.10–5.15) | <0.001 * | 2.40 (1.56–3.68) | <0.001 * |
| Household income | ||||
| <$30,000 | Reference | Reference | ||
| $30,000–$49,999 | 0.43 (0.25–0.73) | 0.002 * | 0.40 (0.23–0.69) | 0.001 * |
| $50,000–$99,999 | 0.38 (0.24–0.62) | <0.001 * | 0.40 (0.25–0.65) | <0.001 * |
| $100,000–$349,999 | 0.45 (0.28–0.73) | 0.001 * | 0.47 (0.29–0.77) | 0.002 * |
| >$350,000 | 0.39 (0.18–0.83) | 0.01 * | 0.46 (0.21–0.98) | 0.04 * |
| Number of people in household | ||||
| 1–2 people | Reference | Reference | ||
| 3–4 people | 0.92 (0.70–1.22) | 0.56 | 0.87 (0.66–1.15) | 0.33 |
| 5+ people | 0.77 (0.44–1.36) | 0.37 | 0.96 (0.55–1.67) | 0.87 |
| US region | ||||
| Northeast | Reference | Reference | ||
| Midwest | 4.03 (1.32–12.37) | 0.02 * | 2.67 (0.95–7.52) | 0.06 |
| South | 1.52 (0.97–2.37) | 0.07 | 1.55 (0.99–2.43) | 0.06 |
| West | 3.60 (1.63–7.95) | 0.002 * | 3.65 (1.61–8.26) | 0.002 * |
| Pets Contracting Zoonotic Disease | Transmitting Zoonotic Disease to Pets | Contracting Zoonotic Disease from Pets | ||||
|---|---|---|---|---|---|---|
| Demographic Characteristics | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Sex a | 0.91 (0.59–1.43) | 0.69 | 1.17 (0.76–1.80) | 0.48 | 1.00 (0.64–1.55) | 0.99 |
| Age | ||||||
| 18–29 | Reference | Reference | Reference | |||
| 30–39 | 0.62 (0.42–0.91) | 0.02 * | 0.66 (0.46–0.96) | 0.03 * | 0.69 (0.48–1.00) | 0.05 |
| 40–49 | 0.64 (0.42–0.96) | 0.03 * | 0.56 (0.38–0.84) | 0.01 * | 0.58 (0.38–0.87) | 0.01 * |
| 50–59 | 0.50 (0.34–0.73) | <0.001 * | 0.59 (0.41–0.86) | 0.01 * | 0.64 (0.44–0.93) | 0.02 * |
| 60+ | 0.49 (0.33–0.73) | <0.001 * | 0.51 (0.35–0.75) | 0.001 * | 0.59 (0.40–0.88) | 0.01 * |
| Race | ||||||
| Caucasian | Reference | Reference | Reference | |||
| African American | 0.48 (0.24–0.96) | 0.04 * | 0.79 (0.39–1.58) | 0.50 | 0.83 (0.41–1.68) | 0.60 |
| Asian | 1.60 (0.61–4.15) | 0.34 | 1.25 (0.53–2.99) | 0.61 | 2.03 (0.85–4.86) | 0.11 |
| Ethnicity | ||||||
| Hispanic/Latino/Spanish | 0.59 (0.28–1.24) | 0.16 | 0.77 (0.37–1.61) | 0.49 | 1.06 (0.51–2.22) | 0.87 |
| Education | ||||||
| High school or less | Reference | Reference | Reference | |||
| Undergraduate | 0.70 (0.47–1.04) | 0.08 | 0.75 (0.51–1.11) | 0.15 | 0.67 (0.46–0.99) | 0.04 * |
| Postgraduate | 0.77 (0.50–1.18) | 0.23 | 0.74 (0.49–1.12) | 0.15 | 0.70 (0.46–1.05) | 0.08 |
| Household income | ||||||
| <$30,000 | Reference | Reference | Reference | |||
| $30,000–$49,999 | 1.01 (0.60–1.70) | 0.97 | 1.15 (0.70–1.91) | 0.58 | 1.16 (0.71–1.91) | 0.55 |
| $50,000–$99,999 | 0.79 (0.50–1.25) | 0.32 | 0.84 (0.54–1.30) | 0.43 | 0.87 (0.56–1.35) | 0.54 |
