Rodent–Human Interface: Behavioral Risk Factors and Leptospirosis in a Province in the Central Region of Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
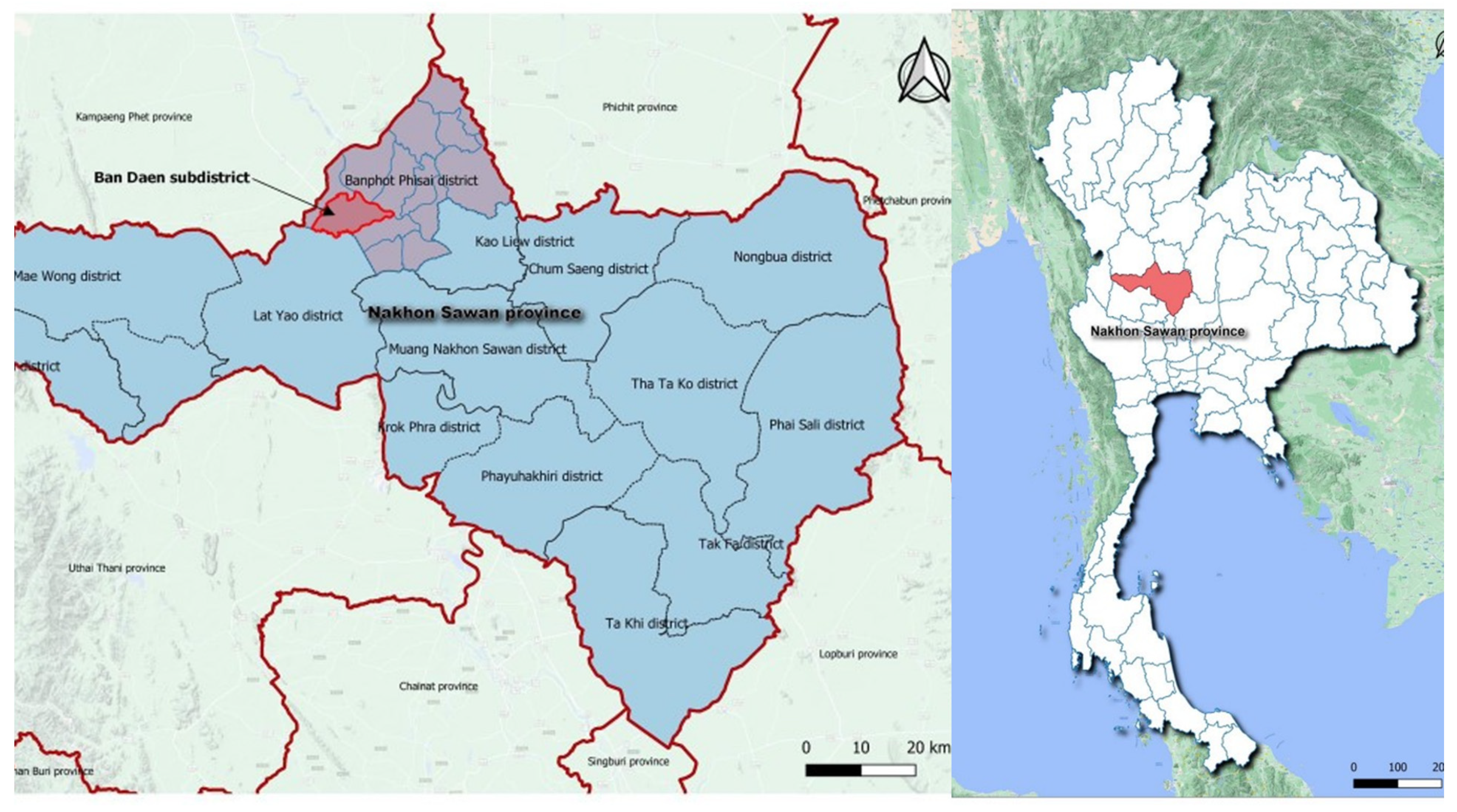
2.2. Study Sites
2.3. Study Participant Criteria
2.4. Sample Size Estimation
2.4.1. Analytic Cross-Sectional Survey and Blood Specimen Collection
2.4.2. Qualitative Study
2.5. Study Tools and Data Collection Procedures
2.6. Study Variables and Data Analysis
2.7. Serological Tests
3. Quantitative Survey Results
3.1. Profiles of Quantitative Survey Participants
3.2. Animal Contact Experiences
3.3. Hunted, Killed, and Prepared Rodents as Food in the Past 12 Months
3.4. Consumed Animals (Either Raw or Cooked) in the Past 12 Months
3.5. Being Bitten by Rodents, including the Period, and Practices toward Wounds
3.6. Feeling toward Animals and Zoonotic Diseases
3.7. Medical Histories
3.8. Leptospirosis Prevalence Study among the Study Subjects
3.9. Rodent Exposure Levels
4. Qualitative Data Collection Results
4.1. Profiles of Qualitative Study Participants
4.2. Rodent Contact Characteristics among the Qualitative Participants
4.3. Rodent Contact Activities and Their Perception toward Rodents
4.4. Benefits and Disadvantages of Rodents
4.5. Mitigation Strategies to Be Protected from Rodents and Diseases
5. Discussion and Conclusions
5.1. Rodent as Food
5.2. KAP and Mitigation Measures for Interacting with Rodents
5.3. Seroprevalence of Leptospirosis among the Study Participants
5.4. Study Limitations
5.5. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Racaniello, V.R. Emerging infectious diseases. J. Clin. Investig. 2004, 113, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, N.D. Bushmeat Hunting, Deforestation, and Prediction of Zoonotic Disease Emergence. Emerg. Infect. Dis. 2005, 11, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Keawcharoen, J.; van den Broek, J.; Bouma, A.; Tiensin, T.; Osterhaus, A.D.; Heesterbeek, H. Wild birds and increased transmission of highly pathogenic avian influenza (H5N1) among poultry, Thailand. Emerg. Infect. Dis. 2011, 17, 1016–1022. [Google Scholar] [CrossRef]
- Baudel, H.; De Nys, H.; Mpoudi Ngole, E.; Peeters, M.; Desclaux, A. Understanding Ebola virus and other zoonotic transmission risks through human-bat contacts: Exploratory study on knowledge, attitudes and practices in Southern Cameroon. Zoonoses Public Health 2019, 66, 288–295. [Google Scholar] [CrossRef]
- Oosterom, J. Epidemiological studies and proposed preventive measures in the fight against human salmonellosis. Int. J. Food Microbiol. 1991, 12, 41–51. [Google Scholar] [CrossRef]
- Blasdell, K.; Cosson, J.F.; Chaval, Y.; Herbreteau, V.; Douangboupha, B.; Jittapalapong, S.; Lundqvist, A.; Hugot, J.P.; Morand, S.; Buchy, P. Rodent-borne hantaviruses in Cambodia, Lao PDR, and Thailand. Ecohealth 2011, 8, 432–443. [Google Scholar] [CrossRef]
- Victoriano, A.F.; Smythe, L.D.; Gloriani-Barzaga, N.; Cavinta, L.L.; Kasai, T.; Limpakarnjanarat, K.; Ong, B.L.; Gongal, G.; Hall, J.; Coulombe, C.A.; et al. Leptospirosis in the Asia Pacific region. BMC Infect. Dis. 2009, 9, 147. [Google Scholar] [CrossRef]
