Suspected Pituitary Apoplexy: Clinical Presentation, Diagnostic Imaging Findings and Outcome in 19 Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Clinical and Neurological Evaluation
2.3. Diagnostic Imaging Findings
2.4. Outcome
3. Results
3.1. Case Population
3.2. Onset of Clinical Signs
3.3. Neurological Findings
3.4. Imaging Findings
3.5. Treatment and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Valle, M.M.; Jesus, O. De Pituitary Apoplexy; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Pyrgelis, E.S.; Mavridis, I.; Meliou, M. Presenting Symptoms of Pituitary Apoplexy. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2018, 79, 052–059. [Google Scholar] [CrossRef]
- Briet, C.; Salenave, S.; Bonneville, J.F.; Laws, E.R.; Chanson, P. Pituitary Apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef] [PubMed]
- Glezer, A.; Bronstein, M.D. Pituitary apoplexy: Pathophysiology, diagnosis and management. Arch. Endocrinol. Metab. 2015, 59, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biousse, V.; Newman, N.J.; Oyesiku, N.M. Precipitating factors in pituitary apoplexy. J. Neurol. Neurosurg. Psychiatry 2001, 71, 542–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, E.H.; Lindholm, J.; Bjerre, P.; Christiansen, J.S.; Hagen, C.; Juul, S.; Jorgensen, J.; Kruse, A.; Laurberg, P. Frequent occurrence of pituitary apoplexy in patients with non-functioning pituitary adenoma. Clin. Endocrinol. 2006, 64, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Mohr, G.; Hardy, J. Hemorrhage, necrosis, and apoplexy in pituitary adenomas. Surg. Neurol. 1982, 18, 181–189. [Google Scholar] [CrossRef]
- Mohanty, S.; Tandon, P.N.; Banerji, A.K.; Prakash, B. Haemorrhage into pituitary adenomas. J. Neurol. Neurosurg. Psychiatry 1977, 40, 987–991. [Google Scholar] [CrossRef] [Green Version]
- Murad-Kejbou, S.; Eggenberger, E. Pituitary apoplexy: Evaluation, management, and prognosis. Curr. Opin. Ophthalmol. 2009, 20, 456–461. [Google Scholar] [CrossRef]
- Nawar, R.N.; AbdelMannan, D.; Selman, W.R.; Arafah, B.M. Pituitary Tumor Apoplexy: A Review. J. Intensive Care Med. 2008, 23, 75–90. [Google Scholar] [CrossRef]
- Semple, P.L.; Jane, J.A.; Lopes, M.B.S.; Laws, E.R. Pituitary apoplexy: Correlation between magnetic resonance imaging and histopathological results. J. Neurosurg. 2008, 108, 909–915. [Google Scholar] [CrossRef]
