Vet-OncoNet: Developing a Network of Veterinary Oncology and Reporting a Pioneering Portuguese Experience
Abstract
:1. Introduction
2. The Vet-OncoNet project
2.1. Data Processing
2.2. Data Delivery
3. Databases’ Preliminary Results
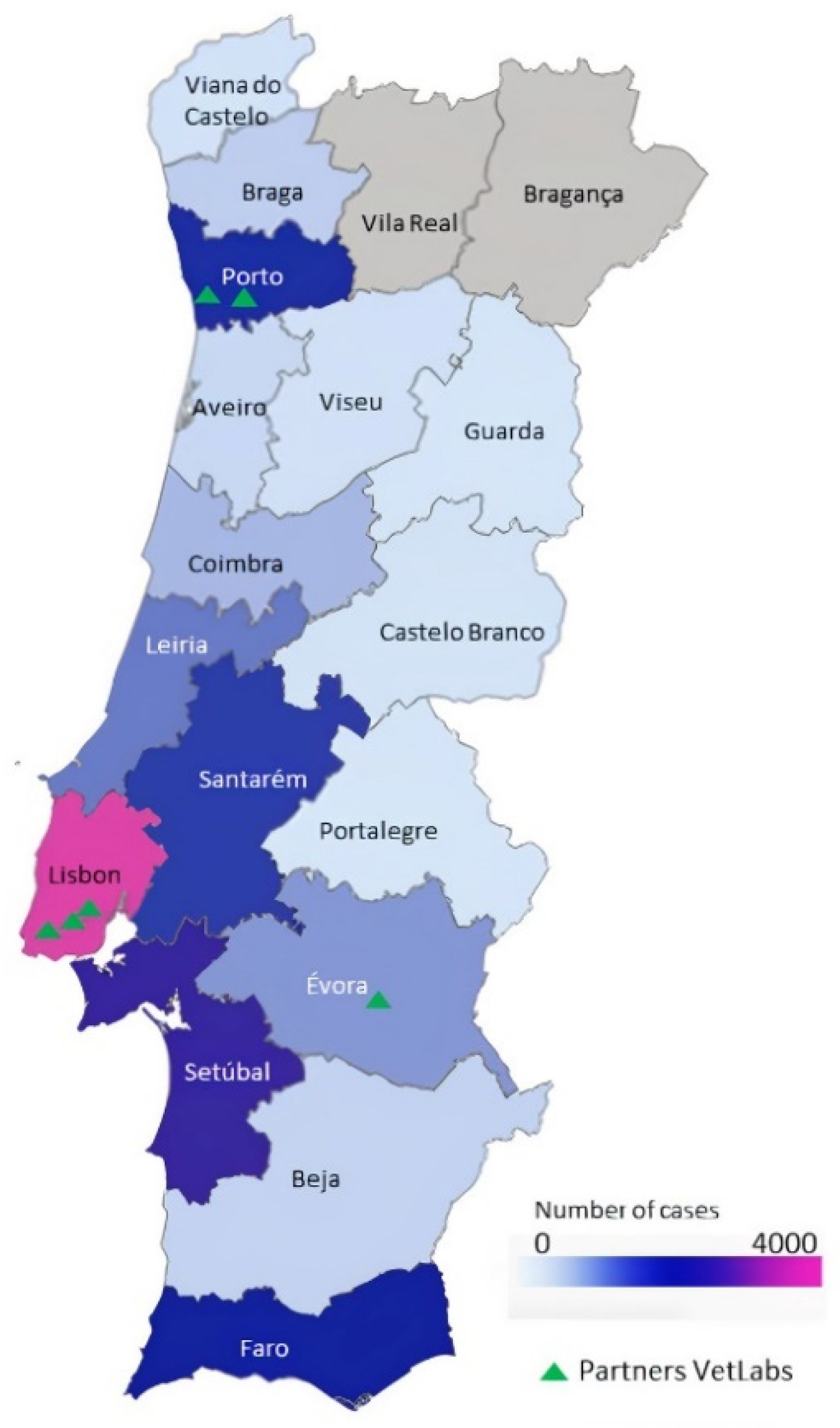
3.1. Animal Cancer Registry
3.2. Clinical Oncology Registry
3.3. Risk Factors Registry
4. Vet-OncoNet and Animal Census
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeBlanc, A.K.; Mazcko, C.N. Improving human cancer therapy through the evaluation of pet dogs. Nat. Rev. Cancer 2020, 20, 727–742. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 1–37. [Google Scholar] [CrossRef] [PubMed]
- MacVean, D.W.; Monlux, A.W.; Anderson, P.S., Jr.; Silberg, S.L.; Roszel, J.F. Frequency of canine and feline tumors in a defined population. Vet. Pathol. 1978, 15, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, A.A.; Modiano, J.F.; Canter, R.J. Characterization and Potential Applications of Dog Natural Killer Cells in Cancer Immunotherapy. J. Clin. Med. 2019, 8, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, A.K.; Breen, M.; Choyke, P.; Dewhirst, M.; Fan, T.M.; Gustafson, D.L.; Helman, L.J.; Kastan, M.B.; Knapp, D.W.; Levin, W.J.; et al. Perspectives from man’s best friend: National Academy of Medicine’s Workshop on Comparative Oncology. Sci. Transl. Med. 2016, 8, 324–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastorinho, M.R.; Sousa, A.C. Pets as Sentinels, Forecasters and Promoters of Human Health, 1st ed.; Springer: Cham, Switzerland, 2020; p. 375. [Google Scholar]
- Rabinowitz, P.; Scotch, M.; Conti, L. Human and animal sentinels for shared health risks. Vet. Ital. 2009, 45, 23–24. [Google Scholar] [PubMed]
- Reif, J.S. Animal sentinels for environmental and public health. Public Health Rep. 2011, 126 (Suppl. 1), 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, P.L. Companion animals as sentinels for public health. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Bonnett, B.N.; Page, R. Epidemiology and the Evidence-Based Medicine Approach. In Withrow and MacEwen’s Small Animal Clinical Oncology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 81–97. [Google Scholar]
- Dorn, C.R.; Taylor, D.O.; Frye, F.L.; Hibbard, H.H. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J. Natl. Cancer Inst. 1968, 40, 295–305. [Google Scholar] [PubMed]
- Dorn, C.R.; Taylor, D.O.; Schneider, R.; Hibbard, H.H.; Klauber, M.R. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J. Natl. Cancer Inst. 1968, 40, 307–318. [Google Scholar] [PubMed]
- Nodtvedt, A.; Berke, O.; Bonnett, B.N.; Bronden, L. Current status of canine cancer registration—Report from an international workshop. Vet. Comp. Oncol. 2012, 10, 95–101. [Google Scholar] [CrossRef] [PubMed]
