Methodology and Neuromarkers for Cetaceans’ Brains
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Workflow for Cetaceans’ Brain Examination: From Fresh to Fixed Samples
2.2. Opening of the Skull
- one dorsal parallel to the nuchal ridge (crista occipitalis externa) at 1 cm from it (slightly more caudally in some species such as beaked whales or BW and pilot whales),
- another ventral and parallel to (a), bypassing the occipital condyles; and
- two lateral and perpendicular to (a) and (b), passing through the parietal and the squamosal bones, in the temporal fossa.
2.3. Cautious Sampling of Fresh Brain at Necropsy: A Key Step
2.4. Fixation of Cetaceans’ Brains: A Challenge
2.5. Sectioning of the Whole Brain and Postfixation
2.6. Routinary Neuropathologic Investigations (FFPE)
- -
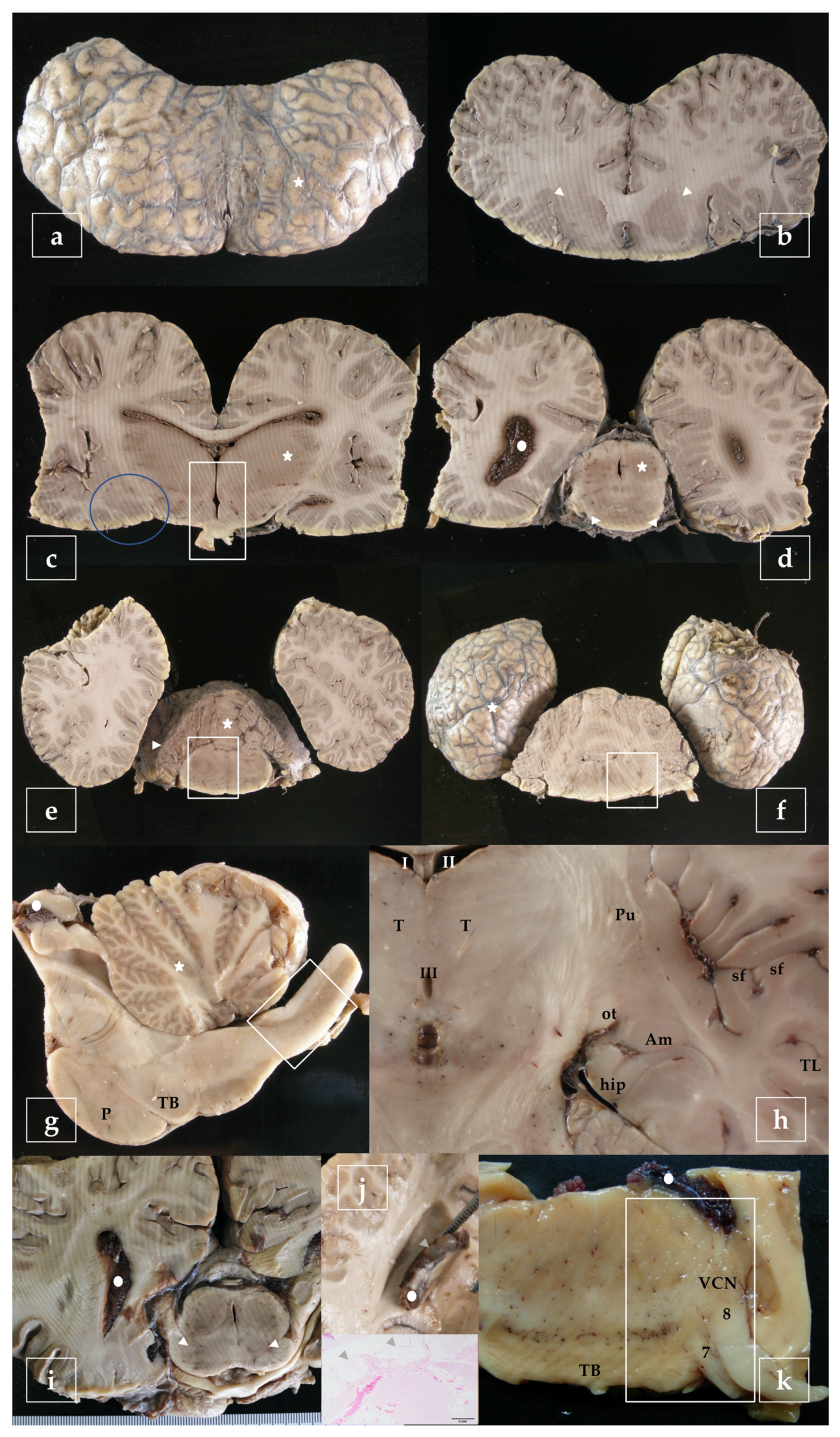