- Randeva, H.S.; Schoebel, J.; Byrne, J.; Esiri, M.; Adams, C.B.T.; Wass, J.A.H. Classical pituitary apoplexy: Clinical features, management and outcome. Clin. Endocrinol. 1999, 51, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Ball, S.G.; Connolly, V.; James, R.A.; Kane, P.; Kelly, W.F.; Kendall-Taylor, P.; Mathias, D.; Perros, P.; Quinton, R.; et al. Pituitary Apoplexy: A Review of Clinical Presentation, Management and Outcome in 45 Cases. Pituitary 2004, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Boellis, A.; Di Napoli, A.; Romano, A.; Bozzao, A. Pituitary apoplexy: An update on clinical and imaging features. Insights Imaging 2014, 5, 753–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, A.; Clayton, J.; Kumar, S.; Robertson, I.; Howlett, T.; Mansell, P. Pituitary apoplexy: Retrospective review of 30 patients--is surgical intervention always necessary? Br. J. Neurosurg. 2006, 20, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, J.; McGregor, E.J.; Mitchell, R.D.; Gittoes, N.J.L. Acute management of pituitary apoplexy--surgery or conservative management? Clin. Endocrinol. 2004, 61, 747–752. [Google Scholar] [CrossRef]
- Leyer, C.; Castinetti, F.; Morange, I.; Gueydan, M.; Oliver, C.; Conte-Devolx, B.; Dufour, H.; Brue, T. A conservative management is preferable in milder forms of pituitary tumor apoplexy. J. Endocrinol. Investig. 2011, 34, 502–509. [Google Scholar] [CrossRef]
- Bujawansa, S.; Thondam, S.K.; Steele, C.; Cuthbertson, D.; Gilkes, C.E.; Noonan, C.; Bleaney, C.W.; Macfarlane, I.A.; Javadpour, M.; Daousi, C. Presentation, management and outcomes in acute pituitary apoplexy: A large single-centre experience from the United Kingdom. Clin. Endocrinol. 2014, 80, 419–424. [Google Scholar] [CrossRef]
- Long, S.N.; Michieletto, A.; Anderson, T.J.; Williams, A.; Knottenbelt, C.M. Suspected pituitary apoplexy in a German shorthaired pointer. J. Small Anim. Pract. 2003, 44, 497–502. [Google Scholar] [CrossRef]
- Bertolini, G.; Rossetti, E.; Caldin, M. Pituitary Apoplexy-Like Disease in 4 Dogs. J. Vet. Intern. Med. 2007, 21, 1251–1257. [Google Scholar] [CrossRef]
- Beltran, E.; Dennis, R.; Foote, A.; De Risio, L.; Matiasek, L. Imaging Diagnosis: Pituitary Apoplexy in a Cat. Vet. Radiol. Ultrasound 2012, 53, 417–419. [Google Scholar] [CrossRef]
- Briola, C.; Galli, G.; Menchetti, M.; Caldin, M.; Bertolini, G. Pituitary tumour apoplexy due to pituitary adenoma in a dog: Clinical, 3T MRI and CT features. Vet. Rec. Case Rep. 2020, 8, e001052. [Google Scholar] [CrossRef]
- Kooistra, H.S.; Voorhout, G.; Mol, J.A.; Rijnberk, A. Correlation between impairment of glucocorticoid feedback and the size of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism. J. Endocrinol. 1997, 152, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, E.; Barthez, P.Y.; Van Der Vlugt-Meijer, R.H.; Voorhout, G.; Meij, B.P. Computed tomography and low-field magnetic resonance imaging of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism: 11 cases (2001–2003). J. Am. Vet. Med. Assoc. 