| $100,000–$349,999 | 1.05 (0.66–1.67) | 0.85 | 1.02 (0.65–1.59) | 0.95 | 0.90 (0.58–1.40) | 0.64 |
| >$350,000 | 0.89 (0.42–1.87) | 0.75 | 0.79 (0.38–1.64) | 0.53 | 0.67 (0.32–1.43) | 0.30 |
| Number of people in household | ||||||
| 1–2 people | Reference | Reference | Reference | |||
| 3–4 people | 0.86 (0.65–1.13) | 0.28 | 0.91 (0.69–1.19) | 0.48 | 1.11 (0.84–1.46) | 0.46 |
| 5+ people | 0.81 (0.46–1.41) | 0.45 | 0.90 (0.52–1.56) | 0.72 | 1.21 (0.70–2.10) | 0.49 |
| US region | ||||||
| Northeast | Reference | Reference | Reference | |||
| Midwest | 1.02 (0.41–2.52) | 0.97 | 1.12 (0.46–2.73) | 0.80 | 1.27 (0.52–3.07) | 0.60 |
| South | 0.97 (0.63–1.50) | 0.90 | 0.74 (0.48–1.12) | 0.15 | 0.81 (0.53–1.25) | 0.35 |
| West | 0.84 (0.42–1.67) | 0.62 | 0.85 (0.43–1.68) | 0.64 | 0.92 (0.46–1.84) | 0.81 |
| Demographic Characteristics | Contracting COVID-19 | Pets Contracting SARS-CoV-2 a | Transmitting SARS-CoV-2 to Pets a | Contracting SARS-CoV-2 from Pets a | Comfort Having SARS-CoV-2+ Pet in Home a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Sex b | 1.67 (0.88–3.19) | 0.12 | 0.75 (0.47–1.20) | 0.23 | 0.91 (0.57–1.46) | 0.69 | 0.72 (0.43–1.23) | 0.23 | 1.19 (0.76–1.86) | 0.46 |
| Age | ||||||||||
| 18–29 | Reference | Reference | Reference | Reference | Reference | |||||
| 30–39 | 0.95 (0.46–1.95) | 0.88 | 0.97 (0.65–1.44) | 0.87 | 0.84 (0.57–1.25) | 0.40 | 1.05 (0.65–1.69) | 0.85 | 1.25 (0.85–1.83) | 0.26 |
| 40–49 | 0.41 (0.21–0.78) | 0.01 * | 1.06 (0.68–1.66) | 0.78 | 0.92 (0.60–1.42) | 0.70 | 1.08 (0.63–1.86) | 0.77 | 1.03 (0.68–1.56) | 0.90 |
| 50–59 | 0.43 (0.23–0.79) | 0.01 * | 1.17 (0.78–1.76) | 0.45 | 0.91 (0.61–1.37) | 0.65 | 1.28 (0.79–2.07) | 0.32 | 1.59 (1.07–2.36) | 0.02 * |
| 60+ | 0.68 (0.34–1.33) | 0.26 | 1.06 (0.70–1.60) | 0.79 | 0.78 (0.52–1.18) | 0.24 | 1.28 (0.78–2.08) | 0.33 | 0.86 (0.58–1.28) | 0.46 |
| Race c | ||||||||||
| Caucasian | Reference | Reference | Reference | Reference | ||||||
| African American | 1.31 (0.62–2.78) | 0.48 | 1.48 (0.71–3.11) | 0.30 | 1.80 (0.78–4.15) | 0.17 | 0.55 (0.27–1.13) | 0.10 | ||
| Asian | 1.09 (0.44–2.66) | 0.84 | 1.47 (0.60–3.62) | 0.40 | 0.99 (0.35–2.84) | 0.99 | 1.19 (0.50–2.82) | 0.69 | ||
| Ethnicity | ||||||||||
| Hispanic/Latino/Spanish | 1.03 (0.35–3.03) | 0.96 | 0.46 (0.19–1.07) | 0.07 | 1.15 (0.52–2.52) | 0.73 | 0.87 (0.33–2.26) | 0.77 | 2.01 (0.91–4.47) | 0.09 |
| Education | ||||||||||
| High school or less | Reference | Reference | Reference | Reference | Reference | |||||
| Undergraduate | 1.11 (0.64–1.92) | 0.70 | 0.41 (0.26–0.63) | <0.001 * | 0.35 (0.11–0.54) | <0.001 * | 0.54 (0.34–0.87) | 0.01 * | 1.16 (0.77–1.73) | 0.48 |