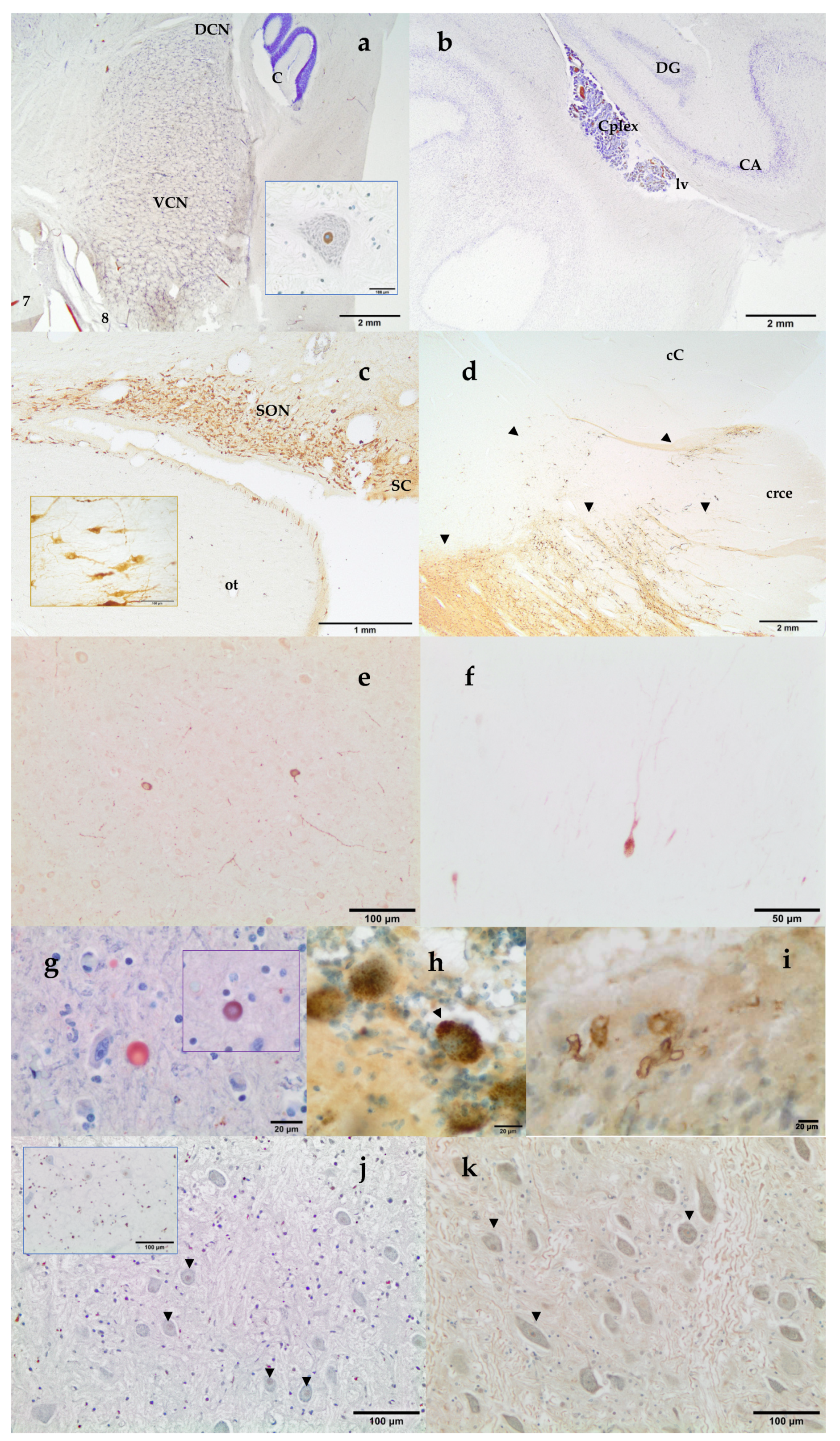
- telencephalon: cortex (2 to 4 samples, at least frontopolar and occipital cortex) (a and f, stars), corpus striatum (b, arrowheads), amygdala (c, blue circle; h, Am), and hippocampus (h, hip);
- -
- diencephalon: thalamus and hypothalamus (c, star and rectangle);
- -
- mesencephalon: tectum (colliculus rostralis and caudalis) (d, star) and tegmentum (with the substantia nigra) (d and i, arrowheads);
- -
- rhombencephalon: at least pons (with the locus ceruleus) (e, rectangle), trapezoid body (with the cochlear nuclei) (f and k, rectangle), medulla oblongata (g, rectangle) and at least two samples of the cerebellum (included a sagittal section of the vermis) (e and g, arrowhead and star);
- -
- choroid plexus (d, g, i, j, and k, circle);
- -
- spinal cord (Figure 2, left): at least pars cervicalis and pars thoracica.
