Author Contributions
Conceptualization, D.E.; methodology, J.X., D.A. and D.E.; software, X.X. and C.W.; validation, J.X., D.A., X.J. and T.H.; formal analysis, J.X., D.A., X.J. and T.H.; investigation, X.X. and C.W.; resources, X.X., C.W., X.J. and T.H.; data curation, D.A.; writing—original draft preparation, J.X.; writing—review and editing, D.E.; visualization, X.X. and C.W.; supervision, X.J. and T.H.; project administration, D.E.; funding acquisition, D.E. All authors have read and agreed to the published version of the manuscript.
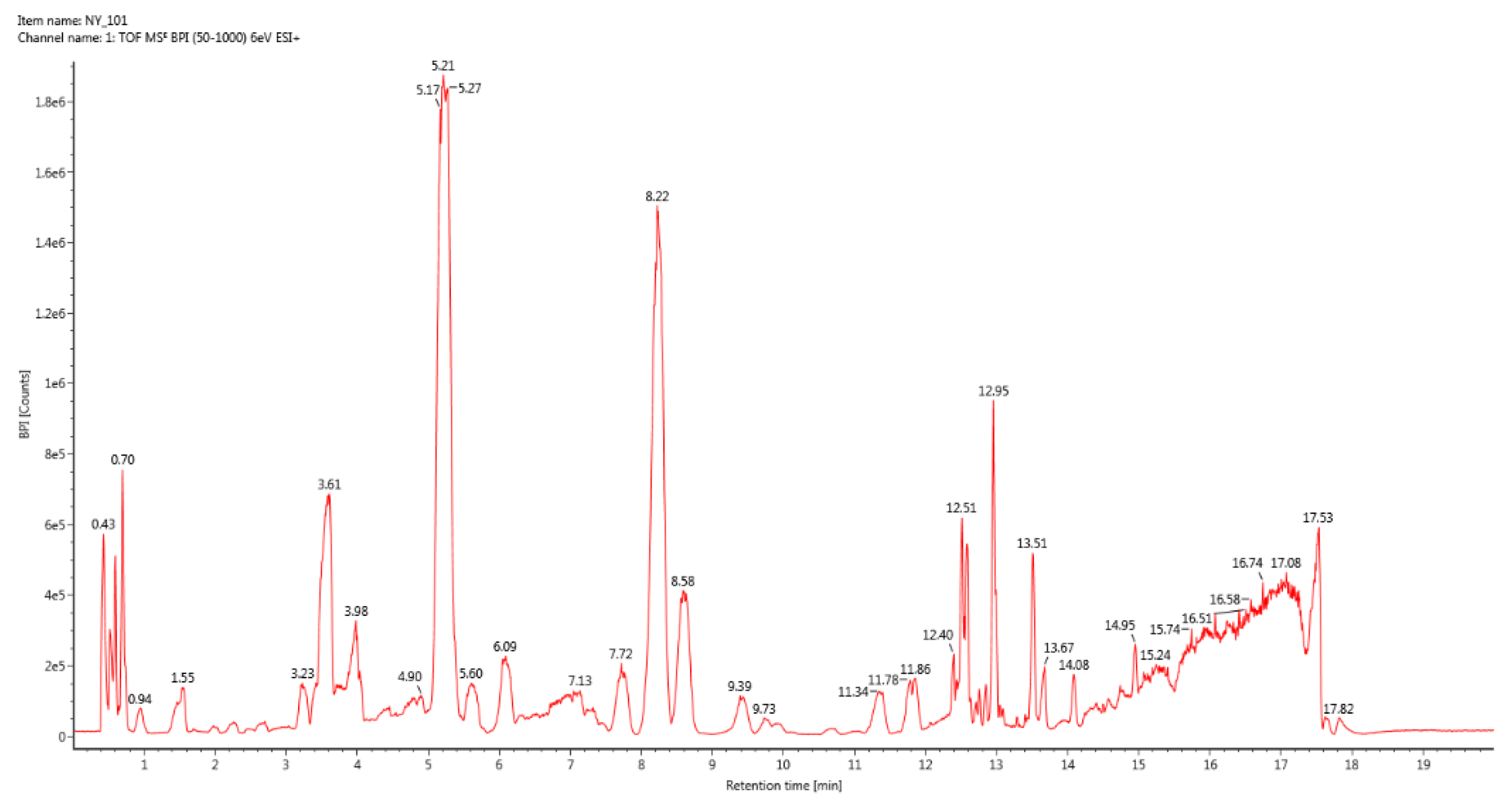
Figure 1.
