Granulomatous Inflammation and Pericarditis Induced by Silk Granuloma Related to Previous Surgical Ligation of Patent Ductus Arteriosus in a Dog
Abstract
Simple Summary
Abstract
1. Introduction
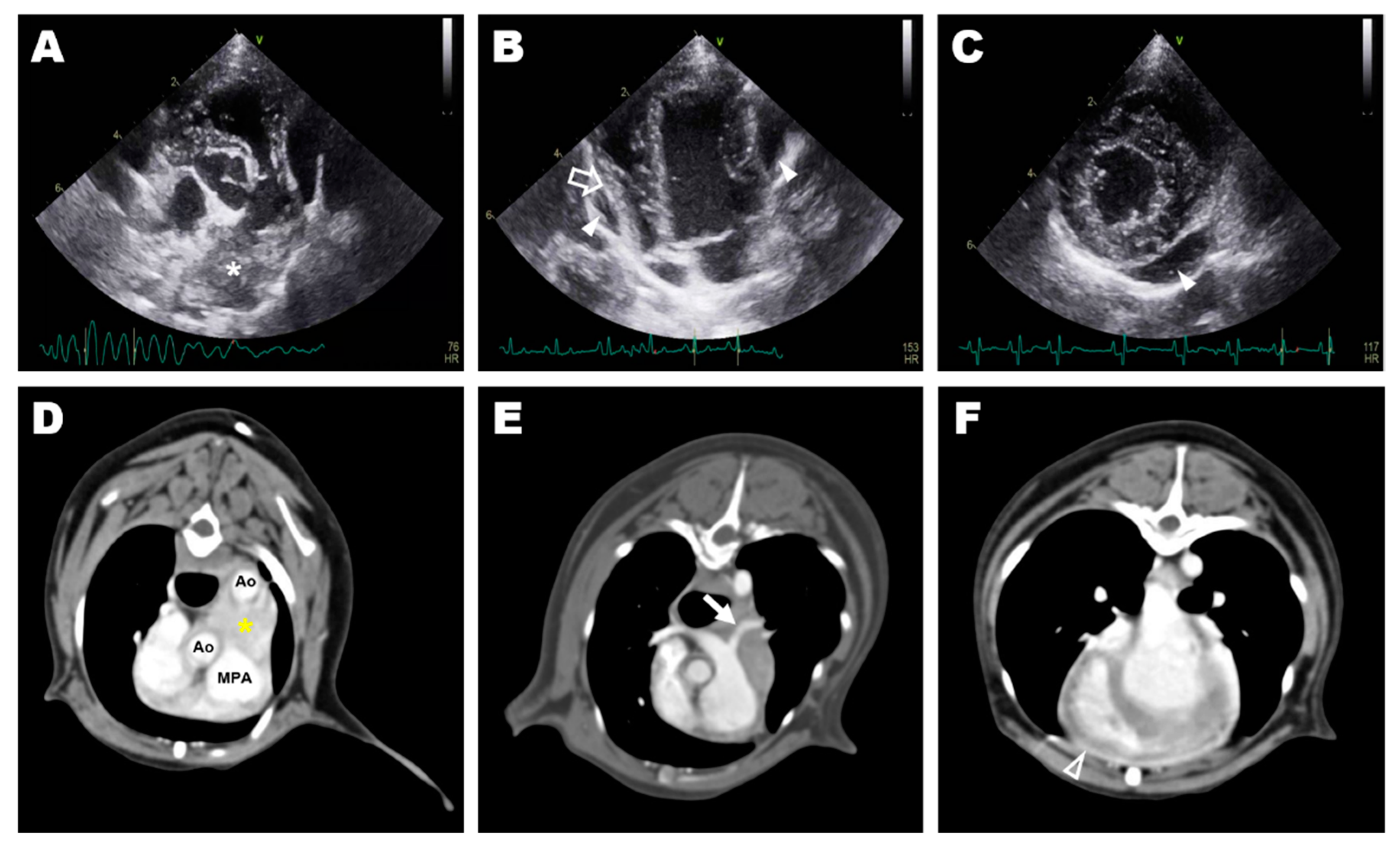
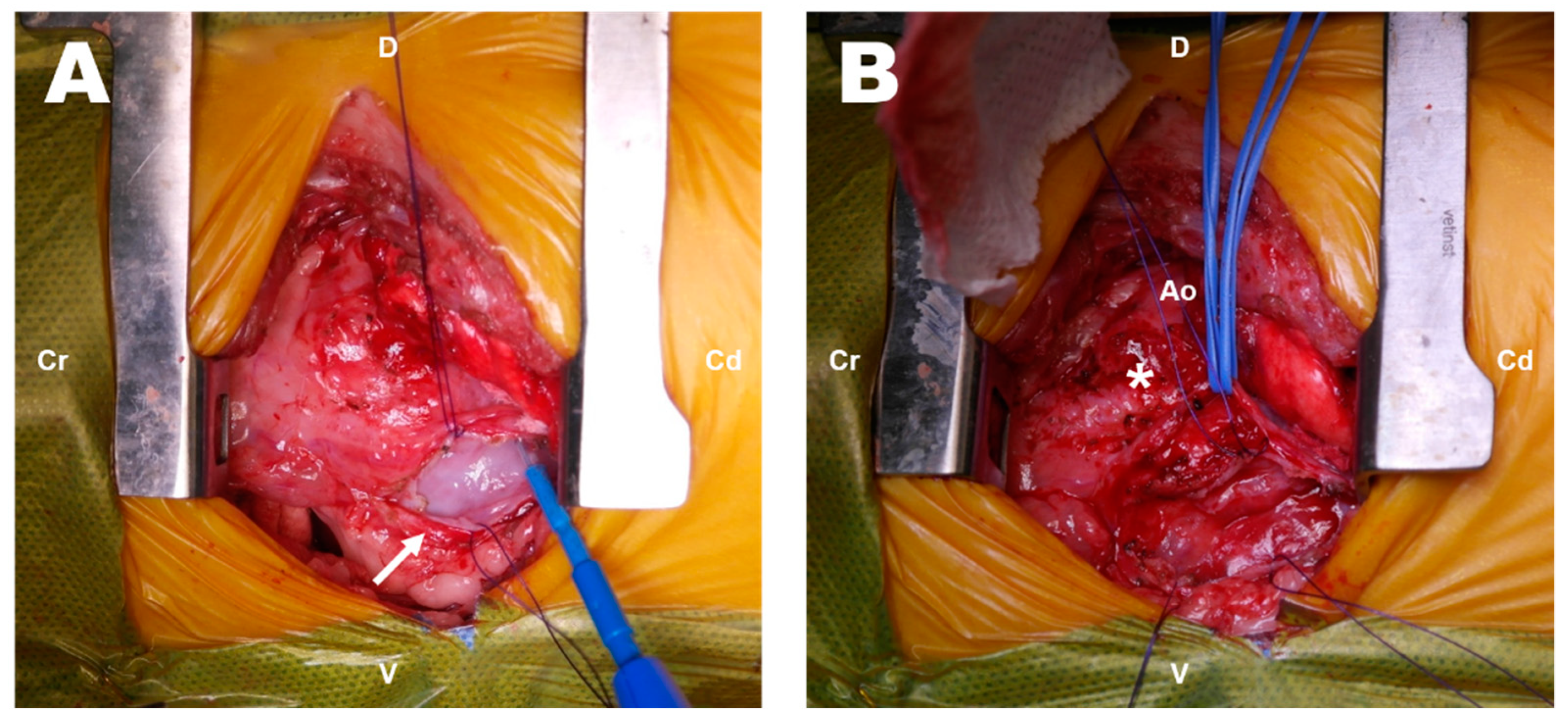
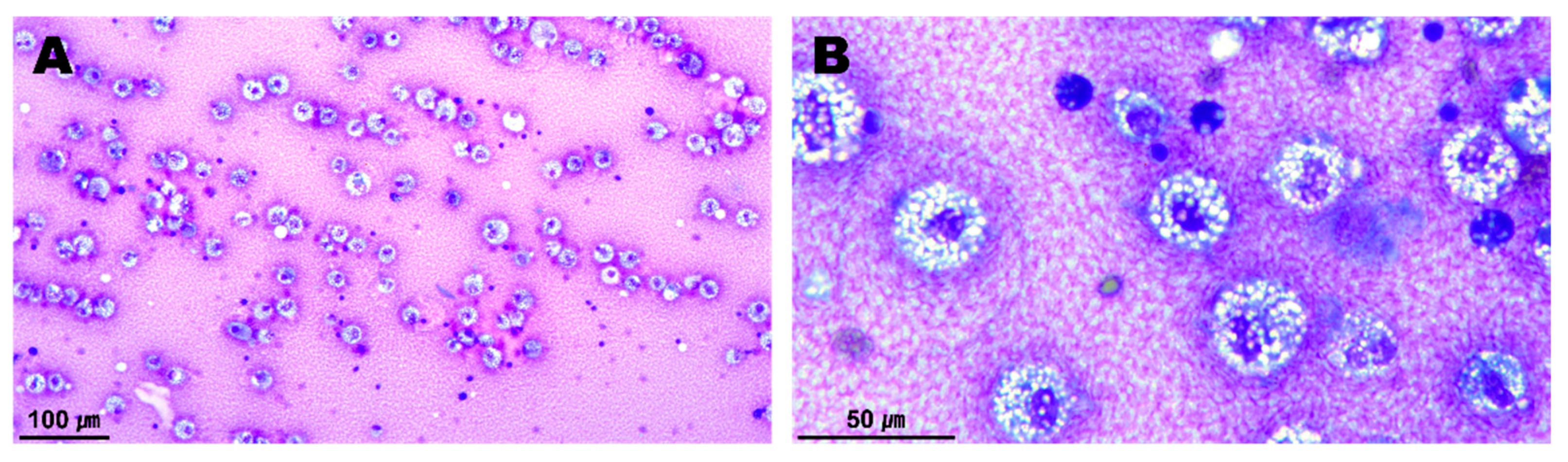
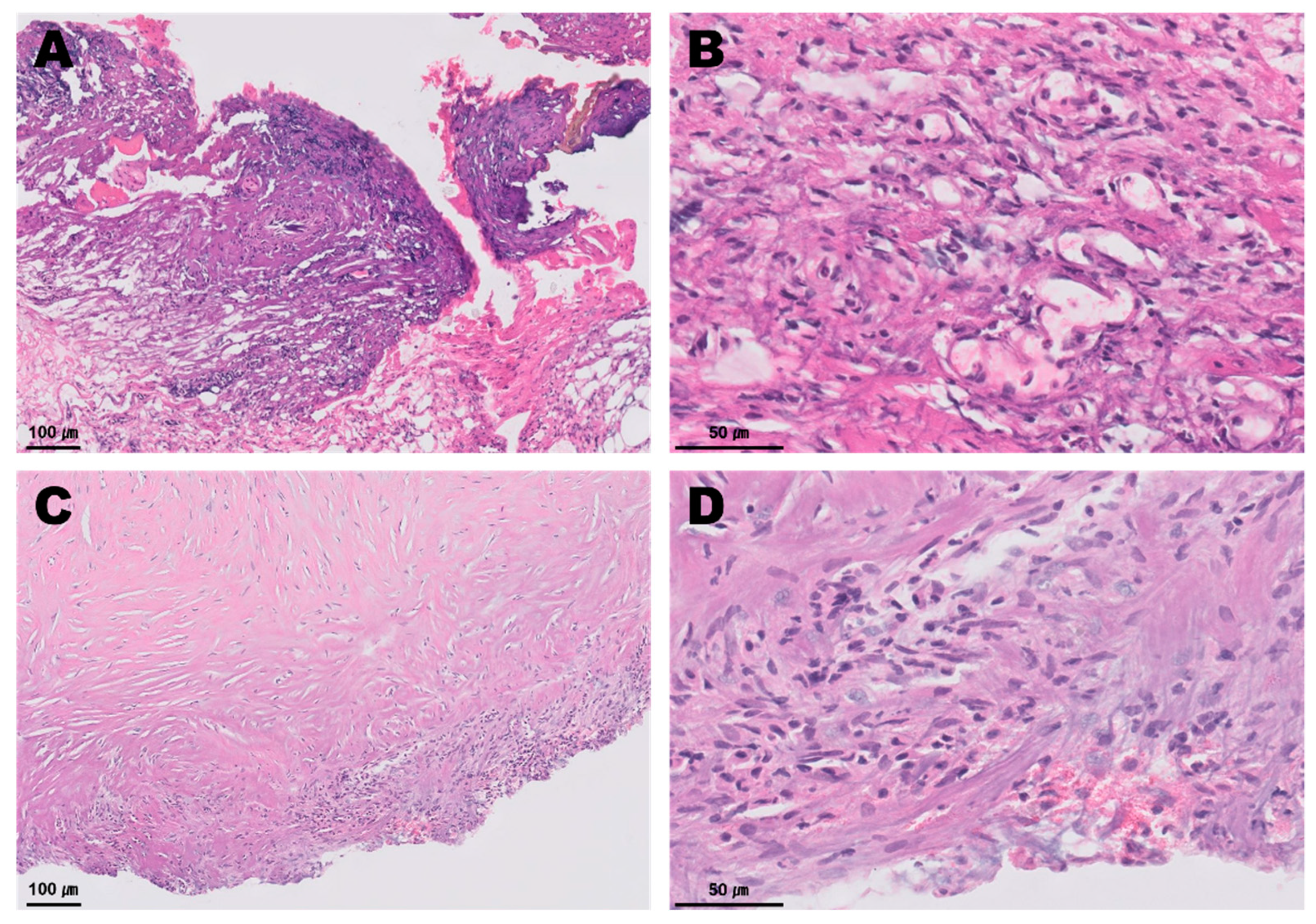
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchanan, J.W. Prevalence of Cardiovascular Disorders. In Textbook of Canine and Feline Cardiology, 2nd ed.; Fox, P.R., Sisson, D.D., Moise, N.S., Eds.; Saunders: Philadelphia, PA, USA, 1999; pp. 457–470. [Google Scholar]
- Detweiler, D.K.; Patterson, D.F. The prevalence and types of cardiovascular disease in dogs. Ann. N. Y. Acad. Sci. 1965, 127, 481–516. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Domenech, O.; Silva, J.; Vannini, S.; Bussadori, R.; Bussadori, C. Retrospective review of congenital heart disease in 976 dogs. J. Vet. Intern. Med. 2011, 25, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, A.; Orton, E.C. Cardiac Surgery. In Veterinary Surgery: Small Animal, 2nd ed.; Johnston, S.A., Tobias, K.M., Eds.; Elsevier: St. Louis, MO, USA, 2018; Volume 2, pp. 2051–2084. [Google Scholar]
- Eyster, G.E.; Eyster, J.T.; Cords, G.B.; Johnston, J. Patent ductus arteriosus in the dog: Characteristics of occurrence and results of surgery in one hundred consecutive cases. J. Am. Vet. Med. Assoc. 1976, 168, 435–438. [Google Scholar] [PubMed]
- Orton, E.C.; Monnet, E. Small Animal Thoracic Surgery; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 177–182. [Google Scholar]