- Quinn, E.K.; Massey, P.D.; Cox-Witton, K.; Paterson, B.J.; Eastwood, K.; Durrheim, D.N. Understanding human-bat interactions in NSW, Australia: Improving risk communication for prevention of Australian bat lyssavirus. BMC Vet. Res. 2014, 10, 144. [Google Scholar] [CrossRef]
- Gurley, E.S.; Hegde, S.T.; Hossain, K.; Sazzad, H.M.S.; Hossain, M.J.; Rahman, M.; Sharker, M.A.Y.; Salje, H.; Islam, M.S.; Epstein, J.H.; et al. Convergence of Humans, Bats, Trees, and Culture in Nipah Virus Transmission, Bangladesh. Emerg. Infect. Dis. 2017, 23, 1446–1453. [Google Scholar] [CrossRef]
- Robertson, K.; Lumlertdacha, B.; Franka, R.; Petersen, B.; Bhengsri, S.; Henchaichon, S.; Peruski, L.F.; Baggett, H.C.; Maloney, S.A.; Rupprecht, C.E. Rabies-related knowledge and practices among persons at risk of bat exposures in Thailand. PLoS Negl. Trop. Dis. 2011, 5, e1054. [Google Scholar] [CrossRef] [PubMed]
- de Zwart, O.; Veldhuijzen, I.K.; Elam, G.; Aro, A.R.; Abraham, T.; Bishop, G.D.; Voeten, H.A.; Richardus, J.H.; Brug, J. Perceived threat, risk perception, and efficacy beliefs related to SARS and other (emerging) infectious diseases: Results of an international survey. Int. J. Behav. Med. 2009, 16, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.M.; Liu, L.P.; Chung, W.C.; Ong, S.J.; Wang, C.C. Human babesiosis in Taiwan: Asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J. Clin. Microbiol. 1997, 35, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Kutz, S.J.; Smith, A. Parasite zoonoses and wildlife: Emerging issues. Int. J. Environ. Res. Public Health 2009, 6, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; Kijlstra, A. Zoonotic risk of rodents in livestock production. Tijdschr. Voor Diergeneeskd. 2006, 131, 445–447. [Google Scholar]
- Kruse, H.; Kirkemo, A.M.; Handeland, K. Wildlife as source of zoonotic infections. Emerg. Infect. Dis. 2004, 10, 2067–2072. [Google Scholar] [CrossRef]
- Ripple, W.J.; Abernethy, K.; Betts, M.G.; Chapron, G.; Dirzo, R.; Galetti, M.; Levi, T.; Lindsey, P.A.; Macdonald, D.W.; Machovina, B.; et al. Bushmeat hunting and extinction risk to the world’s mammals. R. Soc. Open Sci. 2016, 3, 160498. [Google Scholar] [CrossRef]
- Ordaz-Nemeth, I.; Arandjelovic, M.; Boesch, L.; Gatiso, T.; Grimes, T.; Kuehl, H.S.; Lormie, M.; Stephens, C.; Tweh, C.; Junker, J. The socio-economic drivers of bushmeat consumption during the West African Ebola crisis. PLoS Negl. Trop. Dis. 2017, 11, e0005450. [Google Scholar] [CrossRef]
- Meng, X.; Liu, D.; Feng, J.; Meng, Z. Asian medicine: Exploitation of wildlife. Science 2012, 335, 1168. [Google Scholar] [CrossRef] [PubMed]
- Wanger, T.C.; Darras, K.; Bumrungsri, S.; Tscharntke, T.; Klein, A.-M. Bat pest control contributes to food security in Thailand. Biol. Conserv. 2014, 171, 220–223. [Google Scholar] [CrossRef]
- Travis, D.A.; Watson, R.P.; Tauer, A. The spread of pathogens through trade in wildlife. Rev. Sci. Tech. 2011, 30, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Joyjinda, Y.; Rodpan, A.; Chartpituck, P.; Suthum, K.; Yaemsakul, S.; Cheun-Arom, T.; Bunprakob, S.; Olival, K.J.; Stokes, M.M.; Hemachudha, T.; et al. First Complete Genome Sequence of Human Coronavirus HKU1 from a Nonill Bat Guano Miner in Thailand. Microbiol. Resour. Announc. 2019, 8, e01457-18. [Google Scholar] [CrossRef] [PubMed]