2.7. Immunoperoxidase Staining: Paraffin Embedded Tissues (p-IHC)
2.8. Cryoprotection and Preparation of the Sample (FFCS)
2.9. Immunoperoxidase Staining: Free-Floating Immunohistochemistry (ff-IHC)
2.10. Nissl Staining
2.11. TUNEL Staining
3. Results
3.1. Evaluation of Brain Quality
- -
- Provide longitudinal cuts to expose the lateral ventricles and allow the entry of the fixative;
- -
- Make cross-sections of the brain after at least 72 h of immersion in the fixative. Once a great percentage of the fixation process was achieved, serial cuts of the brain allowed a greater fixation;
- -
- Finally, the post-fixation of the selected samples permitted to complete the fixation of the tissues and provide the necessary firmness for their next processing;
- -
- Reposition of new fixative was made after cross sectioning the brain (immersion in a smaller container during 48 h) and then after sampling (postfixed during 24 h).
- -
- Tissue rupture and wearing, dark, shrunken and pycnotic neurons of that brains incorrectly handled during necropsy;
- -
- Poor fixation of the deepest subcortical structures of those brains, which did not receive cross-sections. This resulted in poor tissue quality, predisposing to easy rupture of the sections, especially during the continuous manipulation in ff-IHC, poor immunoreactivity to the neuromarkers, and a very low affinity to thionine;
- -
- The prolonged permanence in the fixative resulted in the loss of tissue quality, which predisposed it to an easy rupture of the sections, loss of antigenicity, and a very low affinity to thionine. In addition, formalin pigment accumulation was observed, as a background deposition and occasionally within the neurons mimicking the neuromelanin pigment;
3.2. Neuromarkers for Neuroanatomical Studies
3.3. Neuromarkers for Neuropathological Studies
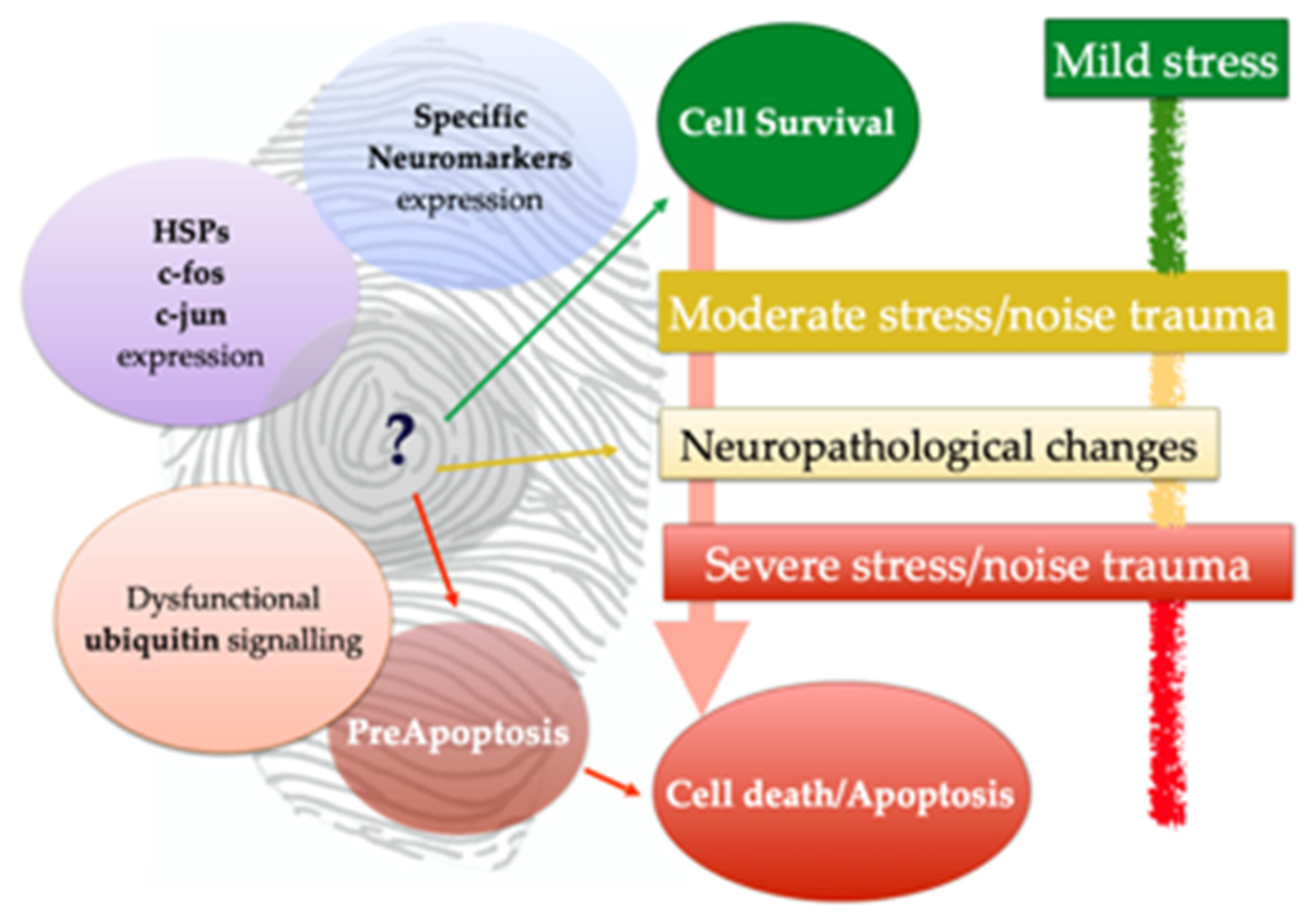
3.4. Neuromarkers for Acoustic Trauma Research
4. Discussion
- -
- Several infectious pathogens including virus, bacteria, fungi, and parasites might cross the blood-cerebrospinal fluid barrier, entering the central nervous system and leading to inflammatory infectious diseases like meningitis and meningoencephalitis [42], very common in these animals;
- -
- Opening the ventricular system is a crucial step which allows its checking for exudates, space-occupying lesions, asymmetries, abnormal cerebrospinal fluid, and/or any alterations affecting the choroid plexus (i.e., cystic lesions as in Figure 3j or swelling). It is highly important to sample the choroid plexus, as a fundamental site of invasion of bacteria, virus (distemper), and protozoa. In fact, lesions may only be confined to the periventricular areas [43];
- -
- Thus, the opening of the ventricular system ensures a rapid uniform penetration of the fixative and the best possible preservation of the tissues. It is important to respect the proportions of fixative because of the large size and rounded shape of cetaceans’ brains. As fixative molecules bind to the tissue, they are depleted. Inadequate fixative volume will result in inadequate tissue fixation [44];
- -
- No macroscopic changes may be detected during necropsy and sampling but severe histopathological hallmarks may be present;
- -
- Random slicing and sampling of the brain may result in confused neuropathological interpretations. In addition, a strong knowledge of neuroanatomical structures is a critical advantage in order to boost the interpretation of neuropathological changes and their etiopathogenesis. Hence, another important aspect is respecting the international anatomical terminology (Nomina Anatomica) of the International Committee on Veterinary Gross Anatomical Nomenclature [45], which evolves over time;
- -
- A strong sampling protocol should not forbear to preserve the bilaterality of the brain, which permits to draw the specific pattern of distributions of the lesions, the first important step in neuropathological diagnosis [41];