- Achen, S.E.; Miller, M.W.; Gordon, S.G.; Saunders, A.B.; Roland, R.M.; Drourr, L.T. Transarterial ductal occlusion with the Amplatzer vascular plug in 31 dogs. J. Vet. Intern. Med. 2008, 22, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, K.R.; Kyles, A.E.; Kass, P.H.; Campbell, F. Retrospective comparison of surgical ligation and transarterial catheter occlusion for treatment of patent ductus arteriosus in two hundred and four dogs (1993–2003). Vet. Surg. 2007, 36, 43–49. [Google Scholar] [CrossRef]
- Ranganathan, B.; LeBlanc, N.L.; Scollan, K.F.; Townsend, K.L.; Agarwal, D.; Milovancev, M. Comparison of major complication and survival rates between surgical ligation and use of a canine ductal occluder device for treatment of dogs with left-to-right shunting patent ductus arteriosus. J. Am. Vet. Med. Assoc. 2018, 253, 1046–1052. [Google Scholar] [CrossRef]
- Van Israël, N.V.; Dukes-McEwan, J.; French, A.T. Long-term follow-up of dogs with patent ductus arteriosus. J. Small Anim. Pract. 2003, 44, 480–490. [Google Scholar] [CrossRef]
- Saunders, A.B.; Gordon, S.G.; Boggess, M.M.; Miller, M.W. Long-term outcome in dogs with patent ductus arteriosus: 520 cases (1994–2009). J. Vet. Intern. Med. 2014, 28, 401–410. [Google Scholar] [CrossRef]
- Buchanan, J.W. Patent ductus arteriosus. Semin. Vet. Med. Surg. Small Anim. 1994, 9, 168–176. [Google Scholar]
- Bureau, S.; Monnet, E.; Orton, E.C. Evaluation of survival rate and prognostic indicators for surgical treatment of left-to-right patent ductus arteriosus in dogs: 52 cases (1995–2003). J. Am. Vet. Med. Assoc. 2005, 227, 1794–1799. [Google Scholar] [CrossRef]