| Postgraduate | 3.81 (1.84–7.86) | <0.001 * | 0.29 (0.18–0.46) | <0.001 * | 0.36 (0.22–0.57) | <0.001 * | 0.50 (0.30–0.82) | 0.01 * | 0.98 (0.64–1.51) | 0.93 |
| Household income | ||||||||||
| <$30,000 | Reference | Reference | Reference | Reference | Reference | |||||
| $30,000–$49,999 | 0.91 (0.42–1.98) | 0.81 | 1.02 (0.63–1.86) | 0.77 | 1.20 (0.70–2.05) | 0.51 | 1.73 (0.89–3.39) | 0.11 | 1.03 (0.62–1.74) | 0.90 |
| $50,000–$99,999 | 1.10 (0.55–2.21) | 0.79 | 1.21 (0.73–1.88) | 0.52 | 1.08 (0.68–1.74) | 0.74 | 1.53 (0.84–2.81) | 0.17 | 0.97 (0.62–1.53) | 0.90 |
| $100,000–$349,999 | 1.30 (0.63–2.70) | 0.48 | 1.12 (0.68–1.78) | 0.71 | 1.10 (0.68–1.77) | 0.70 | 1.26 (0.68–2.35) | 0.46 | 0.79 (0.50–1.26) | 0.32 |
| >$350,000 | 2.41 (0.50–11.63) | 0.27 | 1.51 (0.70–3.29) | 0.30 | 1.25 (0.58–2.70) | 0.58 | 1.41 (0.56–3.58) | 0.47 | 0.57 (0.27–1.22) | 0.15 |
| Number of people in household | ||||||||||
| 1–2 people | Reference | Reference | Reference | Reference | Reference | |||||
| 3–4 people | 1.16 (0.72–1.85) | 0.54 | 0.84 (0.63–1.13) | 0.25 | 0.91 (0.68–1.22) | 0.52 | 1.02 (0.72–1.44) | 0.93 | 0.98 (0.74–1.30) | 0.91 |
| 5+ people | 0.86 (0.36–2.06) | 0.74 | 0.82 (0.45–1.50) | 0.52 | 0.84 (0.46–1.50) | 0.55 | 1.22 (0.61–2.43) | 0.58 | 0.76 (0.43–1.34) | 0.34 |
| US region | ||||||||||
| Northeast | Reference | Reference | Reference | Reference | Reference | |||||
| Midwest | 1.68 (0.22–13.05) | 0.62 | 0.61 (0.23–1.59) | 0.31 | 0.75 (0.30–1.88) | 0.53 | 0.89 (0.29–2.76) | 0.84 | 2.26 (0.84–6.06) | 0.11 |
| South | 1.26 (0.55–2.86) | 0.58 | 0.69 (0.44–1.10) | 0.12 | 0.91 (0.58–1.43) | 0.69 | 0.61 (0.33–1.10) | 0.10 | 0.76 (0.49–1.17) | 0.21 |
| West | 0.77 (0.22–2.68) | 0.69 | 0.51 (0.23–1.18) | 0.09 | 0.66 (0.32–1.37) | 0.26 | 0.53 (0.19–1.45) | 0.22 | 0.67 (0.33–1.36) | 0.26 |
| Free Test for SARS-CoV-2 for Pets | Paid Test for SARS-CoV-2 for Pets | |||
|---|---|---|---|---|
| Demographic Characteristics | OR (95% CI) | p | OR (95% CI) | p |
| Sex a | 0.91 (0.58–1.43) | 0.68 | 1.18 (0.72–1.92) | 0.51 |
| Age | ||||
| 18–29 | Reference | Reference | ||
| 30–39 | 0.55 (0.37–0.81) | 0.002 * | 1.02 (0.68–1.54) | 0.91 |
| 40–49 | 0.73 (0.48–1.10) | 0.14 | 1.20 (0.77–1.89) | 0.42 |
| 50–59 | 0.54 (0.37–0.80) | 0.002 * | 1.08 (0.71–1.63) | 0.73 |
| 60+ | 0.67 (0.45–1.00) | 0.05 * | 1.27 (0.83–1.93) | 0.27 |
| Race | ||||
| Caucasian | Reference | Reference | ||
| African American | 1.66 (0.80–3.46) | 0.18 | 0.69 (0.29–1.65) | 0.40 |
| Asian | 2.00 (0.75–5.31) | 0.17 | 1.60 (0.68–3.78) | 0.29 |
| Ethnicity | ||||