- BSAVA. Small Animal Veterinary Surveillance Network (SAVSNET). Available online: https://www.liverpool.ac.uk/savsnet/ (accessed on 18 October 2021).
- Royal Veterinary College. The Veterinary Companion Animal Surveillance System (VetCompass). Available online: https://www.vetcompass.org/ (accessed on 18 October 2021).
- National Cancer Institute; College of Veterinary Medicine-University of Missouri. The Veterinary Medical DataBase. Available online: https://vmdb.org/ (accessed on 18 October 2021).
- ICBAS—Instituto de Ciências Biomédicas Abel Salazar. Vet-OncoNet, Veterinary Oncology Network. Available online: www.vetonconet.pt (accessed on 26 January 2022).
- Arnesen, K.; Gamlem, H.; Glattre, E.; Moe, L.; Nordstoga, K. Registration of canine cancer. Tidsskr. Nor. Laegeforening 1995, 115, 714–717. [Google Scholar]
- Baioni, E.; Scanziani, E.; Vincenti, M.C.; Leschiera, M.; Bozzetta, E.; Pezzolato, M.; Desiato, R.; Bertolini, S.; Maurella, C.; Ru, G. Estimating canine cancer incidence: Findings from a population-based tumour registry in northwestern Italy. BMC Vet. Res. 2017, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Bronden, L.B.; Nielsen, S.S.; Toft, N.; Kristensen, A.T. Data from the Danish veterinary cancer registry on the occurrence and distribution of neoplasms in dogs in Denmark. Vet. Rec. 2010, 166, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Gruntzig, K.; Graf, R.; Hassig, M.; Welle, M.; Meier, D.; Lott, G.; Erni, D.; Schenker, N.S.; Guscetti, F.; Boo, G.; et al. The Swiss Canine Cancer Registry: A retrospective study on the occurrence of tumours in dogs in Switzerland from 1955 to 2008. J. Comp. Pathol. 2015, 152, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuali, E.; Morgante, R.A.; Maresca, C.; Leonardi, L.; Purificato, I.; Giaimo, M.D.; Giovannini, G. A web-based tumor registration system for a regional Canine Cancer Registry in Umbria, central Italy. Ann. Ist. Super. Sanita 2019, 55, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Tedardi, M.V.; Veneziano, D.B.; Kimura, K.C.; Pedra-Mendonca, P.; Biondi, L.R.; Grandi, F.; Latorre Mdo, R.; Dagli, M.L. Sao Paulo Animal Cancer Registry, the first in Latin America. Vet. Comp. Oncol. 2015, 13, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Pinello, K.C.; Queiroga, F.; de Matos, A.; Santos, A.; Ribeiro, J.N.; Guscetti, F.; Palmieri, C.; Soberano, M.; Momanyi, K.; Torres, J.R.; et al. The Global Initiative for Veterinary Cancer Surveillance (GIVCS): Report of the first meeting and future perspectives. Vet. Comp. Oncol. 2020, 18, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Vet-OncoNet. Portuguese Animal Cancer Registry, 2020; Intituto de Ciências Biomédicas de Abel Salazar, Universidade do Porto: Porto, Portugal, 2021; p. 10. [Google Scholar]
- Cicchelero, L.; Belgian Cancer Fund for Animals. Oncowaf. Available online: https://oncowaf.be/en/Home (accessed on 18 November 2021).