BPI total ion chromatogram of group 1.
Figure 1.
BPI total ion chromatogram of group 1.
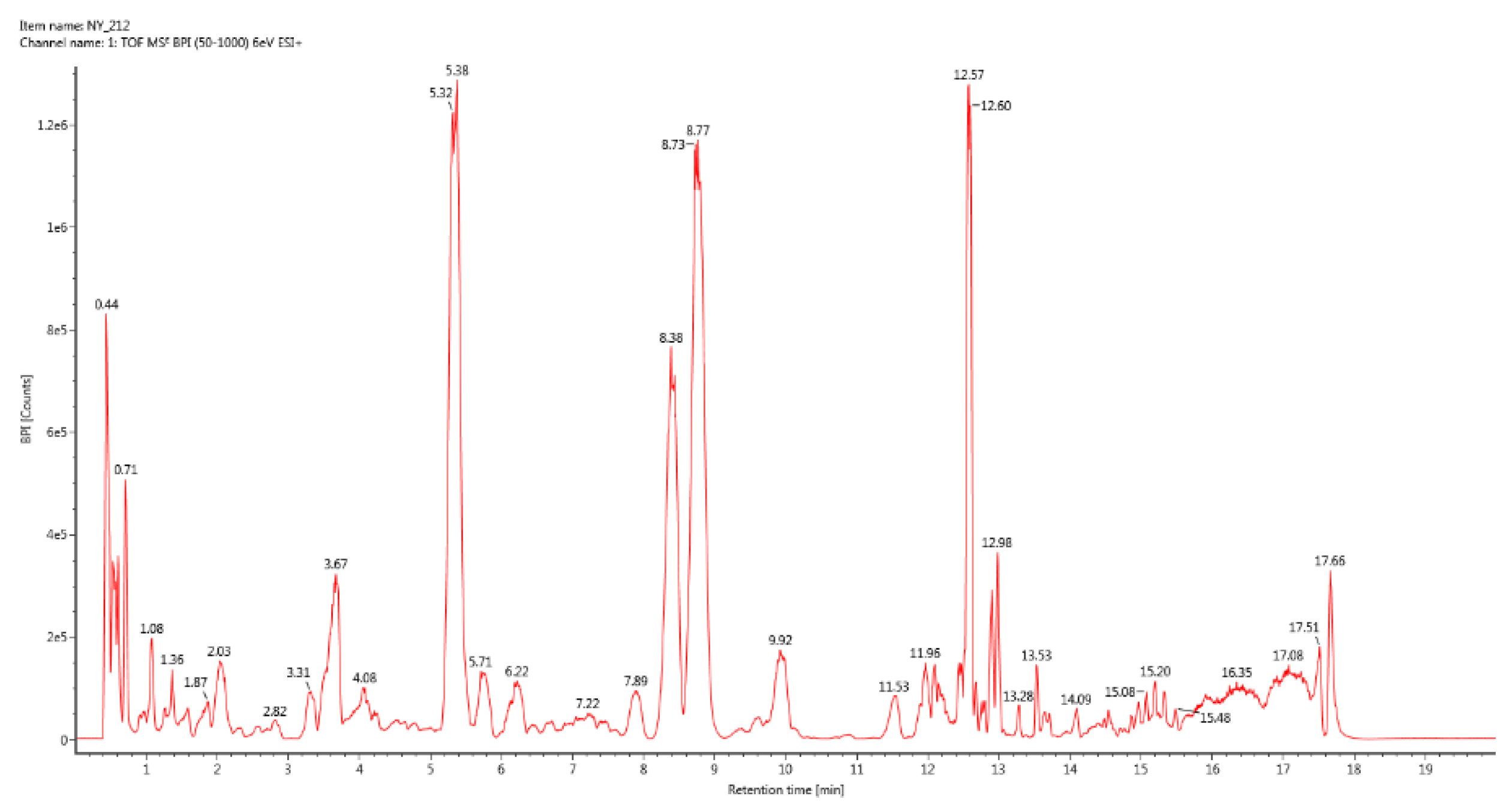
Figure 2.