| Hispanic/Latino/Spanish | 0.50 (0.23–1.08) | 0.08 | 0.52 (0.21–1.34) | 0.17 |
| Education | ||||
| High school or less | Reference | Reference | ||
| Undergraduate | 0.76 (0.51–1.13) | 0.18 | 0.87 (0.56–1.34) | 0.52 |
| Postgraduate | 0.74 (0.48–1.14) | 0.18 | 0.94 (0.60–1.49) | 0.80 |
| Household income | ||||
| <$30,000 | Reference | Reference | ||
| $30,000–$49,999 | 0.96 (0.57–1.62) | 0.88 | 2.27 (1.26–4.11) | 0.01 * |
| $50,000–$99,999 | 0.85 (0.53–1.34) | 0.48 | 1.66 (0.97–2.85) | 0.07 |
| $100,000–$349,999 | 0.90 (0.56–1.44) | 0.66 | 1.95 (1.13–3.36) | 0.01 * |
| >$350,000 | 0.78 (0.37–1.65) | 0.52 | 3.18 (1.43–7.08) | 0.01 * |
| Number of people in household | ||||
| 1–2 people | Reference | Reference | ||
| 3–4 people | 0.93 (0.70–1.23) | 0.61 | 0.83 (0.61–1.12) | 0.22 |
| 5+ people | 0.57 (0.32–1.02) | 0.06 | 0.66 (0.35–1.24) | 0.19 |
| US region | ||||
| Northeast | Reference | Reference | ||
| Midwest | 0.44 (0.17–1.13) | 0.09 | 0.78 (0.29–2.09) | 0.62 |
| South | 0.80 (0.52–1.25) | 0.33 | 0.97 (0.61–1.55) | 0.90 |
| West | 1.00 (0.49–2.02) | 1.00 | 0.70 (0.32–1.51) | 0.36 |
| Concern regarding COVID-19 | ||||
| Not concerned | Reference | Reference | ||
| Somewhat concerned | 2.50 (1.45–4.29) | 0.001 * | 2.42 (1.20–4.86) | 0.01 * |
| Moderately concerned | 3.47 (2.03–5.93) | <0.001 * | 3.00 (1.51–5.97) | 0.002 * |
| Fairly concerned | 5.55 (3.22–9.59) | <0.001 * | 4.32 (2.18–8.59) | <0.001 * |
| Very concerned | 6.68 (3.71–12.04) | <0.001 * | 7.37 (3.61–15.03) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, L.; Lavender, T.M.; Reinhard, C.L.; Watson, B. Pet Owners’ Perceptions of COVID-19, Zoonotic Disease, and Veterinary Medicine: The Impact of Demographic Characteristics. Vet. Sci. 2022, 9, 195. https://doi.org/10.3390/vetsci9050195
Powell L, Lavender TM, Reinhard CL, Watson B. Pet Owners’ Perceptions of COVID-19, Zoonotic Disease, and Veterinary Medicine: The Impact of Demographic Characteristics. Veterinary Sciences. 2022; 9(5):195. https://doi.org/10.3390/vetsci9050195
Chicago/Turabian StylePowell, Lauren, Tyler M. Lavender, Chelsea L. Reinhard, and Brittany Watson. 2022. "Pet Owners’ Perceptions of COVID-19, Zoonotic Disease, and Veterinary Medicine: The Impact of Demographic Characteristics" Veterinary Sciences 9, no. 5: 195. https://doi.org/10.3390/vetsci9050195
APA StylePowell, L., Lavender, T. M., Reinhard, C. L., & Watson, B. (2022). Pet Owners’ Perceptions of COVID-19, Zoonotic Disease, and Veterinary Medicine: The Impact of Demographic Characteristics. Veterinary Sciences, 9(5), 195. https://doi.org/10.3390/vetsci9050195