- Platto, S.; Zhou, J.; Wang, Y.; Wang, H.; Carafoli, E. Biodiversity loss and COVID-19 pandemic: The role of bats in the origin and the spreading of the disease. Biochem. Biophys. Res. Commun. 2021, 538, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Meeyam, T.; Tablerk, P.; Petchanok, B.; Pichpol, D.; Padungtod, P. Seroprevalence and risk factors associated with leptospirosis in dogs. S. Asian J. Trop. Med. Public Health 2006, 37, 148–153. [Google Scholar]
- Sprissler, F.; Jongwattanapisan, P.; Luengyosluechakul, S.; Pusoonthornthum, R.; Prapasarakul, N.; Kurilung, A.; Goris, M.; Ahmed, A.; Reese, S.; Bergmann, M.; et al. Leptospira infection and shedding in cats in Thailand. Transbound. Emerg. Dis. 2018, 66, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Kurilung, A.; Chanchaithong, P.; Lugsomya, K.; Niyomtham, W.; Wuthiekanun, V.; Prapasarakul, N. Molecular detection and isolation of pathogenic Leptospira from asymptomatic humans, domestic animals and water sources in Nan province, a rural area of Thailand. Res. Vet. Sci. 2017, 115, 146–154. [Google Scholar] [CrossRef]
- Munoz-Zanzi, C.; Mason, M.; Encina, C.; Gonzalez, M.; Berg, S. Household characteristics associated with rodent presence and Leptospira infection in rural and urban communities from Southern Chile. Am. J. Trop. Med. Hyg. 2014, 90, 497–506. [Google Scholar] [CrossRef]
- Suwannarong, K.; Schuler, S. Bat consumption in Thailand. Infect. Ecol. Epidemiol. 2016, 6, 29941. [Google Scholar] [CrossRef]
- Suwannarong, K.; Chapman, R.S. Rodent consumption in Khon Kaen Province, Thailand. S. Asian J. Trop. Med. Public Health 2014, 45, 1209–1220. [Google Scholar]
- Suwannarong, K.; Chapman, R.S.; Lantican, C.; Michaelides, T.; Zimicki, S. Hunting, Food Preparation, and Consumption of Rodents in Lao PDR. PLoS ONE 2015, 10, e0133150. [Google Scholar] [CrossRef][Green Version]
- Thipmontree, W.; Suputtamongkol, Y.; Tantibhedhyangkul, W.; Suttinont, C.; Wongswat, E.; Silpasakorn, S. Human leptospirosis trends: Northeast Thailand, 2001–2012. Int. J. Environ. Res. Public Health 2014, 11, 8542–8551. [Google Scholar] [CrossRef]
- Suwannarong, K.; Chapman, R.S. Characteristics associated with contact with rodents in, around, and outside homes in khon kaen province, Thailand. Am. J. Trop. Med. Hyg. 2015, 92, 784–790. [Google Scholar] [CrossRef]
- Suwannarong, K.; Chanabun, S.; Kanthawee, P.; Khiewkhern, S.; Boonyakawee, P.; Suwannarong, K.; Saengkul, C.; Bubpa, N.; Amonsin, A. Risk factors for bat contact and consumption behaviors in Thailand; a quantitative study. BMC Public Health 2020, 20, 841. [Google Scholar]
- Suwannarong, K.; Janetanakit, T.; Kanthawee, P.; Suwannarong, K.; Theamboonlers, A.; Poovorawan, Y.; Tun, H.M.; Chanabun, S.; Amonsin, A. Coronavirus seroprevalence among villagers exposed to bats in Thailand. Zoonoses Public Health 2021, 68, 464–473. [Google Scholar] [CrossRef]
- Nowell, L.S.; Norris, J.M.; White, D.E.; Moules, N.J. Thematic analysis: Striving to meet the trustworthiness criteria. Int. J. Qual. Methods 2017, 16, 1609406917733847. [Google Scholar]