BPI total ion chromatogram of group 2.
Figure 2.
BPI total ion chromatogram of group 2.
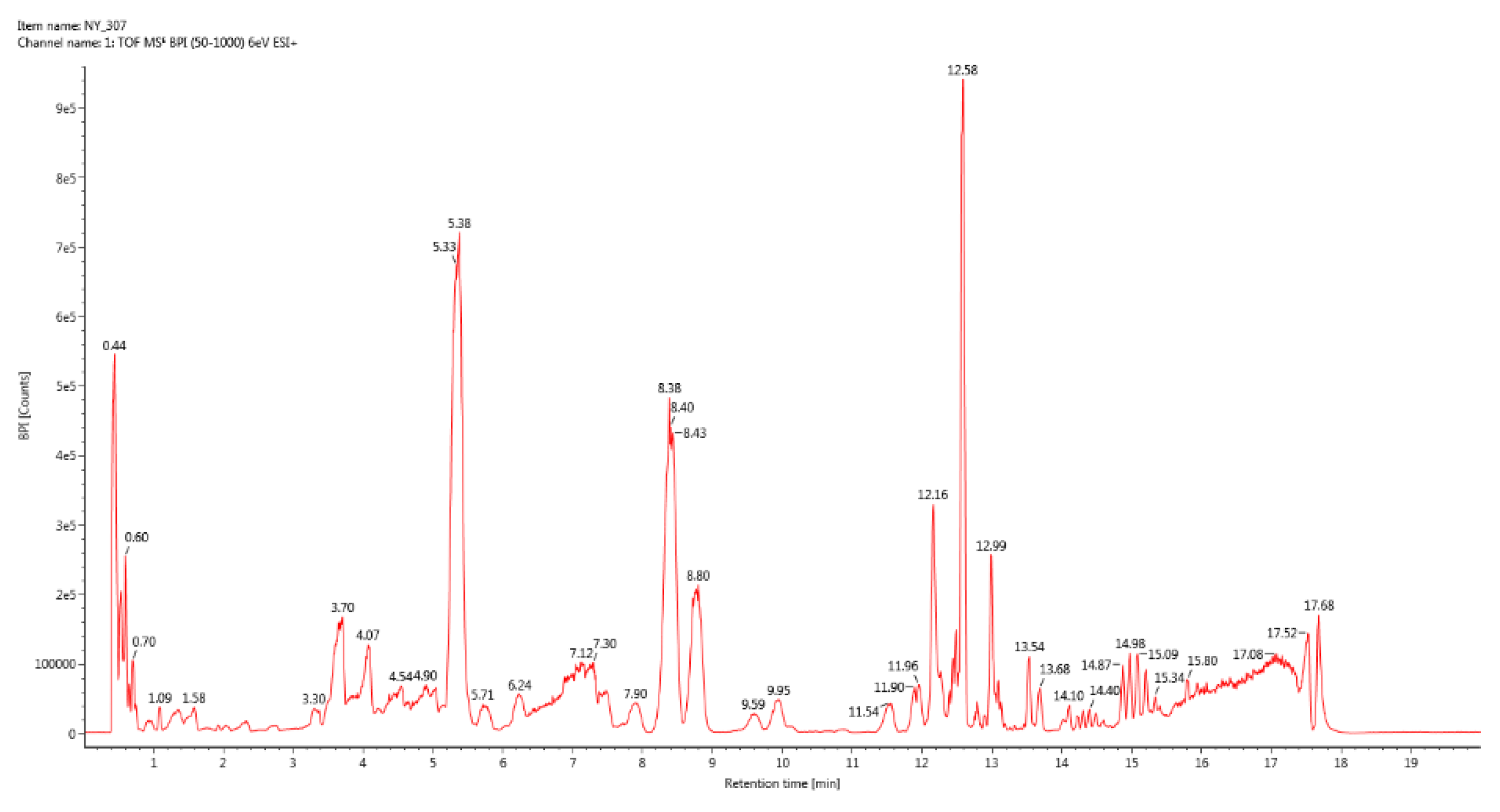
Figure 3.