- -
- Even if we usually lack clinical data on stranded animals, when brain lesions are the cause of the stranding, they are usually severe enough, large, and/or diffused.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cozzi, B.; Huggenberger, S.; Oelschläger, H. Chapter 3. Locomotion (Including Osteology and Myology). In Anatomy of Dolphins; Academic Press: San Diego, CA, USA, 2017; pp. 33–89. [Google Scholar]
- Ridgway, S.H.; Carlin, K.P.; Van Alstyne, K.R.; Hanson, A.C.; Tarpley, R.J. Comparison of Dolphins’ Body and Brain Measurements with Four Other Groups of Cetaceans Reveals Great Diversity. Brain, Behav. Evol. 2016, 88, 235–257. [Google Scholar] [CrossRef]
- Zilles, K.; Palomero-Gallagher, N.; Amunts, K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013, 36, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, S. The size and complexity of dolphin brains—a paradox? J. Mar. Biol. Assoc. 2008, 88, 1103–1108. [Google Scholar] [CrossRef]
- Oelschläger, H.H.A.; Oelschläger, J.S. Brain. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 134–149. [Google Scholar] [CrossRef]
- Würsig, B.; Perrin, W.F.; Thewissen, J.G.M. History of Marine Mammal Research. In Encyclopedia of Marine Mammals, 2nd ed.; Academic Press: London, UK, 2009; pp. 565–569. [Google Scholar]
- Jacobs, M.S.; Jensen, A.V. Gross aspects of the brain and a fiber analysis of cranial nerves in the great whale. J. Comp. Neurol. 1964, 123, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, B.; Mazzariol, S.; Podestà, M.; Zotti, A.; Huggenberger, S. An Unparalleled Sexual Dimorphism of Sperm Whale Encephalization. Int. J. Comp. Psychol. 2016, 29, 29. [Google Scholar] [CrossRef]
- Fix, A.S.; Garman, R.H. Practical Aspects of Neuropathology: A Technical Guide for Working with the Nervous System. Toxicol. Pathol. 2000, 28, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cammermeyer, J. Nonspecific Changes of the Central Nervous System in Normal and Experimental Material. In The Structure and Function of Nervous Tissue V6–Structure IV and Physiology IV, 1st ed.; Bourne, G., Ed.; Academic Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Sarasa, M.; Pesini, P. Natural non-trasgenic animal models for research in Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Gunn-Moore, D.; Kaidanovich-Beilin, O.; Gallego Iradi, M.C.; Gunn-Moore, F.; Lovestone, S. Alzheimer’s disease in humans and other animals: A consequence of postreproductive life span and longevity rather than aging. Alzheimers Dement. J. Alzheimers Assoc. 2017, 14, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Di Guardo, G. Alzheimer’s disease, cellular prion protein, and dolphins. Alzheimer’s Dement. 2018, 14, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Sacchini, S.; Arbelo, M.; Bombardi, C.; Fernández, A.; Cozzi, B.; de Quirós, Y.B.; Herráez, P. Locus coeruleus complex of the family Delphinidae. Sci. Rep. 2018, 8, 5486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacchini, S.; Díaz-Delgado, J.; Espinosa de Los Monteros, A.; Paz, Y.; Bernaldo de Quirós, Y.; Sierra, E.; Arbelo, M.; Herráez, P.; Fernández, A. Amyloid-beta peptide and phosphorylated tau in the frontopolar cerebral cortex and in the cerebellum of toothed whales: Aging vs hypoxia. Biol. Open 2020, 9, bio054734. [Google Scholar] [CrossRef]
- Morgane, P.J.; Jacobs, M.S. Comparative Anatomy of the Cetacean Nervous System. In Functional Anatomy of Marine, Mammals; Harrison, R.J., Ed.; Academic Press: New York, NY, USA, 1972; Volume 1, pp. 117–244. [Google Scholar]
- Knudsen, S.K.; Mørk, S.; Øen, E.O. A novel method for in situ fixation of whale brains. J. Neurosci. Methods 2002, 120, 35–44. [Google Scholar] [CrossRef]
- Hof, P.R.; Van Der Gucht, E. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae). Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 1–31. [Google Scholar] [CrossRef]