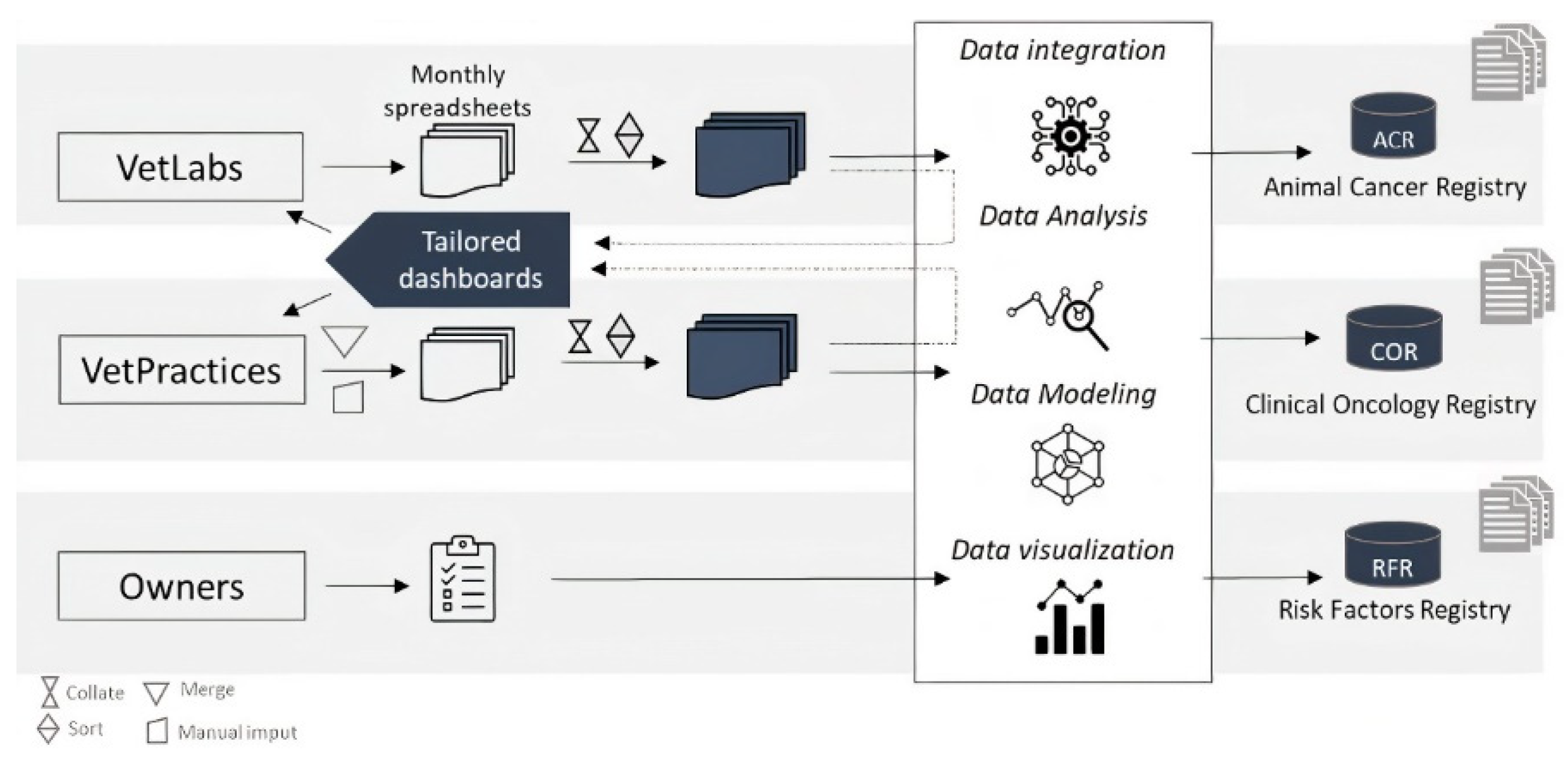
| Name of Data System | Data Source | Variables |
|---|---|---|
| Animal Cancer Registry (ACR) | Pathology reports from Veterinary Pathological Laboratories | Laboratory |
| -Vet-OncoNet code | ||
| Practice | ||
| -Postal Code | ||
| -City | ||
| Tumor | ||
| -Report ID | ||
| -Date of diagnosis | ||
| -Species | ||
| -Sex | ||
| -Breed | ||
| -Age | ||
| -Topography | ||
| -Diagnosis | ||
| -Grade | ||
| -Method of diagnosis (histopathology, cytology, necropsy) | ||
| Clinical Oncology Registry (COR) | Data from Veterinary Practices, after oncology routine | Practice |
| -Vet-OncoNet code | ||
| Owner | ||
| -Postal Code | ||
| -City | ||
| Animal | ||
| -Species | ||
| -Breed | ||
| -Age | ||
| -Sex | ||
| Tumor | ||
| -Topography | ||
| -Diagnosis | ||
| -Grade | ||
| -Method of diagnosis | ||
| -Treatment | ||
| -Outcome | ||
| Risk Factors Registry (RFR) | Owners of oncologic patients | Questionnaire prepared to collect data from several risk factors from the animal, feeding habits, its environment, owners and family behavior. |
| Number of VetPractices | 27 |
|---|---|
| Number of laboratories | 6 † |
| Number of tumor registries | 10,137 |
| Number of animal groups | 10 ‡ |
| Proportion of dogs | 80.2% |
| Proportion of cats | 18.7% |
| Ratio cats: dogs | 1:4.3 |
| Ratio male: female | 1:1.5 |
| Dogs (n = 6877) | Cats (n = 1624) | ||
|---|---|---|---|
| Top 5 topographies | % | % | |
| 1. Skin 2. Mammary gland 3. Subcutaneous and soft tissue 4. Testis 5. Gum | 50.9 21.9 7.3 4.2 3.3 | 1. Skin 2. Mammary gland 3. Digestive organs 4. Nasal Cavity and middle ear 5. Subcutaneous and soft tissue | 38.7 35.5 6.1 3.1 3.0 |
