Bacterial Contamination of the Surgical Site at the Time of Elective Caesarean Section in Belgian Blue Cows—Part 1: Identified by Bacterial Culture
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Description and Samples Utilisation
2.2. Caesarean Section Realisation
2.2.1. Cow Preparation for Caesarean Section
2.2.2. Operator and Material Preparation
2.2.3. Surgical Procedure
2.3. Samples Taken
2.4. Bacterial Culture and Laboratory Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herd Book Blanc Bleu Belge (HBBB). Caractéristiques. 2022. Available online: https://www.hbbbb.be/fr/pages/caracteristique (accessed on 15 April 2022).
- Coopman, F.; De Smet, S.; Gengler, N.; Haegeman, A.; Jacobs, K.; Van Poucke, M.; Laevens, H. Estimating internal pelvic sizes using external body measurements in the double-muscled Belgian Blue beef breed. Anim. Sci. 2003, 76, 229–235. [Google Scholar] [CrossRef]
- Djebala, S.; Moula, N.; Bayrou, C.; Sartelet, A.; Bossaert, P. Prophylactic antibiotic usage by Belgian veterinarians during elective caesarean section in Belgian Blue cattle. Prev. Vet. Med. 2019, 172, 104785. [Google Scholar] [CrossRef]
- De Coensel, E.; Sarrazin, S.; Opsomer, G.; Dewulf, J. Antimicrobial use in the uncomplicated cesarean section in cattle in Flanders. Vlaams Diergeneeskd. Tijdschr. 2020, 89, 41–51. [Google Scholar] [CrossRef]
- Hoeben, D.; Mijten, P.; De Kruif, A. Factors influencing complications during caesarean section on the standing cow. Vet. Q. 1997, 19, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Mijten, P. Puerperal complications after cesarean section in dairy cows and in double- muscled cows. Reprod. Domest. Anim. 1998, 33, 175–179. [Google Scholar] [CrossRef]
- Hanzen, C.; Théron, L.; Detilleux, J. Modalités de réalisation de la césarienne dans l’espèce bovine en Europe. Bull. GTV 2011, 59, 15–26. [Google Scholar]
- Djebala, S.; Evrard, J.; Moula, N.; Sartelet, A.; Bossaert, P. Atypical case of parietal fibrinous peritonitis in a Belgian Blue heifer without a history of laparotomy. Vet. Rec. Case Rep. 2020, 8, e001086. [Google Scholar] [CrossRef]
- Djebala, S.; Evrard, J.; Moula, N.; Gille, L.; Bayrou, C.; Eppe, J.; Casalta, H.; Sartelet, A.; Bossaert, P. Comparison between generalised peritonitis and parietal fibrinous peritonitis in cows after caesarean section. Vet. Rec. 2020, 187, 105867. [Google Scholar] [CrossRef]
- Djebala, S.; Evrard, J.; Gregoire, F.; Thiry, D.; Bayrou, C.; Moula, N.; Sartelet, A.; Bossaert, P. Infectious Agents Identified by Real-Time PCR, Serology and Bacteriology in Blood and Peritoneal Exudate Samples of Cows Affected by Parietal Fibrinous Peritonitis after Caesarean Section. Vet. Sci. 2020, 7, 134. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Callens, B.; Cargnel, M.; Sarrazin, S.; Dewulf, J.; Hoet, B.; Vermeersch, K.; Wattiau, P.; Welby, S. Associations between a decreased veterinary antimicrobial use and resistance in commensal Escherichia coli from Belgian livestock species (2011–2015). Prev. Vet. Med. 2018, 157, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mijten, P.; van den Bogaard, A.E.J.M.; Hazen, M.J.; de Kruif, A. Bacterial contamination of fetal fluids at the time of caesarean section in the cow. Theriogenology 1996, 97, 513–521. [Google Scholar] [CrossRef]
- Kolkman, I.; De Vliegher, S.; Hoflack, G.; Van Aert, M.; Laureyns, J.; Lips, D.; De Kruif, A.; Opsomer, G. Protocol of the caesarean section as performed in daily bovine practice in Belgium. Reprod. Domest. Anim. 2007, 42, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, I.; Opsomer, G.; Lips, D.; Lindenbergh, B.; De Kruif, A.; De Vliegher, S. Preoperative and operative difficulties during bovine caesarean section in Belgium and associated risk factors. Reprod. Domest. Anim. 2010, 45, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Consumption and Resistance in Animals (AMCRA). 2022. Traitement Antibactérien Péri-Opératoire. Available online: https://formularium.amcra.be/i/79 (accessed on 5 April 2022).