- World Health Organization. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Coyle, A.H.; Berrian, A.M.; van Rooyen, J.; Bagnol, B.; Smith, M.H. Gender Roles and One Health Risk Factors at the Human-Livestock-Wildlife Interface, Mpumalanga Province, South Africa. Ecohealth 2020, 17, 233–247. [Google Scholar]
- Ter Meulen, J.; Lukashevich, I.; Sidibe, K.; Inapogui, A.; Marx, M.; Dorlemann, A.; Yansane, M.L.; Koulemou, K.; Chang-Claude, J.; Schmitz, H. Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of Lassa virus in the Republic of Guinea. Am. J. Trop. Med. Hyg. 1996, 55, 661–666. [Google Scholar]
- Gruber, K. Rodent meat—A sustainable way to feed the world? Using rodents as food has a long tradition in many parts of the world. EMBO Rep. 2016, 17, 630–633. [Google Scholar]
- Assogbadjo, A.E.; Codjia, J.T.C.; Sinsin, B.; Ekue, M.R.M.; Mensah, G.A. Importance of rodents as a human food source in Benin. Belg. J. Zool. 2005, 135, 11–15. [Google Scholar]
- Rathinam, S.; Vedhanayaki, R.; Balagiri, K. A Cross-Sectional Assessment of Knowledge, Attitude, and Practice Toward Leptospirosis among Rural and Urban Population of a South Indian District. Ocul. Immunol. Inflamm. 2019, 16, 1–14. [Google Scholar] [CrossRef]
- De Vries, S.G.; Visser, B.J.; Nagel, I.M.; Goris, M.G.; Hartskeerl, R.A.; Grobusch, M.P. Leptospirosis in Sub-Saharan Africa: A systematic review. Int. J. Infect. Dis. 2014, 28, 47–64. [Google Scholar] [CrossRef]
- Chadsuthi, S.; Bicout, D.J.; Wiratsudakul, A.; Suwancharoen, D.; Petkanchanapong, W.; Modchang, C.; Triampo, W.; Ratanakorn, P.; Chalvet-Monfray, K. Investigation on predominant Leptospira serovars and its distribution in humans and livestock in Thailand, 2010–2015. PLoS Negl. Trop. Dis. 2017, 11, e0005228. [Google Scholar] [CrossRef]
- Gonwong, S.; Chuenchitra, T.; Khantapura, P.; Islam, D.; Ruamsap, N.; Swierczewski, B.E.; Mason, C.J. Nationwide Seroprevalence of Leptospirosis among Young Thai Men, 2007–2008. Am. J. Trop. Med. Hyg. 2017, 97, 1682–1685. [Google Scholar] [CrossRef]
- Suwancharoen, D.; Limlertvatee, S.; Chetiyawan, P.; Tongpan, P.; Sangkaew, N.; Sawaddee, Y.; Inthakan, K.; Wiratsudakul, A. A nationwide survey of pathogenic leptospires in urine of cattle and buffaloes by Loop-mediated isothermal amplification (LAMP) method in Thailand, 2011–2013. J. Vet. Med. Sci. 2016, 78, 1495–1500. [Google Scholar] [CrossRef]
- Tangkanakul, W.; Smits, H.L.; Jatanasen, S.; Ashford, D.A. Leptospirosis: An emerging health problem in Thailand. S. Asian J. Trop. Med. Public Health 2005, 36, 281–288. [Google Scholar]
- Nitatpattana, N.; Chauvancy, G.; Dardaine, J.; Poblap, T.; Jumronsawat, K.; Tangkanakul, W.; Poonsuksombat, D.; Yoksan, S.; Gonzalez, J.P. Serological study of hantavirus in the rodent population of Nakhon Pathom and Nakhon Ratchasima Provinces Thailand. S. Asian J. Trop. Med. Public Health 2000, 31, 277–282. [Google Scholar]
- Tangkanakul, W.; Tharmaphornpil, P.; Plikaytis, B.D.; Bragg, S.; Poonsuksombat, D.; Choomkasien, P.; Kingnate, D.; Ashford, D.A. Risk factors associated with leptospirosis in northeastern Thailand, 1998. Am. J. Trop. Med. Hyg. 2000, 63, 204–208. [Google Scholar] [CrossRef][Green Version]