BPI total ion chromatogram of group 3.
Figure 3.
BPI total ion chromatogram of group 3.
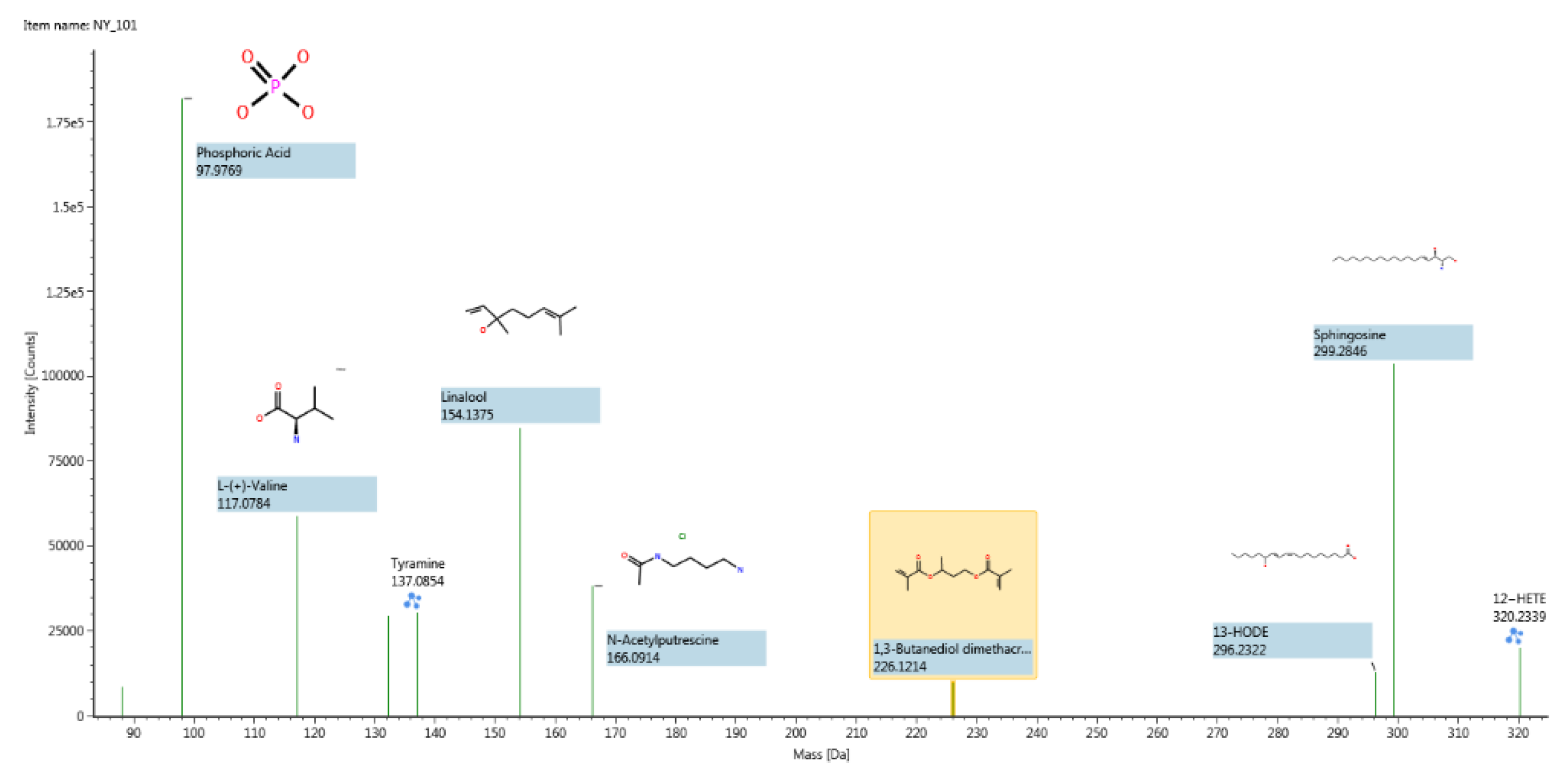
Figure 4.