- Butti, C.; Sherwood, C.C.; Hakeem, A.Y.; Allman, J.M.; Hof, P.R. Total number and volume of Von Economo neurons in the cerebral cortex of cetaceans. J. Comp. Neurol. 2009, 515, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Sacchini, S. Macroscopic and Microscopic, Histochemical and Immunohistochemical Characterization of the Central Nucleus of the Amygdala, Supraoptic and Paraventricular Nuclei of the Hypothalamus, and the Locus Coeruleus of the Brain of Toothed Whales. PhD Thesis, Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain, 2015. [Google Scholar]
- Ridgway, S.H.; Brownson, R.H.; Van Alstyne, K.R.; Hauser, R.A. Higher neuron densities in the cerebral cortex and larger cerebellums may limit dive times of delphinids compared to deep-diving toothed whales. PLoS ONE 2019, 14, e0226206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecozzi, B.; Eroncon, G.; Egranato, A.; Egiurisato, M.; Ecastagna, M.; Eperuffo, A.; Epanin, M.; Eballarin, C.; Emontelli, S.; Epirone, A. The claustrum of the bottlenose dolphin Tursiops truncatus (Montagu 1821). Front. Syst. Neurosci. 2014, 8, 42. [Google Scholar] [CrossRef]
- Patzke, N.; Spocter, M.A.; Karlsson, K.E.; Bertelsen, M.F.; Haagensen, M.; Chawana, R.; Streicher, S.; Kaswera, C.; Gilissen, E.; Alagaili, A.N.; et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Anat. Embryol. 2015, 220, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Manger, P.R.; Ridgway, S.H.; Siegel, J.M. The locus coeruleus complex of the bottlenose dolphin (Tursiops truncatus) as revealed by tyrosine hydroxylase immunohistochemistry. J. Sleep Res. 2003, 12, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Kern, A.; Seidel, K.; Oelschläger, H. The Central Vestibular Complex in Dolphins and Humans: Functional Implications of Deiters’ Nucleus. Brain Behav. Evol. 2009, 73, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.; García-Hartmann, M. In Proceedings of the first European Cetacean Society Workshop on Cetacean Pathology: Dissection Techniques and Tissue Sampling, Leiden, The Netherlands, 13–14 September 1991.
- Nojima, T. Developmental pattern of the bony falx and bony tentorium of spotted dolphins (stenella attenuata) and the relationship between degree of development and age. Mar. Mammal. Sci. 1988, 4, 312–322. [Google Scholar] [CrossRef]
- Cozzi, B.; Huggenberger, S.; Oelschläger, H. Chapter 6. Brain, spinal cord, and cranial nerves. In The Anatomy of dolphins. Insights into Body Structure and Function; Cozzi, B., Huggenberger, S., Oelschläger, H., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 197–304. [Google Scholar]
- Sierra, E.; Fernández, A.; Felipe-Jiménez, I.; Zucca, D.; Díaz-Delgado, J.; Puig-Lozano, R.; Câmara, N.; Consoli, F.; Díaz-Santana, P.; Suárez-Santana, C.; et al. Histopathological Differential Diagnosis of Meningoencephalitis in Cetaceans: Morbillivirus, Herpesvirus, Toxoplasma gondii, Brucella sp., and Nasitrema sp. Front. Veter Sci. 2020, 7, 650. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A. Technical Aspects of Immunohistochemistry. Veter Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.V.; Furness, J.B.; Klemm, H.M.; Pontell, L.; Chan, E.; Hill, A.; Chiocchetti, R. The brain to gut pathway: A possible route of prion transmission. Gut 2010, 59, 1643–1651. [Google Scholar] [CrossRef]
- Bombardi, C.; Grandis, A.; Chiocchetti, R.; Lucchi, M.L. Distribution of calbindin-D28k, neuronal nitric oxide synthase, and nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) in the lateral nucleus of the sheep amygdaloid complex. Z. Für Anat. Und Entwickl. 2006, 211, 707–720. [Google Scholar] [CrossRef]