- Hinjoy, S.; Kongyu, S.; Doung-Ngern, P.; Doungchawee, G.; Colombe, S.D.; Tsukayama, R.; Suwancharoen, D. Environmental and Behavioral Risk Factors for Severe Leptospirosis in Thailand. Trop. Med. Infect. Dis. 2019, 4, 79. [Google Scholar] [CrossRef]


| SES Variables | Males (149, 40.1%) n (%) | Females (223, 59.9%) n (%) | Total (372, 100.0%) n (%) |
|---|---|---|---|
| Age groups | |||
| 20–30 years old | 19 (12.8%) | 26 (11.7%) | 45 (12.1%) |
| 31–40 years old | 14 (9.4%) | 21 (9.4%) | 35 (9.4%) |
| 41–50 years old | 31 (20.8%) | 46 (20.6%) | 95 (20.7%) |
| 51–60 years old | 59 (39.6%) | 87 (39.0%) | 92 (39.2%) |
| 61–65 years old | 26 (17.4%) | 43 (19.3%) | 69 (18.5%) |
| Age groups (Cut-off age at 45 years old) | |||
| 20 to 45 years old | 47 (31.5%) | 66 (29.6%) | 113 (30.4%) |
| >45 years old | 102 (68.5%) | 157 (70.4%) | 259 (69.9%) |
| Range | 20–65 year old | 20–65 year old | 20–65 year old |
| Mean + SD | 49.34 ± 12.15 | 49.52 ± 12.06 | 49.45 ± 12.07 |
| Marital status | |||
| Single | 34 (22.8%) | 52 (23.3%) | 86 (23.1%) |
| Married | 107 (71.1%) | 150 (67.3%) | 257 (69.1%) |
| Other (divorced or widows) | 8 (2.2%) | 21 (9.4%) | 29 (7.8%) |
| Educational attainment levels | |||
| No formal education | 2 (1.3%) | 6 (2.7%) | 8 (2.2%) |
| Primary school | 90 (60.4%) | 134 (60.1%) | 224 (60.2%) |
| Secondary school | 34 (22.8%) | 28 (12.6%) | 62 (16.7%) |
| Vocational education | 18 (12.1%) | 39 (17.5%) | 57 (15.3%) |
| Bachelor’s degree | 5 (3.4%) | 15 (6.7%) | 20 (5.4%) |
| More than Bachelor’s degree | 0 (0.0%) | 1 (0.4%) | 1 (0.3%) |
| Main occupations | |||
| No occupation | 9 (6.0%) | 24 (10.8%) | 33 (8.9%) |
| Agriculture | 79 (53.0%) | 109 (48.9%) | 188 (50.5%) |
| Temporary employee | 35 (23.5%) | 27 (12.1%) | 62 (16.7%) |
| Office worker | 5 (3.4%) | 9 (4.0%) | 14 (3.8%) |
| Vendor | 11 (7.4%) | 30 (13.5%) | 41 (11.0%) |
| Government officer | 1 (0.7%) | 9 (4.0%) | 10 (2.7%) |
| Housemaid | 0 (0.0%) | 8 (3.6%) | 8 (2.2%) |
| Student | 0 (0.0%) | 2 (0.9%) | 2 (0.5%) |
| Other occupation | 9 (6.0%) | 5 (2.2%) | 14 (3.8%) |
| Number of family members | |||
| ≤2 persons | 25 (16.8%) | 51 (22.9%) | 76 (20.4%) |
| 3–6 persons | 115 (77.2%) | 159 (71.3%) | 274 (73.7%) |
| >6 persons | 9 (6.0%) | 13 (5.8%) | 22 (5.9%) |
| Monthly household income | |||
| ≤THB 15,000 or ≤USD 450 | 111 (74.5%) | 172 (77.1%) | 283 (76.1%) |
| THB 15,001–40,000 or USD 450.10–1333.30 | 35 (23.5%) | 46 (20.6%) | 81 (21.8%) |
| THB 40,001–70,000 or USD 1333.40–2333.30 | 3 (2.0%) | 5 (2.2%) | 8 (2.2%) |
| Owned a car or a truck | |||
| Yes | 81 (54.4%) | 135 (60.5%) | 216 (58.1%) |
| No | 68 (45.6%) | 88 (39.5%) | 156 (41.9%) |
| Contact Activities | Rodent Contact Activity Rates n (%) |
|---|---|
| Direct or indirect physical contact activities with either one of the rodent types | 282 (75.8%) |
| Direct physical contact with either one of the rodent types | 90 (24.2%) |
| Hunted | 35 (9.4%) |
| Killed | 41 (11.0%) |
| Prepared rodents as food | 33 (8.9%) |
| Consumed cooked meat | 12 (3.2%) |
| Fed food to rodents | 4 (1.1%) |
| Cleaned feces | 17 (4.6%) |
| Cleaned carcasses | 33 (8.9%) |
