PPARγ/mTOR Regulates the Synthesis and Release of Prostaglandins in Ovine Trophoblast Cells in Early Pregnancy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Culture and Treatments
2.3. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.4. Western Blot Analysis
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistical Analysis
3. Results
3.1. Morphological Characteristics of Sheep Trophoblast Cells (STCs)
3.2. Hormone Treatment of STCs Promoted PPARγ Expression, Inhibited the mTOR Pathway, and Increased Autophagy
3.3. Simulated Intrauterine Treatment of in STCs Increased the Release of PGs
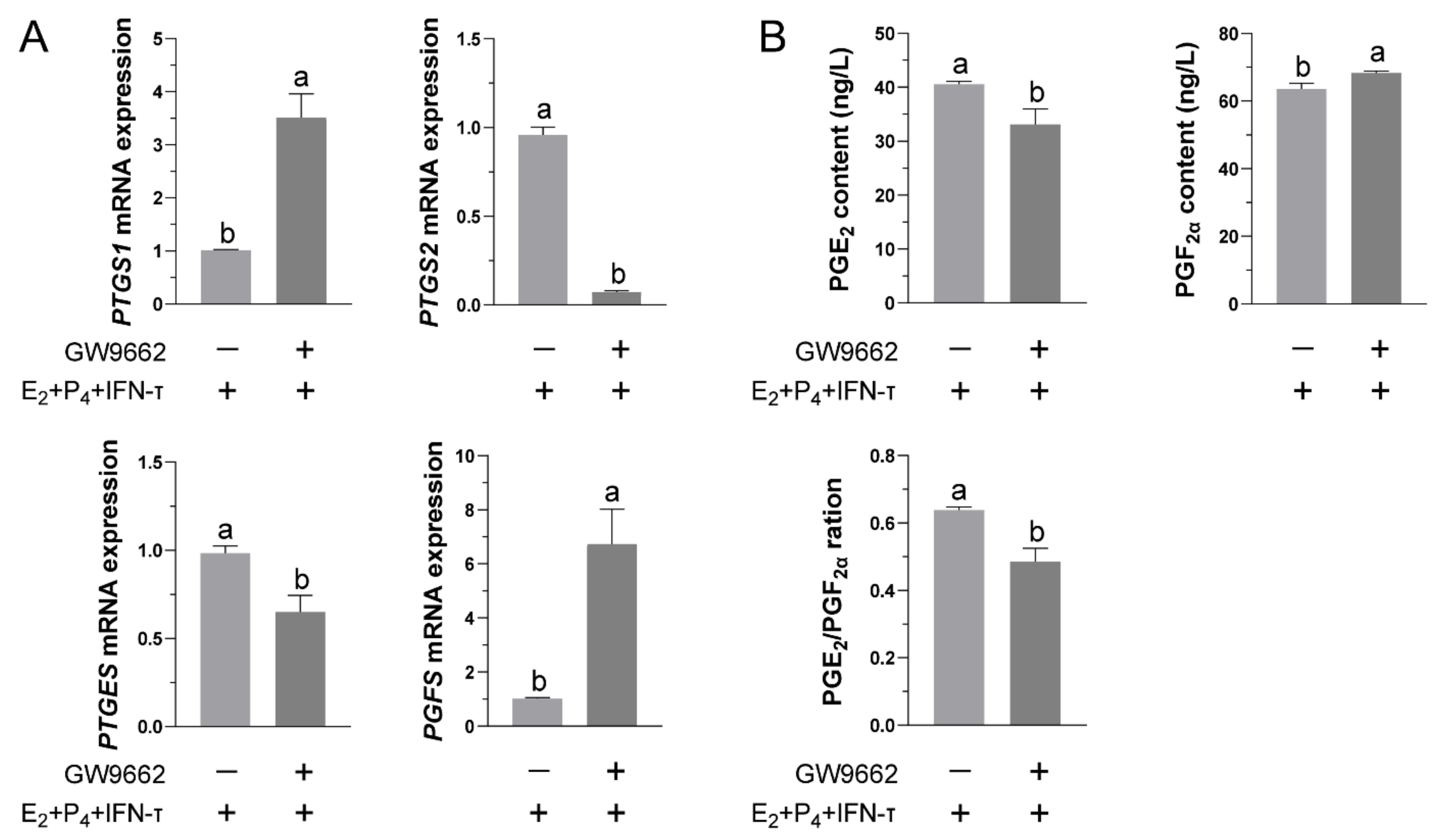
3.4. Inhibition of PPARγ Activity Reduced the Release of PGs from STCs
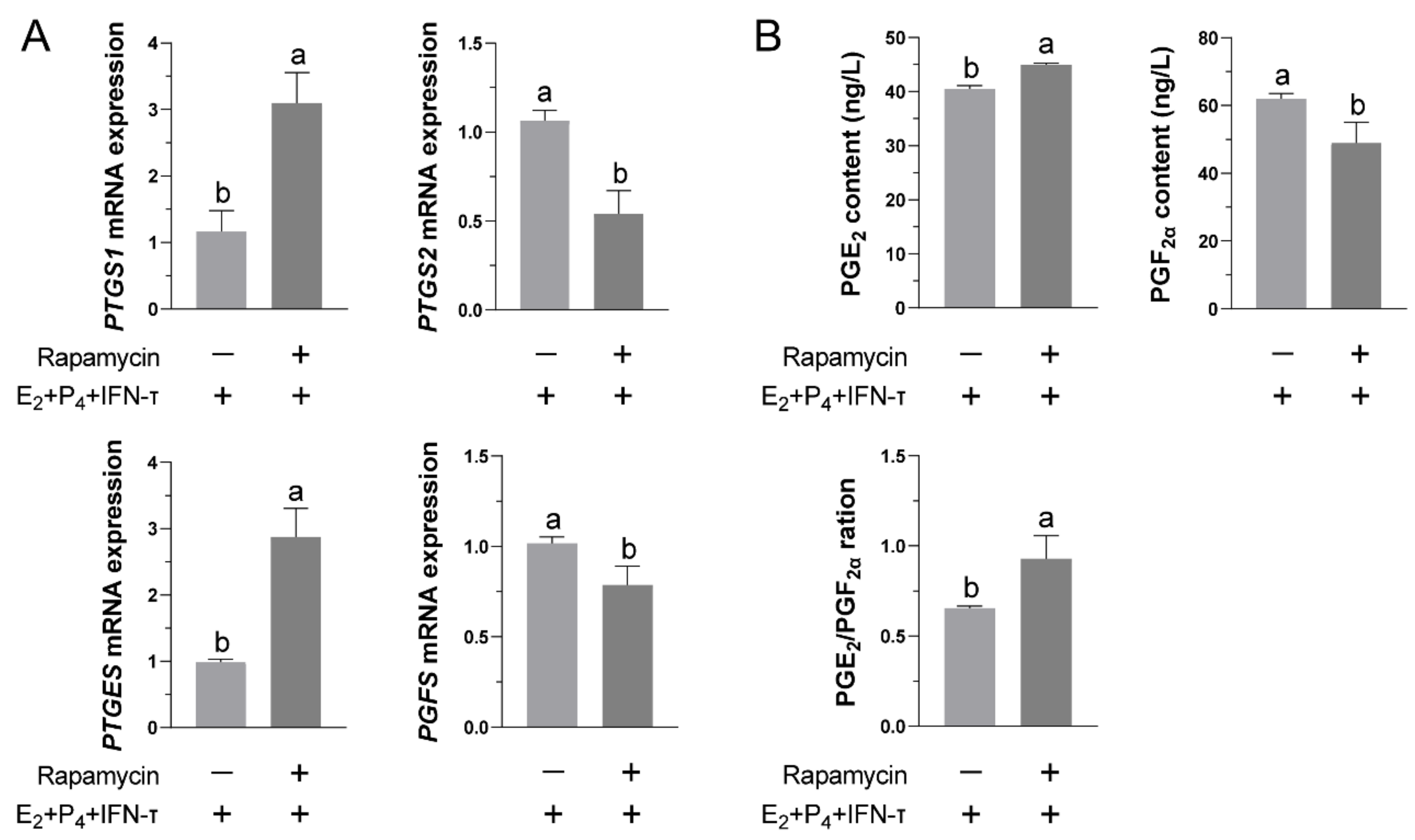
3.5. Blockade of the mTOR Pathway Partially Inhibited the Normal Functional Release of Prostaglandins
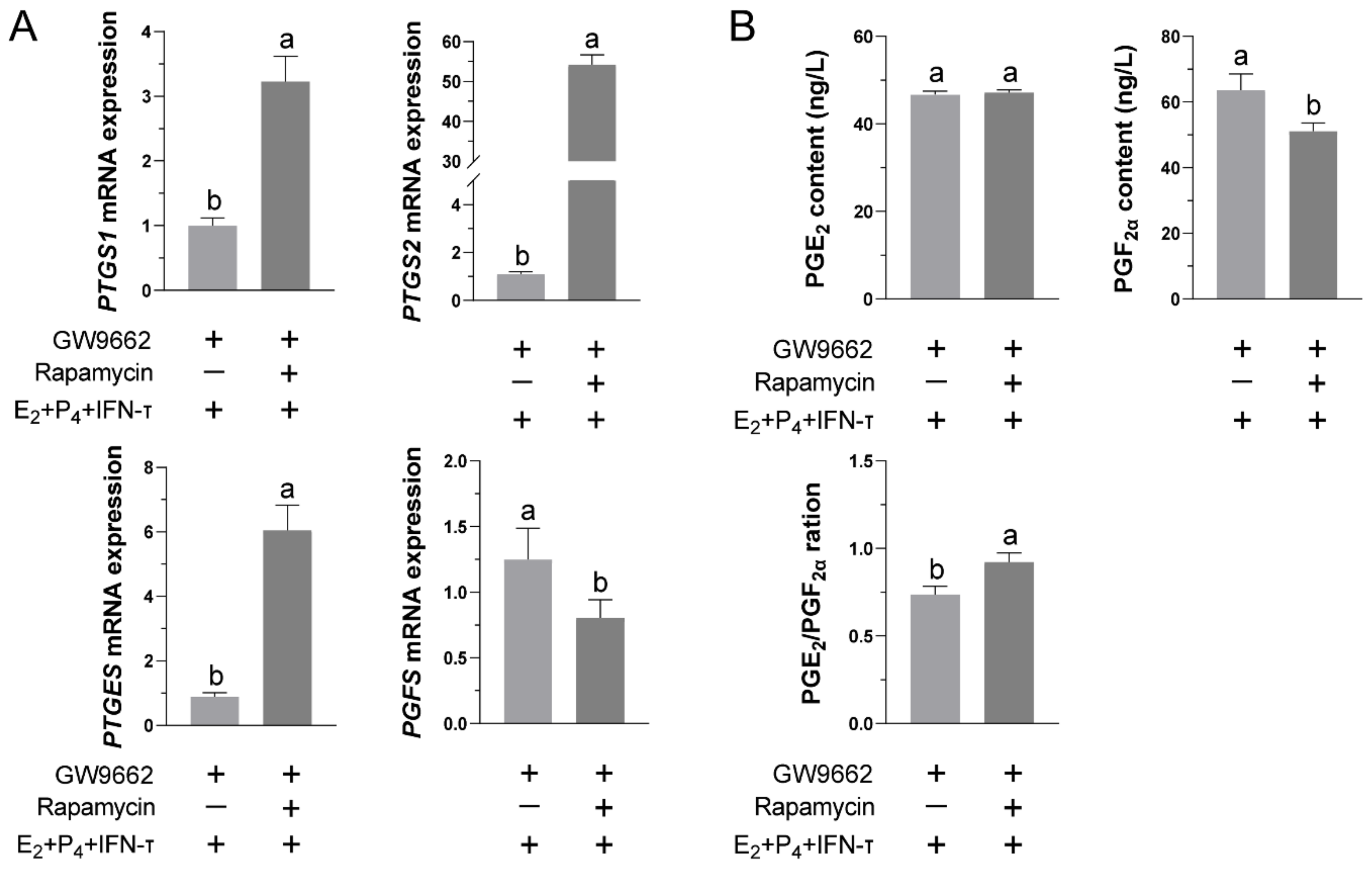
3.6. Blocking the mTOR Pathway Can Restore the Functional Release of PGs in STCs after Inhibiting PPARγ Activity
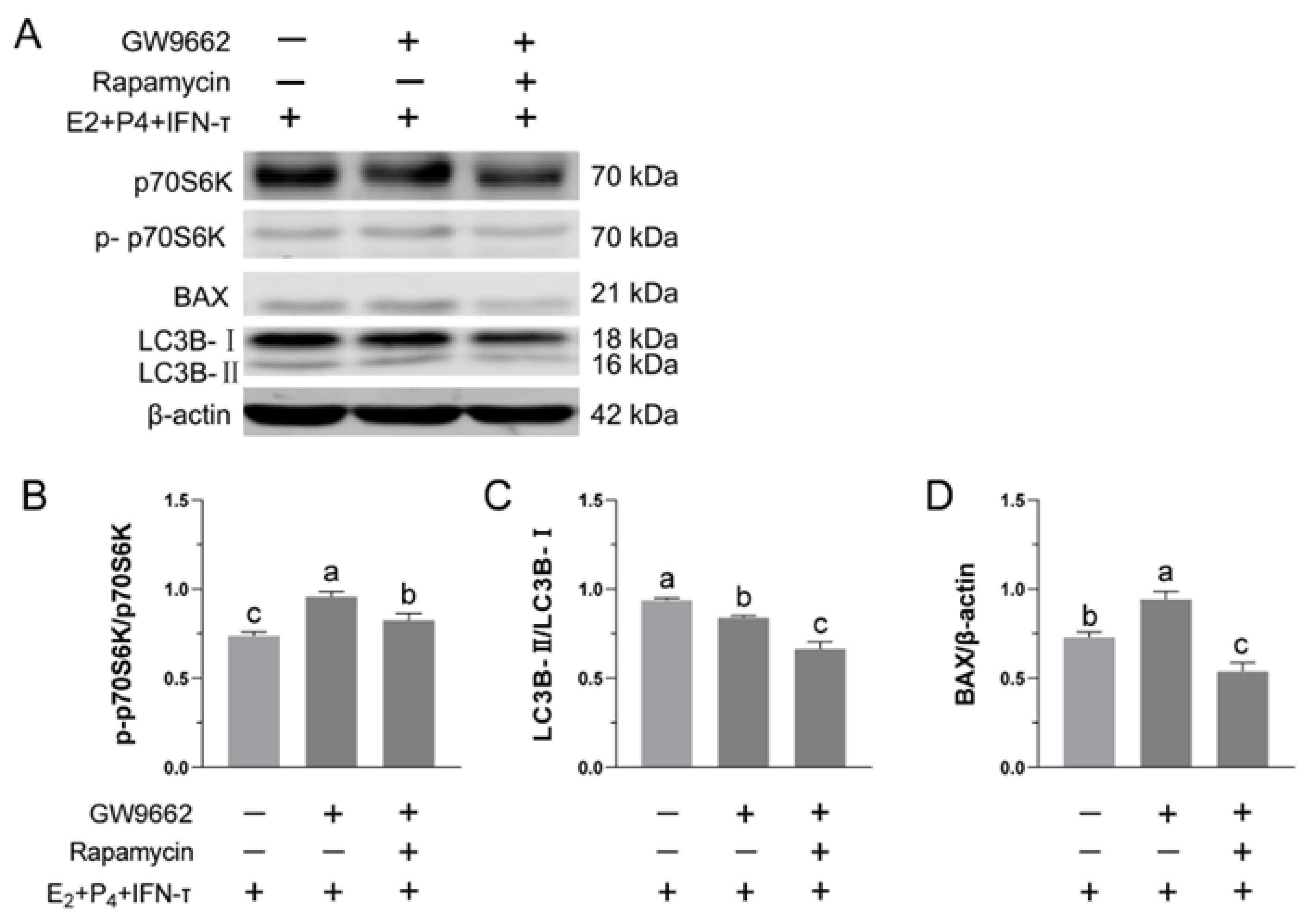
3.7. Blockade of the mTOR Pathway Suppressed GW9662-Induced Inhibition of STCs’ Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padmanabhan, R.A.; Laloraya, M. Estrogen-Initiated Protein Interactomes During Embryo Implantation. Am. J. Reprod. Immunol. 2016, 75, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C. Implantation mechanisms: Insights from the sheep. Reproduction 2004, 128, 657–668. [Google Scholar] [CrossRef]