- Classen, D.C.; Evans, R.S.; Pestotnik, S.L.; Horn, S.D.; Menlove, R.L.; Burke, J.P. The Timing of Prophylactic Administration of Antibiotics and the Risk of Surgical-Wound Infection. N. Engl. J. Med. 1992, 326, 281–286. [Google Scholar] [CrossRef]
- Baaqeel, H.; Baaqeel, R. Timing of administration of prophylactic antibiotics for caesarean section: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2012, 120, 661–669. [Google Scholar] [CrossRef]
- Bollig, C.; Nothacker, M.; Lehane, C.; Motschall, E.; Lang, B.; Meerpohl, J.J.; Schmucker, C.M. Prophylactic antibiotics before cord clamping in cesarean delivery: A systematic review. Acta Obstet. Gynecol. Scand. 2017, 97, 521–535. [Google Scholar] [CrossRef]
- Djebala, S.; Croubels, S.; Cherlet, M.; Martinelle, L.; Thiry, D.; Moula, N.; Sartelet, A.; Bossaert, P. Description of Plasma Penicillin G Concentrations after Intramuscular Injection in Double-Muscled Cows to Optimize the Timing of Antibiotherapy for Caesarean Section. Vet. Sci. 2021, 8, 67. [Google Scholar] [CrossRef]
- Uystepruyst, C.; Coghe, J.; Dorts, T.; Harmegnies, N.; Delsemme, M.H.; Art, T.; Lekeux, P. Optimal timing of elective caesarean section in Belgian White and Blue Breed of cattle: The calf’s point of view. Vet. J. 2002, 163, 267–282. [Google Scholar] [CrossRef]
- Husso, A.; Lietaer, L.; Pessa-Morikawa1, T.; Grönthal, T.; Govaere, J.; Van Soom, A.; Iivanainen, A.; Opsomer, G.; Niku, M. The Composition of the Microbiota in the Full-Term Fetal Gut and Amniotic Fluid: A Bovine Cesarean Section Study. Front. Microbiol. 2021, 12, 626421. [Google Scholar] [CrossRef]
- Schulthess, B.; Brodner, K.; Bloemberg, G.V.; Zbinden, R.; Böttger, E.C.; Hombach, M. Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: Comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J. Clin. Microbiol. 2013, 51, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Laskin, A.I. CRC Handbook of Microbiology: Condensed Edition, 1st ed.; CRC Press: Boca Raton, FL, USA; St. Louis, MI, USA, 1974; p. 940. [Google Scholar] [CrossRef]
- Coenen, M.; Gille, L.; Eppe, J.; Casalta, H.; Bayrou, C.; Dubreucq, P.; Frisée, V.; Moula, N.; Evrard, J.; Martinelle, L.; et al. Blood Inflammatory, Hydro-Electrolytes and Acid-Base Changes in Belgian Blue Cows Developing Parietal Fibrinous Peritonitis or Generalised Peritonitis after Caesarean Section. Vet. Sci. 2022, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Dumas, S.E.; French, H.M.; Lavergne, S.N.; Ramirez, C.R.; Brown, L.J.; Bromfield, C.R.; Garrett, E.F.; French, D.D.; Aldridge, B.M. Judicious use of prophylactic antimicrobials to reduce abdominal surgical site infections in periparturient cows: Part 1—A risk factor review. Vet. Rec. 2016, 178, 654–660. [Google Scholar] [CrossRef]