2009, 235, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, M.; De Risio, L.; Galli, G.; Cherubini, G.B.; Corlazzoli, D.; Baroni, M.; Gandini, G. Neurological abnormalities in 97 dogs with detectable pituitary masses. Vet. Q. 2019, 39, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Imboden, P.N.E.; De Tribolet, N.; Lobrinus, A.; Gaillard, R.C.; Portmann, L.; Pralong, F.; Gomez, F. Apoplexy in pituitary macroadenoma: Eight patients presenting in 12 months. Medicine 2005, 84, 188–196. [Google Scholar] [CrossRef]
- Bills, D.C.; Meyer, F.B.; Laws, E.R.; Davis, D.H.; Ebersold, M.J.; Scheithauer, B.W.; Ilstrup, D.M.; Abboud, C.F. A retrospective analysis of pituitary apoplexy. Neurosurgery 1993, 33, 602–609. [Google Scholar]
- Verrees, M.; Arafah, B.M.; Selman, W.R. Pituitary tumor apoplexy: Characteristics, treatment, and outcomes. Neurosurg. Focus 2004, 16, 1–7. [Google Scholar] [CrossRef]
- De Risio, L. Clinical and diagnostic investigation of the seizure patient. In Canine and Feline Epilepsy, Diagnosis and Management, 1st ed.; De Risio, L., Platt, S., Eds.; CABI: Wallingford, UK, 2014; pp. 274–324. [Google Scholar]
- Meij, B.P. Hypophysectomy as a Treatment for Canine and Feline Cushing’s Disease. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 1015–1041. [Google Scholar] [CrossRef]
- Hullinger, R.L. The endocrine system. In Miller’s Anatomy of the Dog, 4th ed.; Evans, H.E., De Lahunta, A., Eds.; Elsevier Saunders: St Louis, MO, USA, 2013; pp. 406–427. [Google Scholar]
- Rogg, J.M.; Tung, G.A.; Anderson, G.; Cortez, S. Pituitary Apoplexy: Early Detection with Diffusion-Weighted MR Imaging. Am. J. Neuroradiol. 2002, 23, 1240–1245. [Google Scholar]
- Wood, F.D.; Pollard, R.E.; Uerling, M.R.; Feldman, E.C. Diagnostic imaging findings and endocrine test results in dogs with pituitary-dependent hyperadrenocorticism that did or did not have neurologic abnormalities: 157 cases (1989–2005). J. Am. Vet. Med. Assoc. 2007, 231, 1081–1085. [Google Scholar] [CrossRef]
- Nelson, R.W.; Ihle, S.L.; Feldman, E.C. Pituitary macroadenomas and macroadenocarcinomas in dogs treated with mitotane for pituitary-dependent hyperadrenocorticism: 13 cases (1981–1986). J. Am. Vet. Med. Assoc. 1989, 194, 1612–1617. [Google Scholar] [PubMed]
- Duesberg, C.A.; Feldman, E.C.; Nelson, R.W.; Bertoy, E.H.; Dublin, A.B.; Reid, M.H. Magnetic resonance imaging for diagnosis of pituitary macrotumors in dogs. J. Am. Vet. Med. Assoc. 1995, 206, 657–662. [Google Scholar] [PubMed]
- McConnell, F.F. Brain hemorrhage. In Diagnostic MRI in Dogs and Cats, 1st ed.; Mai, W., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 282–308. [Google Scholar]