- Boruszewska, D.; Kowalczyk-Zieba, I.; Sinderewicz, E.; Grycmacher, K.; Staszkiewicz, J.; Woclawek-Potocka, I. The effect of lysophosphatidic acid together with interferon tau on the global transcriptomic profile in bovine endometrial cells. Theriogenology 2017, 92, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Lonergan, P. Interferon-tau and fertility in ruminants. Reproduction 2017, 154, F33–F43. [Google Scholar] [CrossRef] [PubMed]
- Guillomot, M.; Michel, C.; Gaye, P.; Charlier, N.; Trojan, J.; Martal, J. Cellular localization of an embryonic interferon, ovine trophoblastin and its mRNA in sheep embryos during early pregnancy. Biol. Cell 1990, 68, 205–211. [Google Scholar] [CrossRef]
- Lee, J.; Stanley, J.A.; McCracken, J.A.; Banu, S.K.; Arosh, J.A. Intrauterine Coadministration of ERK1/2 Inhibitor U0126 Inhibits Interferon TAU Action in the Endometrium and Restores Luteolytic PGF2alpha Pulses in Sheep1. Biol. Reprod. 2014, 91, 45–47. [Google Scholar] [CrossRef]
- Kucharski, M.; Kaczor, U.; Piórkowska, K. Genetic Variability in the of, and Genes of Sheep Breeds Raised for Different Purposes. Ann. Anim. Sci. 2019, 19, 937–954. [Google Scholar] [CrossRef]
- Abduljabbar, R.; Al-Kaabi, M.M.; Negm, O.H.; Jerjees, D.; Muftah, A.A.; Mukherjee, A.; Lai, C.F.; Buluwela, L.; Ali, S.; Tighe, P.J.; et al. Prognostic and biological significance of peroxisome proliferator-activated receptor-gamma in luminal breast cancer. Breast Cancer Res. Treat. 2015, 150, 511–522. [Google Scholar] [CrossRef]
- Levytska, K.; Drewlo, S.; Baczyk, D.; Kingdom, J. PPAR-γ Regulates Trophoblast Differentiation in the BeWo Cell Model. PPAR Res. 2014, 2014, 637251. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Greco, L.F.; Bisinotto, R.S.; Lima, F.S.; Thatcher, W.W.; Santos, J.E. Biology of Preimplantation Conceptus at the Onset of Elongation in Dairy Cows1. Biol. Reprod. 2016, 94, 17–18. [Google Scholar] [CrossRef]