- Yang, Y.; Keene, C.; Peskind, E.R.; Galasko, D.R.; Hu, S.-C.; Cudaback, E.; Wilson, A.M.; Li, G.; Yu, C.-E.; Montine, K.S.; et al. Cerebrospinal Fluid Particles in Alzheimer Disease and Parkinson Disease. J. Neuropathol. Exp. Neurol. 2015, 74, 672–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparkman, D.R.; Hammon, K.M.; White, C.L. Production and characterization of a monospecific antiserum (A128) to disaggregated Alzheimer paired helical filaments. J. Histochem. Cytochem. 1990, 38, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, T.; Sacchini, S.; Paz, Y.; Rosales, R.S.; Câmara, N.; Andrada, M.; Arbelo, M.; Fernández, A. Comparison of Methods for the Histological Evaluation of Odontocete Spiral Ganglion Cells. Animals 2020, 10, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, E.; Fernandez, A.; Suárez-Santana, C.M.; Xuriach, A.; Zucca, D.; de Quirós, Y.B.; García-Álvarez, N.; De La Fuente, J.; Sacchini, S.; Andrada, M.; et al. Morbillivirus and Pilot Whale Deaths, Canary Islands, Spain, 2015. Emerg. Infect. Dis. 2016, 22, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Bombardi, C. Distribution of 5-HT2A receptor immunoreactivity in the rat amygdaloid complex and colocalization with γ-aminobutyric acid. Brain Res. 2011, 1370, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Bombardi, C. Neuronal localization of 5-HT2A receptor immunoreactivity in the rat hippocampal region. Brain Res. Bull. 2012, 87, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Rosene, D.; Roy, N.J.; Davis, B.J. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J. Histochem. Cytochem. 1986, 34, 1301–1315. [Google Scholar] [CrossRef] [Green Version]
- Figols Ladrón de Guevara, J. Técnica de la autopsia neuropatológica: Técnica macroscópica de realización de la autopsia y procedimiento de obtención de muestras. Rev. Esp. Patol. 2004, 37, 45–56. [Google Scholar]
- Vandevelde, M.; Higgins, R.; Oevermann, A. Veterinary Neuropathology: Essentials of Theory and Practice; Wiley-Blackwell: New York, NY, USA, 2012. [Google Scholar]
- Eschwerk, C.; Etenenbaum, T.; Ekim, K.S.; Eschroten, H. The choroid plexus—a multi-role player during infectious diseases of the CNS. Front. Cell. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Wessels, M.E.; Deaville, R.; Perkins, M.W.; Jepson, P.D.; Penrose, R.; Rocchi, M.S.; Maley, M.; Ballingall, K.T.; Dagleish, M.P. Novel Presentation of DMV-Associated Encephalitis in a Long-Finned Pilot Whale (Globicephala melas). J. Comp. Pathol. 2021, 183, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Knoblaugh, S.E.; Randolph-Habecker, J. 3–Necropsy and Histology. In Comparative Anatomy and Histology, 2nd ed.; Treuting, P.M., Dintzis, S.M., Montine, K.S., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 23–51. [Google Scholar] [CrossRef]
- ICVGAN. International Committee on Veterinary Gross Anatomical Nomenclature–Nomina Anatomica Veterinaria; World Association of Veterinary Anatomists (WAVA): Hannover, Germany; Ghent, Belgium; Columbia, MO, USA; Rio de Janeiro, Brazil, 2017; p. 160. [Google Scholar]
- Korzhevskii, D.; Sukhorukova, E.; Kirik, O.; Grigorev, I. Immunohistochemical demonstration of specific antigens in the human brain fixed in zinc-ethanol-formaldehyde. Eur. J. Histochem. 2015, 59, 2530. [Google Scholar] [CrossRef] [PubMed]
- Sierra, E.; Sánchez, S.; Saliki, J.; Blas-Machado, U.; Arbelo, M.; Zucca, D.; Fernandez, A. Retrospective Study of Etiologic Agents Associated with Nonsuppurative Meningoencephalitis in Stranded Cetaceans in the Canary Islands. J. Clin. Microbiol. 2014, 52, 2390–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Delgado, J.; Sacchini, S.; Suárez-Bonnet, A.; Sierra, E.; Arbelo, M.; Espinosa, A.; Bassas, E.R.-G.; Mompeo, B.; Pérez, L.; Fernandez, A. High-grade Astrocytoma (Glioblastoma Multiforme) in an Atlantic Spotted Dolphin (Stenella frontalis). J. Comp. Pathol. 2015, 152, 278–282. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef] [PubMed]