| Indirect physical contact with either one of the rodent types | 282 (75.8%) |
| Had seen rodents without physical contact at any locations in their lifetime | 114 (30.6%) |
| Had seen rodents without physical contact in households and communities in their lifetime | 214 (57.5%) |
| Rodent Types (Scientific Names) | Hunted | Killed | Prepared as Food | Consumed Cooked Meat |
|---|---|---|---|---|
| (n, %) | (n, %) | (n, %) | (n, %) | |
| At least one species of rodents | 35 (9.4%) | 41 (11.0%) | 33 (8.9%) | 12 (3.2%) |
| Field rat (Rattus argentiventer) | 32 (8.6%) | 37 (9.9%) | 32 (8.6%) | 0 (0.0%) |
| Bandicoot (Peramelemorphia) | 3 (0.8%) | 4 (1.1%) | 4 (1.1%) | 4 (1.1%) |
| Brown rat (Rattus norvegicus) | 1 (0.3%) | 3 (0.8%) | 0 (0.0%) | 6 (1.6%) |
| Ryukyu mouse (Mus caroli) | 1 (0.3%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
| Squirrels (Sciuridae) | 2 (0.5%) | 0 (0.0%) | 0 (0.0%) | 2 (0.5%) |
| Tree shrew (Scandentia) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Serum ID | IgM Titers | IgG Titers |
|---|---|---|
| 56 | 1:100 | <1:50 |
| 171 | 1:100 | <1:50 |
| 174 | 1:200 | <1:50 |
| 196 | 1:100 | 1:100 |
| 197 | 1:100 | 1:100 |
| 198 | 1:100 | 1:50 |
| 199 | 1:100 | 1:200 |
| 200 | 1:100 | 1:50 |
| 203 | 1:100 | 1:100 |
| 273 | 1:100 | 1:200 |
| Variables | Total (n = 372) | Leptospirosis Results | Crude OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Positive (n = 10) | Negative (n = 362) | ||||
| Sociodemographic (SES) information (9 variables) | |||||
| Gender | |||||
| Male | 149 (40.1%) | 5 (50.0%) | 144 (39.8%) | 1.51 (0.43–5.32) | 0.53 |
| Female | 223 (59.9%) | 5 (50.0%) | 218 (60.2%) | 1(ref) | |
| Age groups | |||||
| 20–45 years | 113 (30.4%) | 3 (30.0%) | 110 (30.4%) | 0.98 (0.25–3.87) | 1.00 |
| >45 years | 259 (69.6%) | 7 (70.0%) | 252 (69.6%) | 1(ref) | |
| Marital status | |||||
| Married | 257 (69.1%) | 8 (80.0%) | 249 (68.8%) | 1.86 (0.38–8.69) | 0.73 |
| Other marital status | 115 (30.9%) | 2 (20.0%) | 113 (31.2%) | 1(ref) | |
| Educational attainment | |||||
| Basic educational attainment level | 232 (62.4%) | 5 (50.0%) | 227 (62.7%) | 0.60 (0.17–2.09) | 0.51 |
| Other educational attainment levels | 140 (37.6%) | 5 (50.0%) | 135 (37.3%) | 1(ref) | |
| Main occupations | |||||
| Agriculture-related occupation | 188 (50.5%) | 7 (70.0%) | 181 (50.0%) | 2.33 (0.59–9.17) | 0.34 |
| Non-agriculture-related occupation | 184 (49.5%) | 3 (30.0%) | 181 (50.0%) | 1(ref) | |
| Secondary occupations | |||||
| Agriculture-related occupation | 39 (10.5%) | 2 (20.0%) | 37 (10.2%) | 2.20 (0.45–10.73) | 0.28 |
| Non-agriculture-related occupation | 333 (89.5%) | 8 (80.0%) | 325 (89.8%) | 1(ref) | |
| Numbers of family members | |||||
| ≤2 persons | 76 (20.4%) | 1 (10.0%) | 75 (20.7%) | 0.43 (0.05–3.41) | 0.69 |
| >2 persons | 296 (79.6%) | 9 (90.0%) | 287 (73.2%) | 1(ref) | |
| Monthly household income | |||||
| ≤THB 15,000 or ≤USD 450 | 283 (76.1%) | 9 (90.0%) | 274 (75.7%) | 2.89 (0.36–23.14) | 0.46 |
| >THB 15,001 or >USD 450 | 89 (23.9%) | 1 (10.0%) | 88 (24.3%) | 1(ref) | |
| Owned a car or a truck | |||||
| Yes | 216 (58.1%) | 6 (60.0%) | 210 (58.0%) | 1.09 (0.30–3.91) | 1.00 |