- Cammas, L.; Reinaud, P.; Bordas, N.; Dubois, O.; Germain, G.; Charpigny, G. Developmental regulation of prostacyclin synthase and prostacyclin receptors in the ovine uterus and conceptus during the peri-implantation period. Reproduction 2006, 131, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.E.; Burns, G.W.; Spencer, T.E. Peroxisome Proliferator Activator Receptor Gamma (PPARG) Regulates Conceptus Elongation in Sheep1. Biol. Reprod. 2015, 92, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Yoshimori, T.; Tooze, S.A. The autophagosome: Origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-S.; Kim, G.J. The role of autophagy in the placenta as a regulator of cell death. Clin. Exp. Reprod. Med. 2014, 41, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, J.J.; He, J.L.; Liu, X.Q.; Chen, X.M.; Ding, Y.B.; Tong, C.; Peng, C.; Geng, Y.Q.; Wang, Y.X.; et al. Endometrial autophagy is essential for embryo implantation during early pregnancy. J. Mol. Med. 2020, 98, 555–567. [Google Scholar] [CrossRef]
- Lee, J.E.; Oh, H.A.; Song, H.; Jun, J.H.; Roh, C.R.; Xie, H.; Dey, S.K.; Lim, H.J. Autophagy Regulates Embryonic Survival During Delayed Implantation. Endocrinology 2011, 152, 2067–2075. [Google Scholar] [CrossRef]
- Sobolewska, A.; Gajewska, M.; Zarzyńska, J.; Gajkowska, B.; Motyl, T. IGF-I, EGF, and sex steroids regulate autophagy in bovine mammary epithelial cells via the mTOR pathway. Eur. J. Cell Biol. 2009, 88, 117–130. [Google Scholar] [CrossRef]
- Spencer, T.E.; Forde, N.; Lonergan, P. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. Eur. J. Cell Biol. 2016, 99, 5941–5950. [Google Scholar] [CrossRef]