- Stylianaki, I.; Komnenou, A.T.; Posantzis, D.; Nikolaou, K.; Papaioannou, N. Alzheimer’s disease-like pathological lesions in an aged bottlenose dolphin (Tursiops truncatus). Veter-Rec. Case Rep. 2019, 7, e000700. [Google Scholar] [CrossRef]
- Di Guardo, G. Do dolphins get Alzheimer’s disease? Veter-Rec. 2019, 185, 762. [Google Scholar] [CrossRef]
- Di Guardo, G. Cetaceans, models for human disease? Res. Veter- Sci. 2018, 119, 43–44. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Goedert, M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001, 2, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Del Tredici, K.; Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013, 9, 13–24. [Google Scholar] [CrossRef]
- Takahashi, H.; Iwanaga, K.; Egawa, S.; Ikuta, F. Ultrastructural Relationship between Lewy Bodies and Pale Bodies Studied in Locus Ceruleus Neurons of a Non-Parkinsonian Patient. Neuropathology 1994, 14, 73–80. [Google Scholar] [CrossRef]
- Arbelo, M.; de Los Monteros, A.E.; Herráez, P.; Andrada, M.; Sierra, E.; Rodríguez, F.; Jepson, P.D.; Fernandez, A. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999–2005). Dis. Aquat. Org. 2013, 103, 87–99. [Google Scholar] [CrossRef]
- Fernández, A.; Edwards, J.F.; Rodríguez, F.; de los Monteros, A.E.; Herráez, P.; Castro, P.; Jaber, J.R.; Martín, V.; Arbelo, M. “Gas and Fat Embolic Syndrome” Involving a Mass Stranding of Beaked Whales (Family Ziphiidae) Exposed to Anthropogenic Sonar Signals. Veter-Pathol. 2005, 42, 446–457. [Google Scholar] [CrossRef]
- Olney, J.W. New Insights and New Issues in Developmental Neurotoxicology. NeuroToxicology 2002, 23, 659–668. [Google Scholar] [CrossRef]
- Ochiai, M.; Nguyen, H.T.; Kurihara, N.; Hirano, M.; Tajima, Y.; Yamada, T.K.; Iwata, H. Directly Reprogrammed Neurons as a Tool to Assess Neurotoxicity of the Contaminant 4-Hydroxy-2′,3,5,5′-tetrachlorobiphenyl (4′OH-CB72) in Melon-Headed Whales. Environ. Sci. Technol. 2021, 55, 8159–8168. [Google Scholar] [CrossRef] [PubMed]
- Jesenberger, V.; Jentsch, S. Deadly encounter: Ubiquitin meets apoptosis. Nat. Rev. Mol. Cell Biol. 2002, 3, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Terrin, A.-S.; Jeton, F.; Pichon, A.; Frugière, A.; Richalet, J.-P.; Bodineau, L.; Voituron, N. The c-FOS Protein Immunohistological Detection: A Useful Tool as a Marker of Central Pathways Involved in Specific Physiological Responses In Vivo and Ex Vivo. J. Vis. Exp. 2016, 10, 53613. [Google Scholar] [CrossRef] [Green Version]
- Wessel, T.C.; Joh, T.H.; Volpe, B.T. In situ hybridization analysis of c-fos and c-jun expression in the rat brain following transient forebrain ischemia. Brain Res. 1991, 567, 231–240. [Google Scholar] [CrossRef]
- Thakur, A.; Wang, X.; Siedlak, S.L.; Perry, G.; Smith, M.A.; Zhu, X. c-Jun phosphorylation in Alzheimer disease. J. Neurosci. Res. 2007, 85, 1668–1673. [Google Scholar] [CrossRef]
- Saporito, M.; Thomas, B.A.; Scott, R.W. MPTP Activates c-Jun NH2-Terminal Kinase (JNK) and Its Upstream Regulatory Kinase MKK4 in Nigrostriatal Neurons In Vivo. J. Neurochem. 2002, 75, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Schneuer, M.; Flachsbarth, S.; Czech-Damal, N.; Folkow, L.; Siebert, U.; Burmester, T. Neuroglobin of seals and whales: Evidence for a divergent role in the diving brain. Neuroscience 2012, 223, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Fago, A.; Hundahl, C.; Malte, H.; Weber, R.E. Functional Properties of Neuroglobin and Cytoglobin. Insights into the Ancestral Physiological Roles of Globins. IUBMB Life 2004, 56, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Zavanelli, M.; Miller, A.M.; Goldbeck, A.R.; Morledge, M.; Casper, D.; Pabst, D.A.; McLellan, W.; Cantin, L.P.; Kliger, D.S. Running, swimming and diving modifies neuroprotecting globins in the mammalian brain. Proc. R. Soc. B Boil. Sci. 2008, 275, 751–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, A.; de Quirós, Y.B.; Sacchini, S.; Sierra, E. Pathology of Marine Mammals: What It Can Tell Us About Environment and Welfare. In Marine Mammal Welfare: Human Induced Change in the Marine Environment and its Impacts on Marine Mammal Welfare; Butterworth, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 585–608. [Google Scholar] [CrossRef]