- Hunt, G.B.; Simpson, D.J.; Beck, J.A.; Goldsmid, S.E.; Lawrence, D.; Pearson, M.R.; Bellenger, C.R. Intraoperative hemorrhage during patent ductus arteriosus ligation in dogs. Vet. Surg. 2001, 30, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Scansen, B.A.; Simpson, E.M.; López-Alvarez, J.; Thomas, W.P.; Bright, J.M.; Eason, B.D.; Rush, J.E.; Dukes-McEwan, J.; Green, H.W.; Cunningham, S.M.; et al. Pulmonary artery dissection in eight dogs with patent ductus arteriosus. J. Vet. Cardiol. 2015, 17, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Birchard, S.J.; Bonagura, J.D.; Fingland, R.B. Results of ligation of patent ductus arteriosus in dogs: 201 cases (1969–1988). J. Am. Vet. Med. Assoc. 1990, 196, 2011–2013. [Google Scholar] [PubMed]
- Cooper, R.C.; Webber, W.J.; Goodwin, J.K. The surgical treatment of common congenital heart defects. Vet. Med. 1992, 87, 676–688. [Google Scholar]
- Love, B.A.; Thierry, F.; Schwarz, T.; Martinez-Pereira, Y.; Culshaw, G.J. Aortic sinus aneurysm communicating with the main pulmonary artery, and a concurrent patent ductus arteriosus, in a dog. J. Small Anim. Pract. 2021, 62, 300–304. [Google Scholar] [CrossRef]
- Scansen, B.A.; Schober, K.E.; Bonagura, J.D.; Smeak, D.D. Acquired pulmonary artery stenosis in four dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1172–1180. [Google Scholar] [CrossRef]
- Stanley, B.J.; Luis-Fuentes, V.; Darke, P.G. Comparison of the incidence of residual shunting between two surgical techniques used for ligation of patent ductus arteriosus in the dog. Vet. Surg. 2003, 32, 231–237. [Google Scholar] [CrossRef]
- Takeuchi, A.; Uemura, A.; Goya, S.; Shimada, K.; Yoshida, T.; Hara, S.; Sato, K.; Shiraishi, K.; Yairo, A.; Kto, K.; et al. The utility of patent ductus arteriosus closure with hemostatic clip in dogs. Pol. J. Vet. Sci. 2020, 23, 255–260. [Google Scholar] [CrossRef]