- Lonergan, P.; Forde, N.; Spencer, T. Role of progesterone in embryo development in cattle. Reprod. Fertil. Dev. 2015, 28, 66–74. [Google Scholar] [CrossRef]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated Fatty Acids in Male and Female Reproduction1. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef]
- Ali, A.; Stenglein, M.D.; Spencer, T.E.; Bouma, G.J.; Anthony, R.V.; Winger, Q.A. Trophectoderm-Specific Knockdown of LIN28 Decreases Expression of Genes Necessary for Cell Proliferation and Reduces Elongation of Sheep Conceptus. Int. J. Mol. Sci. 2020, 21, 2549. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jiang, T.; Liu, J.; Hong, J.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Jin, Y. Hormone regulates endometrial function via cooperation of endoplasmic reticulum stress and mTOR-autophagy. J. Cell. Physiol. 2018, 233, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, Y.; Gao, H.; Evans, A.; Zeng, S.-M. Roles of interferon-stimulated gene 15 protein in bovine embryo development. Reprod. Fertil. Dev. 2017, 29, 1209–1216. [Google Scholar] [CrossRef]
- Spencer, T.E.; Forde, N.; Lonergan, P. Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod. Fertil. Dev. 2017, 29, 84–100. [Google Scholar] [CrossRef]
- Dorniak, P.; Welsh, T.H., Jr.; Bazer, F.W.; Spencer, T.E. Cortisol and Interferon Tau Regulation of Endometrial Function and Conceptus Development in Female Sheep. Endocrinology 2013, 154, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Banu, S.K.; McCracken, J.A. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants. J. Dairy Sci. 2016, 99, 5926–5940. [Google Scholar] [CrossRef] [PubMed]