- Koskinen, M.T.; Wellenberg, G.J.; Sampimon, O.C.; Holopainen, J.; Rothkamp, A.; Salmikivi, L.; Van Haeringen, W.A.; Lam, T.J.; Mand, G.; Pyörälä, S. Field comparison of real-time polymerase chain reaction and bacterial culture for identification of bovine mastitis bacteria. J. Dairy Sci. 2010, 93, 5707–5715. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kamagata, Y. The Challenges of Studying the Anaerobic Microbial World. Microbes Environ. 2014, 29, 335–337. [Google Scholar] [CrossRef]
- Gustin, P. Antibactériens. In Répertoire Commenté des Médicaments à Usage Vétérinaire; Gustin, P., Ed.; Centre Belge d’Information Pharmacothérapeutique CBIP-Vétérinaire: Bruxelles, Belgium, 2017; pp. 1–46. [Google Scholar]
- Cunha, F.; Jeon, J.S.; Daetz, R.; Vieira-Neto, A.; Laporta, J.; Jeong, K.C.; Barbet, A.F.; Risco, C.A.; Galvao, K.N. Quantifying known and emerging uterine pathogens, and evaluatingtheir association with metritis and fever in dairy cows. Theriogenology 2018, 114, 25–33. [Google Scholar] [CrossRef]
- Djebala, S.; Evrard, J.; Gregoire, F.; Bayrou, C.; Gille, L.; Eppe, J.; Casalta, H.; Frisée, V.; Moula, N.; Sartelet, A.; et al. Antimicrobial susceptibility profile of several bacteria species identified in the peritoneal exudate of cows affected by parietal fibrinous peritonitis after caesarean section. Vet. Sci. 2021, 8, 295. [Google Scholar] [CrossRef]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef]
- Ferrer, M.; Martínez-Abarca, F.; Golyshin, P.N. Mining genomes and ‘metagenomes’ for novel catalysts. Curr. Opin. Biotechnol. 2005, 16, 588–593. [Google Scholar] [CrossRef]
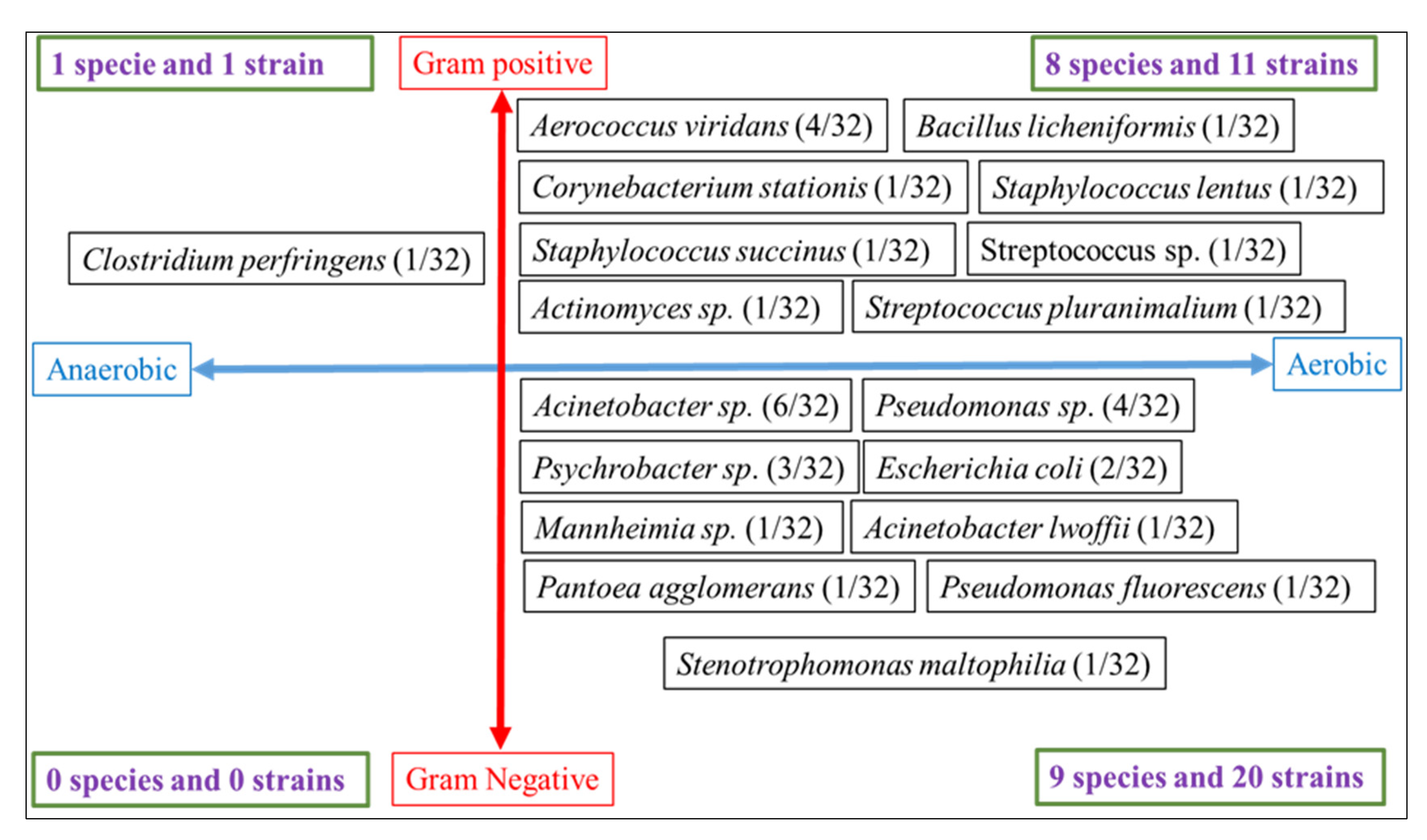

| Origin of the Sample (Farm) | Positive Samples | Number of Species in Each Sample | Bacterium 1 | Bacterium 2 | Bacterium 3 | Bacterium 4 |
|---|---|---|---|---|---|---|
| A (6/7 positive) | 1 | 1 | Pseudomonas sp. | / | / | / |
| 2 | 1 | Psychrobacter sp. | / | / | / | |
| 3 | 1 | Aerococcus viridans | / | / | / | |
| 4 | 2 | Aerococcus viridans | Psychrobacter sp. | / | ||
| 5 | 2 | Pseudomonas sp. | Stenotrophomonas maltophilia | / | / | |
| 6 | 2 | Acinetobacter sp. | Psychrobacter sp. | / | / | |