- Whitlock, J.; Holdsworth, A.; Morales, C.; Garosi, L.; Carrera, I. 1.5 Tesla Magnetic Resonance Imaging Features of Canine Intracranial Intra-axial Hematomas. Front. Vet. Sci. 2021, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, S.J.; Galac, S.; Tryfonidou, M.A.; Hesselink, J.W.; Penning, L.C.; Kooistra, H.S.; Meij, B.P. The Influence of Pituitary Size on Outcome After Transsphenoidal Hypophysectomy in a Large Cohort of Dogs with Pituitary-Dependent Hypercortisolism. J. Vet. Intern. Med. 2016, 30, 989–995. [Google Scholar] [CrossRef]
- Meij, B.; Voorhout, G.; Rijnberk, A. Progress in transsphenoidal hypophysectomy for treatment of pituitary-dependent hyperadrenocorticism in dogs and cats. Mol. Cell. Endocrinol. 2002, 197, 89–96. [Google Scholar] [CrossRef]
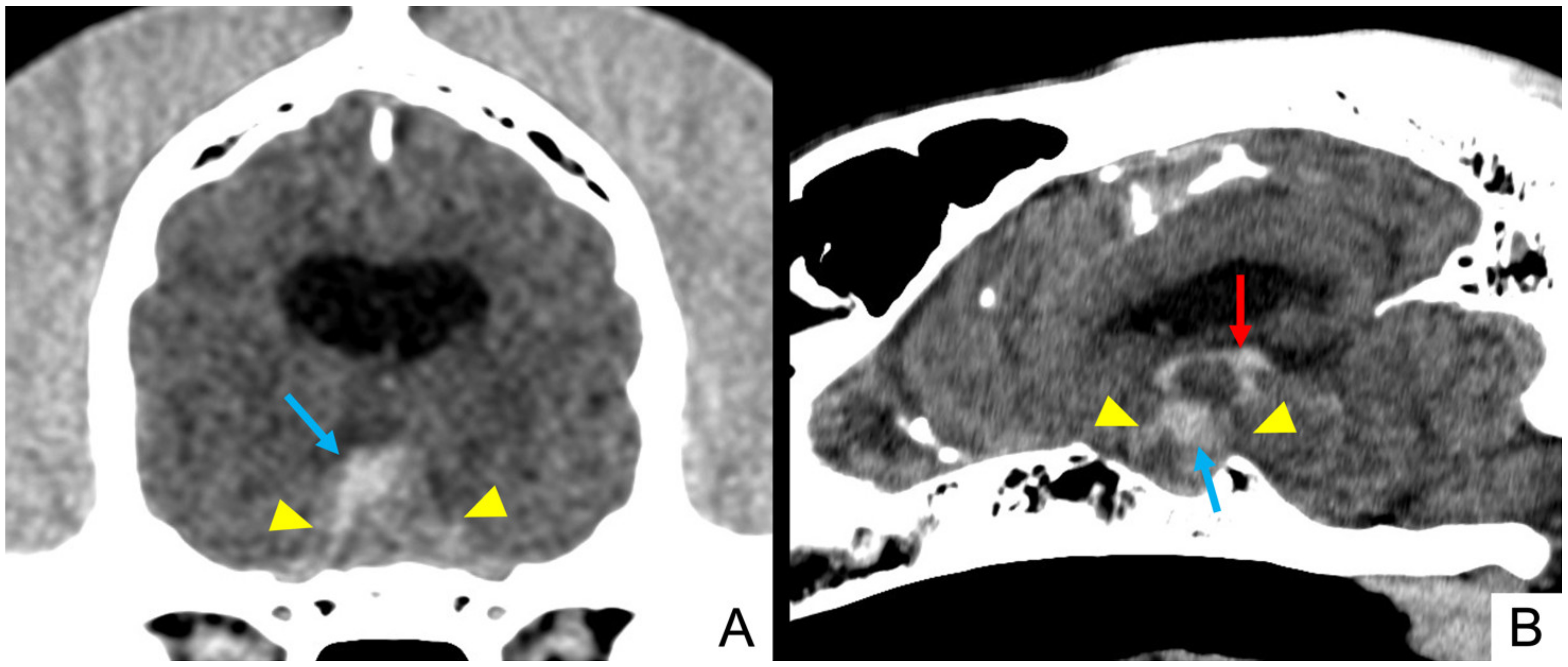
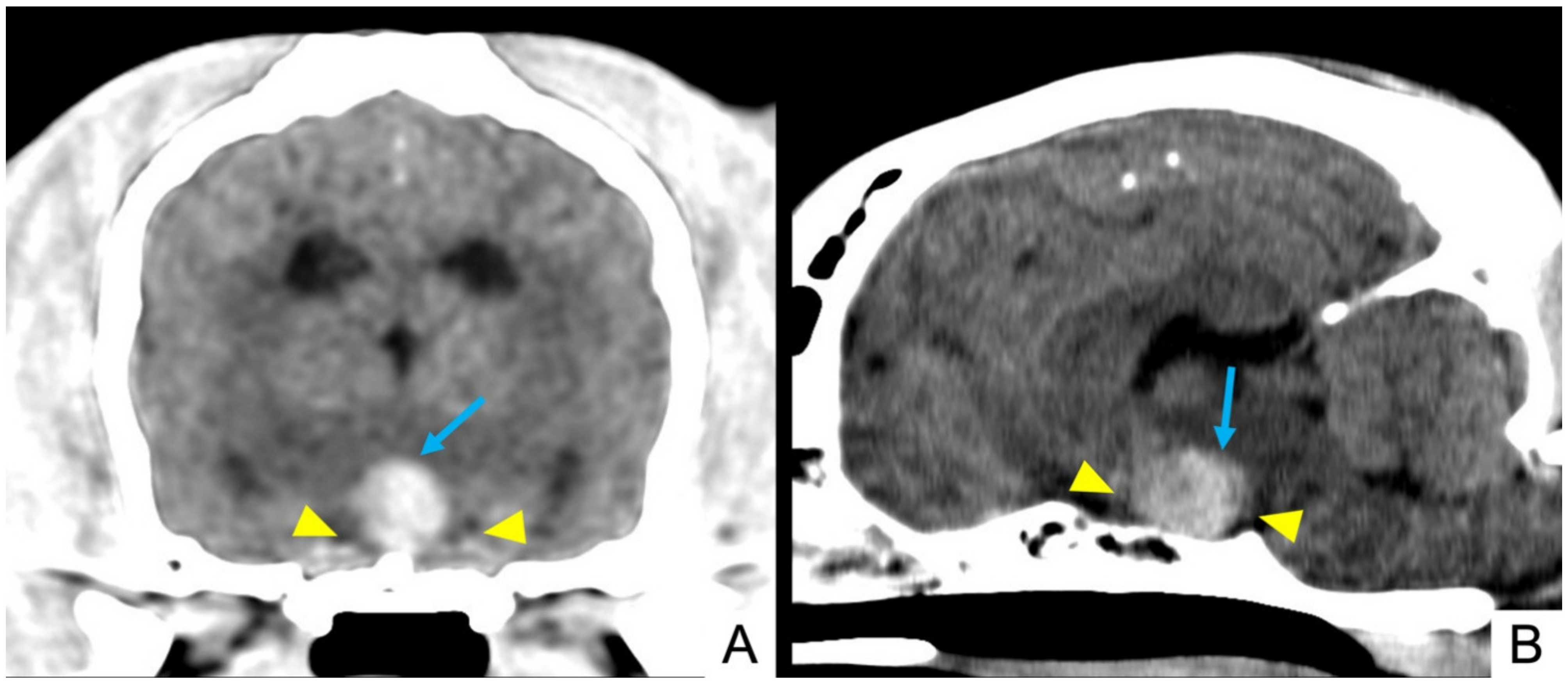

| Signalment | Onset (Per-Acute/Acute/Chronic) | Main Complain | Epileptic Seizures | Accessory Signs |
|---|---|---|---|---|
| 1. Mixed breed MN, 9.75 y, 24.6 Kg | Per-acute | Balance loss | No | \ |
| 2. Italian hound F, 11.5 y, 14.8 Kg | Per-acute | Balance loss | No | Pica |
| 3. Mixed breed FN, 12.7 y, 4.7 Kg | Per-acute | Balance loss | No | \ |
| 4. Italian Bracco dog F, 12.8 y, 27 Kg | Per-acute | Compulsion, circling, obtundation | No | \ |
| 5. Mixed breed FN, 12.7 y, 30 Kg | Per-acute | Vocalization, pathologic nystagmus | No | \ |
| 6. Beagle FN, 7.6 y, 16.6 Kg | Per-acute | Balance loss, pathologic nystagmus | No | \ |
| 7. Labrador Retriever F, 5 y, 31.2 Kg | Per-acute | Anxious behaviour | Yes | PU/PD, weakness |
| 8. Labrador Retriever FN, 10.8 y, 35 Kg | Per-acute | \ | Yes | \ |
| 9. Mixed breed FN, 5.8 y, 21 Kg | Chronic | Aggressive behaviour | No | \ |
| 10. Labrador Retriever FN, 8.6 y, 29 Kg | Per-acute | \ | Yes | \ |
| 11. Boxer FN, 11.4 y, 27.4 Kg | Per-acute | Vocalizations, circling | No | \ |
| 12. Springer Spaniel M, 13.7 y, 18.7 Kg | Acute | Disorientation, anxious behaviour, obtundation | Yes | \ |
| 13. Mixed breed M, 11.1 y, 33 Kg | Per-acute | Circling | No | Weight loss |
| 14. Mixed breed M, 9.8 y, 14.5 Kg | Per-acute | Disorientation, obtundation, balance loss | No | Tremors |
| 15. Corso dog FN, 9.4 y, 43 Kg | Per-acute | Balance loss | No | \ |
| 16. English Bulldog M, 6.1 y, 28 Kg | Per-acute | Aggressive behaviour, vocalization, obtundation | No | Dysorexia, tremors |
| 17. Mixed breed M, 9.3 y, 9.3 Kg | Chronic | Obtundation | No | Weakness, weight loss, tremors |
| 18. Mixed breed M, 3.2 y, 15 Kg | Per-acute | Non-specific changes from normal behaviour | Yes | \ |
| 19. Mixed breed M, 11.1 y, 27.6 Kg | Acute | Vocalization, obtundation | No | PU/PD |
| Neurologic Examination | Behaviour | Mental Status | Posture | Gait | Postural Reactions | Cranial Nerves | Epileptic Seizures | Hyperalgesia |