- Dorniak, P.; Bazer, F.W.; Spencer, T.E. Prostaglandins Regulate Conceptus Elongation and Mediate Effects of Interferon Tau on the Ovine Uterine Endometrium1. Biol. Reprod. 2011, 84, 1119–1127. [Google Scholar] [CrossRef]
- Demmers, K.J.; Derecka, K.; Flint, A. Trophoblast interferon and pregnancy. Reproduction 2001, 121, 41–49. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Santos, J.E.P.; Thatcher, W.W. Role of lipids on elongation of the preimplantation conceptus in ruminants. Reproduction 2016, 152, R115–R126. [Google Scholar] [CrossRef]
- Wahli, W.; Braissant, O.; Desvergne, B. Peroxisome proliferator activated receptors: Transcriptional regulators of adipogenesis, lipid metabolism and more. Chem. Biol. 1995, 2, 261–266. [Google Scholar] [CrossRef]
- Blitek, A.; Szymanska, M. Peroxisome proliferator-activated receptor β/δ and γ agonists differentially affect prostaglandin E2 and cytokine synthesis and nutrient transporter expression in porcine trophoblast cells during implantation. Theriogenology 2020, 152, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.M.; Addiego, A.; Wingert, R.A. Ppargc1a Regulates Prostaglandin Signaling to Control Ciliogenesis and Renal Multiciliated Cell Fate Choice During Development. FASEB J. 2020, 34, 1–3. [Google Scholar] [CrossRef]
- Sun, H.; Kim, J.K.; Mortensen, R.; Mutyaba, L.P.; Hankenson, K.D.; Krebsbach, P.H. Osteoblast-Targeted suppression of PPARγ increases osteogenesis through activation of mTOR signaling. Stem. Cells 2013, 31, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.T.; Xie, Z.S.; Kuang, Y.J.; Liu, S.Y.; Zeng, C.; Li, P.; Liu, E.H. Discovery of a potent FKBP38 agonist that ameliorates HFD-induced hyperlipidemia via mTOR/P70S6K/SREBPs pathway. Acta Pharm. Sin. B 2021, 11, 3542–3552. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T. How to Interpret LC3 Immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef]
- Yamada, T.; Murata, D.; Adachi, Y.; Itoh, K.; Kameoka, S.; Igarashi, A.; Kato, T.; Araki, Y.; Huganir, R.L.; Dawson, T.M.; et al. Mitochondrial Stasis Reveals p62-Mediated Ubiquitination in Parkin-Independent Mitophagy and Mitigates Nonalcoholic Fatty Liver Disease. Cell Metab. 2018, 28, 588–604. [Google Scholar] [CrossRef]
- Ekizceli, G.; Inan, S.; Oktem, G.; Onur, E.; Ozbilgin, K. Assessment of mTOR pathway molecules during implantation in rats. Biotech. Histochem. 2017, 92, 450–458. [Google Scholar] [CrossRef]
- Wu, K.; Hu, Y.; Yan, K.; Qi, Y.; Zhang, C.; Zhu, D.; Liu, D.; Zhao, S. microRNA-10b confers cisplatin resistance by activating AKT/mTOR/P70S6K signaling via targeting PPARγ in esophageal cancer. J. Cell. Physiol. 2020, 235, 1247–1258. [Google Scholar] [CrossRef]
- Bell, A.; Grunder, L.; Sorisky, A. Rapamycin Inhibits Human Adipocyte Differentiation in Primary Culture. Obes. Res. 2000, 8, 249–254. [Google Scholar] [CrossRef]
- Kim, J.E.; Chen, J. Regulation of Peroxisome Proliferator–Activated Receptor-γ Activity by Mammalian Target of Rapamycin and Amino Acids in Adipogenesis. Diabetes 2004, 53, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Dell’Accio, F.; Sherwood, J. PPARγ/mTOR signalling: Striking the right balance in cartilage homeostasis. Ann. Rheum. Dis. 2015, 74, 477–478. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | GenBank | Sequence (5’–3’) | Length (bp) |