| No | 156 (41.9%) | 4 (40.0%) | 152 (42.0%) | 1(ref) | |
| Knowledge, Attitude, and Practice (KAP) (7 variables) | |||||
| Knowledge score on animals and zoonotic diseases | |||||
| ≥80% | 228 (61.3%) | 6 (60.0%) | 222 (61.3%) | 0.95 (0.26–3.41) | 1.00 |
| <80% | 144 (38.7%) | 4 (40.0%) | 140 (38.7%) | 1(ref) | |
| Attitude score toward rodents | |||||
| ≥80% | 66 (17.7%) | 0 (0.0%) | 66 (18.2%) | 1.03 (1.01–1.06) | 0.22 |
| <80% | 306 (82.3%) | 10 (100.0%) | 296 (81.8%) | 1(ref) | |
| Practice score toward rodents | |||||
| ≥80% | 24 (6.5%) | 0 (0.0%) | 24 (6.6%) | 1.03 (1.01–1.05) | 1.00 |
| <80% | 348 (93.5%) | 10 (100.0%) | 338 (93.4%) | 1(ref) | |
| Variables | Total | Leptospirosis Results | Crude OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| (n = 372) | Positive (n = 10) | Negative (n = 362) | |||
| Contacted with the animals in the past 12 months | |||||
| Cats | |||||
| Yes | 211 (56.7%) | 9 (90.0%) | 202 (55.8%) | 7.13 (0.89–56.86) | 0.05 *,** |
| No | 161 (43.3%) | 1 (10.0%) | 160 (44.2%) | 1(ref) | |
| Hunted animals in the past 12 months | |||||
| At least one type of rodents | |||||
| Yes | 35 (9.4%) | 3 (30.0%) | 32 (8.8%) | 4.42 (1.09–17.93) | 0.06 *,** |
| No | 337 (90.6%) | 7 (70.0%) | 330 (91.2%) | 1(ref) | |
| Field rat | |||||
| Yes | 32 (8.6%) | 3 (30.0%) | 29 (8.0%) | 4.92 (1.21–20.05) | 0.05 *,** |
| No | 340 (91.4%) | 7 (70.0%) | 333 (92.0%) | 1(ref) | |
| Killed animals in the past 12 months | |||||
| At least one type of rodents | |||||
| Yes | 41 (11.0%) | 3 (30.0%) | 38 (10.5%) | 3.65 (0.91–14.72) | 0.09 *,** |
| No | 331 (89.0%) | 7 (70.0%) | 324 (89.5%) | 1(ref) | |
| Fed food to rodents | |||||
| Bandicoot | |||||
| Yes | 1 (18.8%) | 1 (10.0%) | 0 (0.0%) | 41.22 (21.62–78.60) | 0.03 *,** |
| No | 371 (81.2%) | 9 (90.0%) | 362 (100.0%) | 1(ref) | |
| Variables | Total (n = 372) | Rodent Exposure Level | Crude OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Direct (n = 90) | Indirect (n = 282) | ||||
| Sociodemographic (SES) information (9 variables) | |||||
| Gender | |||||
| Male | 149 (40.1%) | 55 (61.1%) | 94 (33.3%) | 3.14 (1.92–5.13) | <0.01 *,** |
| Female | 223 (59.9%) | 35 (38.9%) | 188 (66.7%) | 1(ref) | |
| Age groups | |||||
| 20–45 years | 113 (30.4%) | 31 (34.4%) | 82 (29.1%) | 1.28 (0.77–2.12) | 0.36 |
| >45 years | 259 (69.6%) | 59 (65.6%) | 200 (70.9%) | 1(ref) | |
| Marital status | |||||
| Married | 257 (69.1%) | 63 (70.0%) | 194 (68.8%) | 1.06 (0.63–1.77) | 0.90 |
| Other marital status | 115 (30.9%) | 27 (30.0%) | 88 (31.2%) | 1(ref) | |
| Educational attainment levels | |||||
| Basic educational attainment level | 232 (62.4%) | 55 (61.1%) | 177 (62.8%) | 0.93 (0.57–1.16) | 0.80 |
| Other educational attainment levels | 140 (37.6%) | 35 (38.9%) | 105 (37.2%) | 1(ref) | |
| Main occupations | |||||
| Agriculture-related occupation | 188 (50.5%) | 51 (56.7%) | 137 (48.6%) | 1.38 (0.86–2.23) | 0.19 |
| Non-agriculture-related occupation | 184 (49.5%) | 39 (43.3%) | 145 (51.4%) | 1(ref) | |
| Secondary occupations | |||||
| Agriculture-related occupation | 39 (10.5%) | 10 (11.1%) | 29 (10.3%) | 1.09 (0.51–2.34) | 0.84 |
| Non-agriculture-related occupation | 333 (89.5%) | 80 (88.9%) | 253 (89.7%) | 1(ref) | |