|---|---|---|---|---|---|---|---|---|
| Results | Normal (n = 8) Altered (n = 11) | Normal (n = 12) Altered (n = 7) | Normal (n = 18) Altered (n =1) | Normal (n = 15) Altered (n = 4) | Normal (n = 14) Altered (n = 5) | Normal (n = 15) Altered (n =4) | No (n = 13) Yes (n = 6) | No (n = 16) Yes (n = 3) |
| Detail | Disorientation (n = 6) Compulsion (n = 4) Circling (n = 3) Aggressive behaviour (n = 3) Head pressing (n = 2) Anxiety (n = 2) Vocalizations (n = 2) | Obtundation (n = 7) | Neck ventroflexion (n = 1) | Hindlimbs hypometria (n = 1) Hindlimbs proprioceptive ataxia (n = 1) Proprioceptive ataxia 4 limbs (n = 1) Tetraparesis (n = 1) | 4 limbs (n = 3) 1 posterior limb (n = 1) Left side (n = 1) | Altered menace response (n = 4) Internal ophtalmoparesis/plegia (n = 2) | Cervical (n = 2) Diffuse (n = 1) |
| Signalment | Pre-Contrast Heterogeneity | Pre-Contrast Hyperattenuation | Hyperattenuating Foci | Contrast Enhancement | Post-Contrast Hypovascular Areas | CT Diagnosis |
|---|---|---|---|---|---|---|
| 1. Mixed breed MN, 9.75 y, 24.6 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 3. Mixed breed FN, 12.7 y, 4.7 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 4. Italian Bracco dog F, 12.8 y, 27 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 5. Mixed breed FN, 12.7 y, 30 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 6. Beagle FN, 7.6 y, 16.6 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 7. Labrador Retriever F, 5 y, 31.2 Kg | Yes | Yes | Yes | Heterogeneous | No | Pituitary mass with intralesional haemorrhage |
| 8. Labrador Retriever FN, 10.8 y, 35 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 9. Mixed breed FN, 5.8 y, 21 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 10. Labrador Retriever FN, 8.6 y, 29 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 11. Boxer FN, 11.4 y, 27.4 Kg | Yes | Yes | No | Heterogeneous | No | Pituitary mass with intralesional haemorrhage |
| 12. Springer Spaniel M, 13.7 y, 18.7 Kg | Yes | Yes | No | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 13. Mixed breed M, 11.1 y, 33 Kg | Yes | Yes | No | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 14. Mixed breed M, 9.8 y, 14.5 Kg | Yes | Yes | No | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| 15. Corso dog FN, 9.4 y, 43 Kg | Yes | Yes | Yes | Heterogeneous | No | Pituitary mass with intralesional haemorrhage |
| 16. English Bulldog M, 6.1 y, 28 Kg | Yes | Yes | No | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage and necrosis |
| 17. Mixed breed M, 9.3 y, 9.3 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage and necrosis |