| C (3/5 positive) | 14 | 2 | Escherichia coli | Acinetobacter sp. | / | / |
| 15 | 2 | Acinetobacter sp. | Pseudomonas fluorescens | / | / | |
| 16 | More than 4 | / | / | |||
| D (1/5 positive) | 19 | 2 | Actinomyces sp. | Streptococcus sp. | / | / |
| E (1/4 positive) | 24 | 1 | Acinetobacter sp. | / | / | / |
| F (1/4 positive) | 28 | 1 | Bacillus licheniformis | / | / | / |
| G (1/4 positive) | 32 | 1 | Mannheimia sp. | / | / | / |
| H (2/4 positive) | 36 | 3 | Acinetobacter lwoffii | Aerococcus viridans | Pseudomonas sp. | / |
| 37 | 3 | Aerococcus viridans | Pantoea agglomerans | Staphylococcus lentus | / | |
| G (1/4 positive) | 44 | More than 4 | / | / | / | / |
| M (1/3 positive) | 56 | 1 | Clostridium perfringens | / | / | / |
| N (1/3 positive) | 59 | 1 | Streptococcus pluranimalium | / | / | / |
| S (1/1 positive) | 70 | 1 | Pseudomonas sp. | / | / | / |
| W (1/1 positive) | 74 | 4 | Acinetobacter sp. | Corynebacterium stationis | Escherichia coli | Staphylococcus succinus |
| Y (1/1 positive) | 76 | 1 | Acinetobacter sp. | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djebala, S.; Coria, E.; Munaut, F.; Gille, L.; Eppe, J.; Moula, N.; Taminiau, B.; Daube, G.; Bossaert, P. Bacterial Contamination of the Surgical Site at the Time of Elective Caesarean Section in Belgian Blue Cows—Part 1: Identified by Bacterial Culture. Vet. Sci. 2022, 9, 687. https://doi.org/10.3390/vetsci9120687
Djebala S, Coria E, Munaut F, Gille L, Eppe J, Moula N, Taminiau B, Daube G, Bossaert P. Bacterial Contamination of the Surgical Site at the Time of Elective Caesarean Section in Belgian Blue Cows—Part 1: Identified by Bacterial Culture. Veterinary Sciences. 2022; 9(12):687. https://doi.org/10.3390/vetsci9120687
Chicago/Turabian StyleDjebala, Salem, Elise Coria, Florian Munaut, Linde Gille, Justine Eppe, Nassim Moula, Bernard Taminiau, Georges Daube, and Philippe Bossaert. 2022. "Bacterial Contamination of the Surgical Site at the Time of Elective Caesarean Section in Belgian Blue Cows—Part 1: Identified by Bacterial Culture" Veterinary Sciences 9, no. 12: 687. https://doi.org/10.3390/vetsci9120687
APA StyleDjebala, S., Coria, E., Munaut, F., Gille, L., Eppe, J., Moula, N., Taminiau, B., Daube, G., & Bossaert, P. (2022). Bacterial Contamination of the Surgical Site at the Time of Elective Caesarean Section in Belgian Blue Cows—Part 1: Identified by Bacterial Culture. Veterinary Sciences, 9(12), 687. https://doi.org/10.3390/vetsci9120687