| Primary Antibody | Type | Specificity [Published Manuscript] | Diluition ff-IHC p-IHC | Antigen Retrieval (Only for FFPE) | NS | SA |
|---|---|---|---|---|---|---|
| c-Fos (4) Santa Cruz Biotechnology sc-52 | P/R | c-Fos Protein | 1/100 (both) | Pronase, 7 min | G | BaR |
| c-Jun (D) Santa Cruz Biotechnology sc-44 | P/R | c-Jun Protein | 1/100 (both) | Citrate Buffer, 90–95 °C, 10 min (pH 6) | G | BaR |
| HSP70 Abcam Ab6535 | M/Mo | Heat Shock Protein 70 kD [35] | 1/100 (both) | None | H | BaMo |
| Ubiquitin Dako Z045801 | P/R | Human Ubiquitin | 1/100 (p-IHC) | None | G | BaR |
| Neuroglobin Abcam Ab37258 | M/Mo | Neuroglobin | 1/100 (p-IHC) | None | H | BaMo |
| Calretinin Swant 6B3 | M/Mo | Calretinin calcium-binding protein [35] | 1/500 (p-IHC) | Wet autoclave method of Shin 118° C, 5 min | H | BaMo |
| Calbindin D-28k Swant 300 | M/Mo | Calbindin calcium-binding protein | 1/500 (both) | Wet autoclave method of Shin, 118° C, 5 min | H | BaMo |
| Parvalbumin Swant 235 | M/Mo | Parvalbumin calcium-binding protein | 1/500 (p-IHC) | Wet autoclave method of Shin, 118° C, 5 min | H | BaMo |
| GFAP DakoCytomation | P/R | Glial Fibrillary Acidic Protein | 1/120 (p-IHC) | None | G | BaR |
| nNOS Millipore Ab5380 | P/R | Nitric Oxide Synthase | 1/300 (p-IHC) | Wet autoclave method of Shin, 118° C, 5 min | G | BaR |
| TH Monosan MONX10786 | M/Mo | Tyrosine Hydroxylase [14] | 1/200 (ff-IHC) 1/50 (p-IHQ) | Wet autoclave method of Shin, 118° C, 5 min | H | BaMo |
| CRF Abcam Ab59023 | P/G | Corticotropin Releasing Factor | 1/100 (ff-IHC) | N/A | R | BaG |
| Vasopressin Abcam Ab39363 | P/R | Vasopressin | 1/500 (ff-IHC) | N/A | G | BaR |
| HSV1 Abcam Ab9533 | P/R | Herpesvirus type I [29] | 1/50 (p-IHC) | Pronase 10 min | G | BaR |
| CDV VMRD CDV-NP | M/Mo | Nucleoprotein of Canine Distemper Virus [36] | 1/100 (p-IHC) | Wet autoclave method of Shin, 118° C, 5 min | R | BaMo (1/20) |
| Laforin Novus Biologicals NBP2-24474 | P/R | Human Laforin (EPM2A) | 1/100 (p-IHC) | Wet autoclave method of Shin, 118° C, 5 min | G | BaR |
| B-Amyloid Invitrogen 700254 | M/R | Beta Amyloid (H31L21) [15] | 1/100 (ff-IHC) | N/A | G | BaR |
| NFT AHB0161 | P/R | Neurofibrillary Tangles [15] | 1/100 (ff-IHC) | N/A | G | BaR |
| α-Synuclein Abcam Ab27766 | M/Mo | Alpha-synuclein (LB 509) | 1/100 (ff-IHC) | N/A | H | BaMo |
| α-Synuclein Invitrogen 35-8300 | M/Mo | Alpha-synuclein (Syn 505) | 1/100 (p-IHC) | Wet autoclave method of Shin, 118° C, 5 min | H | BaMo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchini, S.; Herráez, P.; Arbelo, M.; Espinosa de los Monteros, A.; Sierra, E.; Rivero, M.; Bombardi, C.; Fernández, A. Methodology and Neuromarkers for Cetaceans’ Brains. Vet. Sci. 2022, 9, 38. https://doi.org/10.3390/vetsci9020038
Sacchini S, Herráez P, Arbelo M, Espinosa de los Monteros A, Sierra E, Rivero M, Bombardi C, Fernández A. Methodology and Neuromarkers for Cetaceans’ Brains. Veterinary Sciences. 2022; 9(2):38. https://doi.org/10.3390/vetsci9020038
Chicago/Turabian StyleSacchini, Simona, Pedro Herráez, Manuel Arbelo, Antonio Espinosa de los Monteros, Eva Sierra, Miguel Rivero, Cristiano Bombardi, and Antonio Fernández. 2022. "Methodology and Neuromarkers for Cetaceans’ Brains" Veterinary Sciences 9, no. 2: 38. https://doi.org/10.3390/vetsci9020038
APA StyleSacchini, S., Herráez, P., Arbelo, M., Espinosa de los Monteros, A., Sierra, E., Rivero, M., Bombardi, C., & Fernández, A. (2022). Methodology and Neuromarkers for Cetaceans’ Brains. Veterinary Sciences, 9(2), 38. https://doi.org/10.3390/vetsci9020038