|---|---|---|---|
| PTGS1 | NM_001009476.1 | F: CATCCACTTTCTGCTGACGC R: GGATAAGGTTGGAACGCACTG | 110 |
| PTGS2 | NM_001009432.1 | F: ATCCCCAGGGCACAAATCT R: TTGAAAAGGCGACGGTTATG | 175 |
| PTGES | XM_027966307.2 | F: AGTCCTGGAGCTAATGAACGG R: TTCTTCCGCAGCCTCACTT | 117 |
| PGFS | XM_004014323.5 | F: CAGTTCTTTGTGCCATTGCC R:CTCTTTGATCCGCTTCTTGTTG | 122 |
| β-actin | U39357.2 | F: AGGTCATCACCATCGGCAAT R: CGTGTTGGCGTAGAGGTCTTT | 155 |
| ISG15 | NM_001009735.1 | F: TGAAGGTGAAGATGCTAGGGG R: GCTGGAAAGCAGGCACATT | 114 |
| PPARγ | KF727439.1 | F: CTCATAATGCCATCAGGTTCG R: CAGCAGACTCTGGGTTCAGTTG | 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, K.; Wang, J.; Li, Z.; Chen, H.; Jia, B.; Hu, G. PPARγ/mTOR Regulates the Synthesis and Release of Prostaglandins in Ovine Trophoblast Cells in Early Pregnancy. Vet. Sci. 2022, 9, 649. https://doi.org/10.3390/vetsci9110649
Hao K, Wang J, Li Z, Chen H, Jia B, Hu G. PPARγ/mTOR Regulates the Synthesis and Release of Prostaglandins in Ovine Trophoblast Cells in Early Pregnancy. Veterinary Sciences. 2022; 9(11):649. https://doi.org/10.3390/vetsci9110649
Chicago/Turabian StyleHao, Kexing, Jing Wang, Zhiyuan Li, Huihui Chen, Bin Jia, and Guangdong Hu. 2022. "PPARγ/mTOR Regulates the Synthesis and Release of Prostaglandins in Ovine Trophoblast Cells in Early Pregnancy" Veterinary Sciences 9, no. 11: 649. https://doi.org/10.3390/vetsci9110649
APA StyleHao, K., Wang, J., Li, Z., Chen, H., Jia, B., & Hu, G. (2022). PPARγ/mTOR Regulates the Synthesis and Release of Prostaglandins in Ovine Trophoblast Cells in Early Pregnancy. Veterinary Sciences, 9(11), 649. https://doi.org/10.3390/vetsci9110649