- Boza, S.; Lucas, X.; Zarelli, M.; Soler, M.; Belda, E.; Agut, A. Late abscess formation caused by silk suture following hysterectomy in a female dog. Reprod. Domest. Anim. 2010, 45, 934–936. [Google Scholar] [CrossRef]
- Lim, J.W.; Tang, C.L.; Keng, G.H. False positive F-18 fluorodeoxyglucose combined PET/CT scans from suture granuloma and chronic inflammation: Report of two cases and review of literature. Ann. Acad. Med. Singap. 2005, 34, 457–460. [Google Scholar]
- Singh, S.K.; Kannan, N.; Talwar, R.; Tyagi, A.K.; Madan, R.; Jaiswal, P.; Kumar, A. Suture granuloma: A rare differential diagnosis of residual/recurrent gastrointestinal stromal tumor of stomach. Int. Cancer. Conf. J. 2015, 31, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Haruki, K.; Tsukihara, S.; Abe, T.; Koyama, M.; Ito, D.; Kanno, H.; Son, K.; Nanyu, N.; Eto, K. Suture granuloma with hydronephrosis caused by ileostomy closure after rectal cancer surgery: A case report. Surg. Case Rep. 2021, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.T.; Williams, W.J. Granulomatous inflammation—A review. J. Clin. Pathol. 1983, 36, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.K.; Pritt, B.S.; Alexander, M.P. Histopathologic review of granulomatous inflammation. J. Clin. Tuberc. Other Mycobact. Dis. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fossum, T.W. Small Animal Surgery, 5th ed.; Elsevier: St. Louis, MO, USA, 2019; pp. 788–832. [Google Scholar]
- Postlethwait, R.W.; Willigan, D.A.; Ulin, A.W. Human tissue reaction to sutures. Ann. Surg. 1975, 181, 144–150. [Google Scholar] [CrossRef]