| Top 5 morphologies | % | % | |
| 1. Mast cell tumors 2. Lipoma 3. Complex adenoma 1 4. Histiocytoma 5. Benign Mixed Tumor 1 | 9.4 5.5 4.7 3.7 3.7 | 1. Squamous cell carcinoma 2. Tubular adenocarcinoma 1 3. Lymphomas 4. Tubule-papillary adenocarcinoma 1 5. Solid carcinoma 1 | 11.5 11.4 6.9 6.5 6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinello, K.; Pires, I.; Castro, A.F.; Carvalho, P.T.; Santos, A.; de Matos, A.; Queiroga, F.; Niza-Ribeiro, J. Vet-OncoNet: Developing a Network of Veterinary Oncology and Reporting a Pioneering Portuguese Experience. Vet. Sci. 2022, 9, 72. https://doi.org/10.3390/vetsci9020072
Pinello K, Pires I, Castro AF, Carvalho PT, Santos A, de Matos A, Queiroga F, Niza-Ribeiro J. Vet-OncoNet: Developing a Network of Veterinary Oncology and Reporting a Pioneering Portuguese Experience. Veterinary Sciences. 2022; 9(2):72. https://doi.org/10.3390/vetsci9020072
Chicago/Turabian StylePinello, Katia, Isabel Pires, Ana Filipa Castro, Paulo Tiago Carvalho, Andreia Santos, Augusto de Matos, Felisbina Queiroga, and João Niza-Ribeiro. 2022. "Vet-OncoNet: Developing a Network of Veterinary Oncology and Reporting a Pioneering Portuguese Experience" Veterinary Sciences 9, no. 2: 72. https://doi.org/10.3390/vetsci9020072
APA StylePinello, K., Pires, I., Castro, A. F., Carvalho, P. T., Santos, A., de Matos, A., Queiroga, F., & Niza-Ribeiro, J. (2022). Vet-OncoNet: Developing a Network of Veterinary Oncology and Reporting a Pioneering Portuguese Experience. Veterinary Sciences, 9(2), 72. https://doi.org/10.3390/vetsci9020072