| 18. Mixed breed M, 3.2 y, 15 Kg | Yes | Yes | Yes | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage and necrosis |
| 19. Mixed breed M, 11.1 y, 27.6 Kg | Yes | Yes | No | Heterogeneous | Yes | Pituitary mass with intralesional haemorrhage |
| Signalment | T1-Weighted | T2-Weighted | FLAIR | T2*/SWI | DWI | ADC | Contrast Enhancement | MRI Diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1. Mixed breed MN, 9.75 y, 24.6 Kg | Homogeneous isointense | Heterogeneous isointense | Heterogeneous isointense | No | Hyperintense | Hypointense | Heterogeneous | Pituitary mass with different stages of intralesional haemorrhage |
| 2. Italian hound F, 11.5 y, 14.8 Kg | Heterogeneous isointense | Heterogeneous isointense | Heterogeneous isointense | Yes | Hyperintense | Hypointense | Heterogeneous | Pituitary mass with intralesional haemorrhage |
| 5. Mixed breed FN, 12.7 y, 30 Kg | Homogeneous isointense | Heterogeneous isointense | Heterogeneous isointense | Yes | Isointense | Isointense | Heterogeneous | Pituitary mass with intralesional haemorrhage |
| 6. Beagle FN, 7.6 y, 16.6 Kg | Heterogeneous hypointense | Heterogeneous hyperintense | Heterogeneous hyperintense | Yes | Hypointense | Hypointense | Heterogeneous | Pituitary mass with intralesional haemorrhage |
| 7. Labrador Retriever F, 5 y, 31.2 Kg | Heterogeneous hypointense | Heterogeneous hyperintense | Heterogeneous hyperintense | \ | Hypointense | Hyperintense | Heterogeneous | Pituitary mass with intralesional haemorrhage |
| 8. Labrador Retriever FN, 10.8 y, 35 Kg | Heterogeneous hypointense | Heterogeneous hyperintense | Heterogeneous hyperintense | No | \ | \ | Heterogeneous | Pituitary mass |
| 9. Mixed breed FN, 5.8 y, 21 Kg | Heterogeneous hypointense | Heterogeneous hyperintense | Heterogeneous hyperintense | Yes | \ | \ | Heterogeneous | Pituitary mass with intralesional haemorrhage |
| 11. Boxer FN, 11.4 y, 27.4 Kg | Homogeneous hyperintense | Heterogeneous hyperintense | Heterogeneous hyperintense | \ | \ | \ | Heterogeneous | Pituitary mass with intralesional haemorrhage |
| 12. Springer Spaniel M, 13.7 y, 18.7 Kg | Heterogeneous isointense | Heterogeneous hyperintense | Heterogeneous hyperintense | Yes | Hypointense | Hyperintense | Heterogeneous | Pituitary mass with intralesional haemorrhage |
| Signalment | Onset (Per-Acute/Acute/Chronic) | Recovery Time | Long Term Follow-Up | MRI/CT | P:B Ratio | Mass Effect (Yes/No) |
|---|---|---|---|---|---|---|
| 1. Mixed breed MN, 9.75 y, 24.6 Kg | Per-acute | <24 h | Alive (11 m) | MRI CT | 0.52 | No |
| 2. Italian hound F, 11.5 y, 14.8 Kg | Per-acute | <24 h | Alive (6 m) | MRI | 0.35 | No |
| 3. Mixed breed FN, 12.7 y, 4.7 Kg | Per-acute | <24 h | Alive (18 m) | CT | 0.75 | Yes |