- Tsuchida, Y.; Park, C.H.; Yasuie, Y.; Isomura, H.; Kozima, D.; Ueki, H.; Ikeda, M.; Oyamada, T. Histopathological study on canine post-operative suture granuloma. J. Jpn. Vet. Med. Assoc. 2009, 62, 388–394. [Google Scholar] [CrossRef]
- Aoki, T.; Sunahara, H.; Sugimoto, K.; Ito, T.; Kanai, E.; Fujii, Y. Peripheral pulmonary artery stenosis in three cats. J. Vet. Med. Sci. 2015, 77, 487–491. [Google Scholar] [CrossRef][Green Version]
- Adzick, N.S.; Harrison, M.R.; deLorimier, A.A. Surgical clip ligation of patent ductus arteriosus in premature infants. J. Pediatr. Surg. 1986, 21, 158. [Google Scholar] [CrossRef]
- Traugott, R.C.; Will, R.J.; Schuchmann, G.F.; Treasure, R.L. A simplified method of ligation of patent ductus arteriosus in premature infants. Ann. Thorac. Surg. 1980, 29, 263. [Google Scholar] [CrossRef]
- Mandhan, P.L.; Samarakkody, U.; Brown, S.; Kukkady, A.; Maoate, K.; Blakelock, R.; Beasley, S. Comparison of suture ligation and clip application for the treatment of patent ductus arteriosus in preterm neonates. J. Thorac. Cardiovasc. Surg. 2006, 132, 672–674. [Google Scholar] [CrossRef][Green Version]
- Corti, L.B.; Merkley, D.; Nelson, O.L.; Ware, W.A. Retrospective evaluation of occlusion of patent ductus arteriosus with hemoclips in 20 dogs. J. Am. Anim. Hosp. Assoc. 2000, 36, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Ozai, Y.; Uemura, A.; Tanaka, R.; Takeuchi, A.; Hamabe, L.; Shimada, K.; Yokoi, A.; Hirose, M.; Watanabe, M.; Uehara, K. Clip ligation for treatment of patent ductus arteriosus occlusion in three cats. J. Vet. Sci. 2022, 23, e39. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Sun, M.; Liu, Y.; Xin, L. A case of foreign body granuloma caused by titanium clips after breast conserving surgery. Asian J. Surg. 2021, 44, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Nihon-Yanagi, Y.; Ishiwatari, T.; Otsuka, Y.; Okubo, Y.; Tochigi, N.; Wakayama, M.; Nemoto, T.; Watanabe, M.; Kaneko, H.; Sumino, Y.; et al. A case of postoperative hepatic granuloma presumptively caused by surgical staples/clipping materials. Diagn. Pathol. 2015, 10, 90. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, C.-H.; Kim, W.-J.; Lee, W.-J.; Kim, K.-M.; Lee, H.-B.; Jeong, S.-M.; Kim, D.-H. Granulomatous Inflammation and Pericarditis Induced by Silk Granuloma Related to Previous Surgical Ligation of Patent Ductus Arteriosus in a Dog. Vet. Sci. 2022, 9, 694. https://doi.org/10.3390/vetsci9120694
Moon C-H, Kim W-J, Lee W-J, Kim K-M, Lee H-B, Jeong S-M, Kim D-H. Granulomatous Inflammation and Pericarditis Induced by Silk Granuloma Related to Previous Surgical Ligation of Patent Ductus Arteriosus in a Dog. Veterinary Sciences. 2022; 9(12):694. https://doi.org/10.3390/vetsci9120694
Chicago/Turabian StyleMoon, Chang-Hwan, Woo-Jin Kim, Won-Jong Lee, Kyung-Min Kim, Hae-Beom Lee, Seong-Mok Jeong, and Dae-Hyun Kim. 2022. "Granulomatous Inflammation and Pericarditis Induced by Silk Granuloma Related to Previous Surgical Ligation of Patent Ductus Arteriosus in a Dog" Veterinary Sciences 9, no. 12: 694. https://doi.org/10.3390/vetsci9120694
APA StyleMoon, C.-H., Kim, W.-J., Lee, W.-J., Kim, K.-M., Lee, H.-B., Jeong, S.-M., & Kim, D.-H. (2022). Granulomatous Inflammation and Pericarditis Induced by Silk Granuloma Related to Previous Surgical Ligation of Patent Ductus Arteriosus in a Dog. Veterinary Sciences, 9(12), 694. https://doi.org/10.3390/vetsci9120694