| Numbers of family members | |||||
| ≤2 persons | 76 (20.4%) | 20 (22.2%) | 56 (19.9%) | 1.15 (0.65–2.05) | 0.65 |
| >2 persons | 296 (79.6%) | 70 (77.8%) | 226 (80.1%) | 1(ref) | |
| Monthly household income | |||||
| ≤THB 15,000 or ≤USD 450 | 283 (76.1%) | 61 (67.8%) | 222 (78.7%) | 0.57 (0.34–0.96) | 0.05 *,** |
| >THB 15,001 or >USD 450 | 89 (23.9%) | 29 (32.2%) | 60 (21.3%) | 1(ref) | |
| Sociodemographic (SES) information (9 variables) | |||||
| Owned a car or a truck | |||||
| Yes | 216 (58.1%) | 53 (58.9%) | 163 (57.8%) | 1.05 (0.65–1.69) | 0.90 |
| No | 156 (41.9%) | 37 (41.1%) | 119 (42.2%) | 1(ref) | |
| Knowledge, Attitude, and Practice (KAP) (7 variables) | |||||
| Knowledge score on animals and zoonotic diseases | |||||
| ≥80% | 228 (61.3%) | 57 (63.3%) | 171 (60.6%) | 1.12 (0.69–1.83) | 0.71 |
| <80% | 144 (38.7%) | 33 (36.7%) | 111 (39.4%) | 1(ref) | |
| Attitude score toward rodents | |||||
| ≥80% | 66 (17.7%) | 17 (18.9%) | 49 (17.4%) | 1.11 (0.60–2.04) | 0.75 |
| <80% | 306 (82.3%) | 73 (81.1%) | 233 (82.6%) | 1(ref) | |
| Practice score toward rodents | |||||
| ≥80% | 24 (6.5%) | 4 (4.4%) | 20 (7.1%) | 0.61 (0.20–1.83) | 0.47 |
| <80% | 348 (93.5%) | 86 (95.6%) | 262 (92.9%) | 1(ref) | |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Gender | ||||
| Male | 3.143 (1.924–5.134) | <0.001 ** | 3.137 (1.914–5.139) | <0.001 ** |
| Female | 1(ref) | |||
| Monthly household income | ||||
| ≤THB 15,000 or ≤USD 450 | 0.568 (0.336–0.962) | 0.046 ** | 0.57 (0.332–0.985) | 0.044 ** |
| >THB 15,001 or >USD 450.10 | 1(ref) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannarong, K.; Soonthornworasiri, N.; Maneekan, P.; Yimsamran, S.; Balthip, K.; Maneewatchararangsri, S.; Saisongkorh, W.; Saengkul, C.; Sangmukdanun, S.; Phunta, N.; et al. Rodent–Human Interface: Behavioral Risk Factors and Leptospirosis in a Province in the Central Region of Thailand. Vet. Sci. 2022, 9, 85. https://doi.org/10.3390/vetsci9020085
Suwannarong K, Soonthornworasiri N, Maneekan P, Yimsamran S, Balthip K, Maneewatchararangsri S, Saisongkorh W, Saengkul C, Sangmukdanun S, Phunta N, et al. Rodent–Human Interface: Behavioral Risk Factors and Leptospirosis in a Province in the Central Region of Thailand. Veterinary Sciences. 2022; 9(2):85. https://doi.org/10.3390/vetsci9020085
Chicago/Turabian StyleSuwannarong, Kanokwan, Ngamphol Soonthornworasiri, Pannamas Maneekan, Surapon Yimsamran, Karnsunaphat Balthip, Santi Maneewatchararangsri, Watcharee Saisongkorh, Chutarat Saengkul, Suntaree Sangmukdanun, Nittaya Phunta, and et al. 2022. "Rodent–Human Interface: Behavioral Risk Factors and Leptospirosis in a Province in the Central Region of Thailand" Veterinary Sciences 9, no. 2: 85. https://doi.org/10.3390/vetsci9020085
APA StyleSuwannarong, K., Soonthornworasiri, N., Maneekan, P., Yimsamran, S., Balthip, K., Maneewatchararangsri, S., Saisongkorh, W., Saengkul, C., Sangmukdanun, S., Phunta, N., & Singhasivanon, P. (2022). Rodent–Human Interface: Behavioral Risk Factors and Leptospirosis in a Province in the Central Region of Thailand. Veterinary Sciences, 9(2), 85. https://doi.org/10.3390/vetsci9020085