| 4. Italian Bracco dog F, 12.8 y, 27 Kg | Per-acute | 48–72 h | Unrelated survival time: death 7 m | CT | 0.78 | Yes |
| 5. Mixed breed FN, 12.7 y, 30 Kg | Per-acute | <24 h | Alive (32 m) | MRI CT | 0.37 | No |
| 6. Beagle FN, 7.6 y, 16.6 Kg | Per-acute | 48–72 h | Related survival time: death 23 m | MRI CT | 0.48 | No |
| 7. Labrador Retriever F, 5 y, 31.2 Kg | Per-acute | <24 h | Alive (34 m) | MRI CT | 0.35 | No |
| 8. Labrador Retriever FN, 10.8 y, 35 Kg | Per-acute | D | Related death for cardiovascular arrest 3 days after PA diagnosis | MRI CT | 0.39 | No |
| 9. Mixed breed FN, 5.8 y, 21 Kg | Chronic | Persistent behavioural changes | Related survival time: death 8 m | MRI CT | 1.3 | Yes |
| 10. Labrador Retriever FN, 8.6 y, 29 Kg | Per-acute | <24 h | Unknown | CT | 0.33 | No |
| 11. Boxer FN, 11.4 y, 27.4 Kg | Per-acute | 24–48 h | Related survival time: death 1 m | MRI CT | 1.2 | yes |
| 12. Springer Spaniel M, 13.7 y, 18.7 Kg | Acute | <24 h | Unrelated survival time: death 25 m | MRI CT | 0.8 | Yes |
| 13. Mixed breed M, 11.1 y, 33 Kg | Per-acute | D | Euthanised 4 days after PA diagnosis | CT | 0.88 | Yes |
| 14. Mixed breed M, 9.8 y, 14.5 Kg | Per-acute | Unknown | Unknown | CT | 1.1 | Yes |
| 15. Corso dog FN, 9.4 y, 43 Kg | Per-acute | Unknown | Unknown | CT | 0.44 | No |
| 16. English Bulldog M, 6.1 y, 28 Kg | Acute | Unknown | Unknown | CT | 1.35 | Yes |
| 17. Mixed breed M, 9.3 y, 9.3 Kg | Chronic | 24–48 h | Related survival time: death 102 m | CT | 1.3 | Yes |
| 18. Mixed breed M, 3.2 y, 15 Kg | Per-acute | D | Euthanised 1 day after PA diagnosis | CT | 1.3 | Yes |
| 19. Mixed breed M, 11.1 y, 27.6 Kg | Acute | Unknown | Unknown | CT | 1.03 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, G.; Bertolini, G.; Dalla Serra, G.; Menchetti, M. Suspected Pituitary Apoplexy: Clinical Presentation, Diagnostic Imaging Findings and Outcome in 19 Dogs. Vet. Sci. 2022, 9, 191. https://doi.org/10.3390/vetsci9040191
Galli G, Bertolini G, Dalla Serra G, Menchetti M. Suspected Pituitary Apoplexy: Clinical Presentation, Diagnostic Imaging Findings and Outcome in 19 Dogs. Veterinary Sciences. 2022; 9(4):191. https://doi.org/10.3390/vetsci9040191
Chicago/Turabian StyleGalli, Greta, Giovanna Bertolini, Giulia Dalla Serra, and Marika Menchetti. 2022. "Suspected Pituitary Apoplexy: Clinical Presentation, Diagnostic Imaging Findings and Outcome in 19 Dogs" Veterinary Sciences 9, no. 4: 191. https://doi.org/10.3390/vetsci9040191
APA StyleGalli, G., Bertolini, G., Dalla Serra, G., & Menchetti, M. (2022). Suspected Pituitary Apoplexy: Clinical Presentation, Diagnostic Imaging Findings and Outcome in 19 Dogs. Veterinary Sciences, 9(4), 191. https://doi.org/10.3390/vetsci9040191