Structure determination of the major compounds in positive ion mode for Bactrian camels’ vaginal secretion samples.
Figure 4.
Structure determination of the major compounds in positive ion mode for Bactrian camels’ vaginal secretion samples.
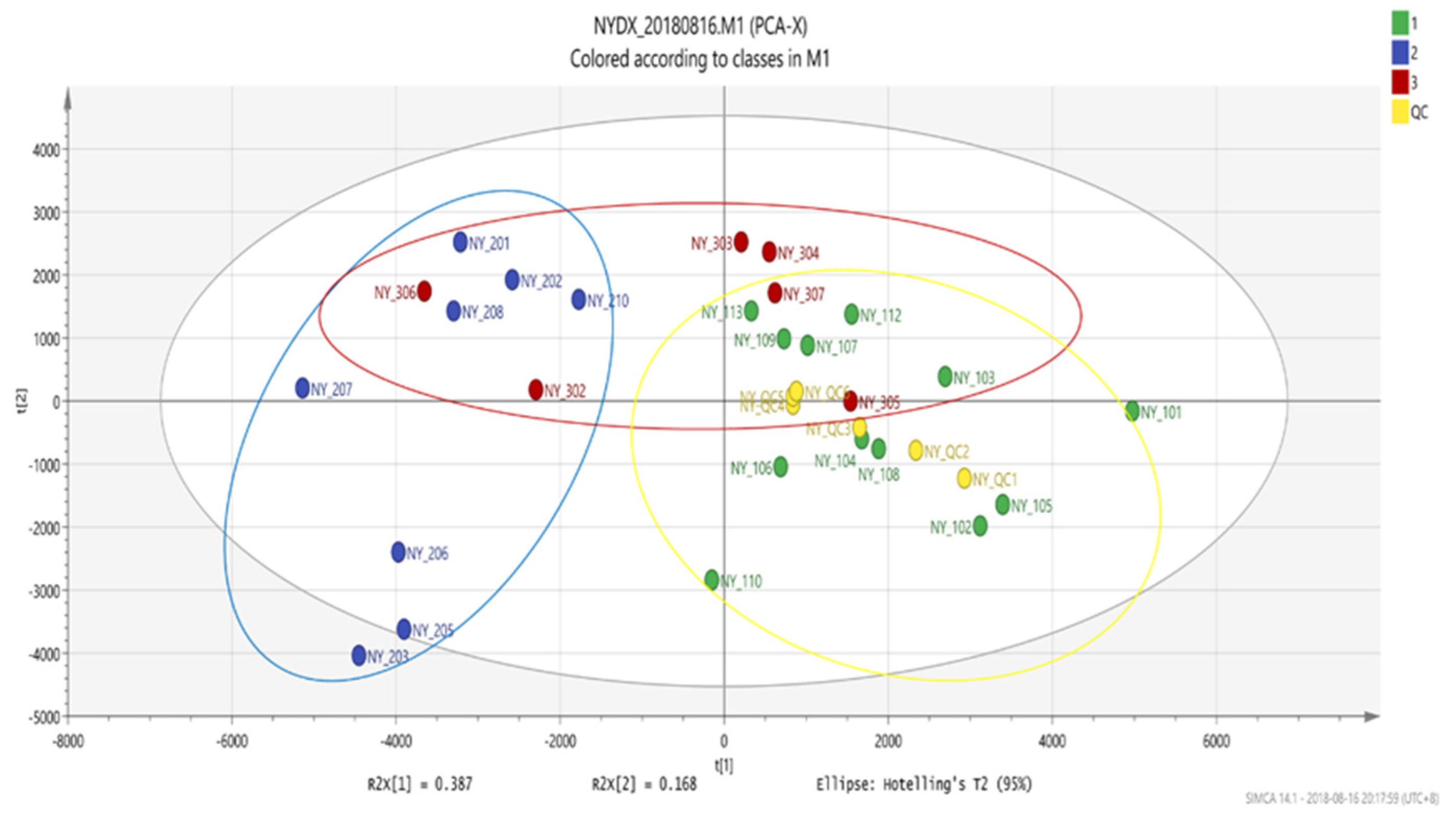
Figure 5.
Score plot of PCA.
Figure 5.
Score plot of PCA.
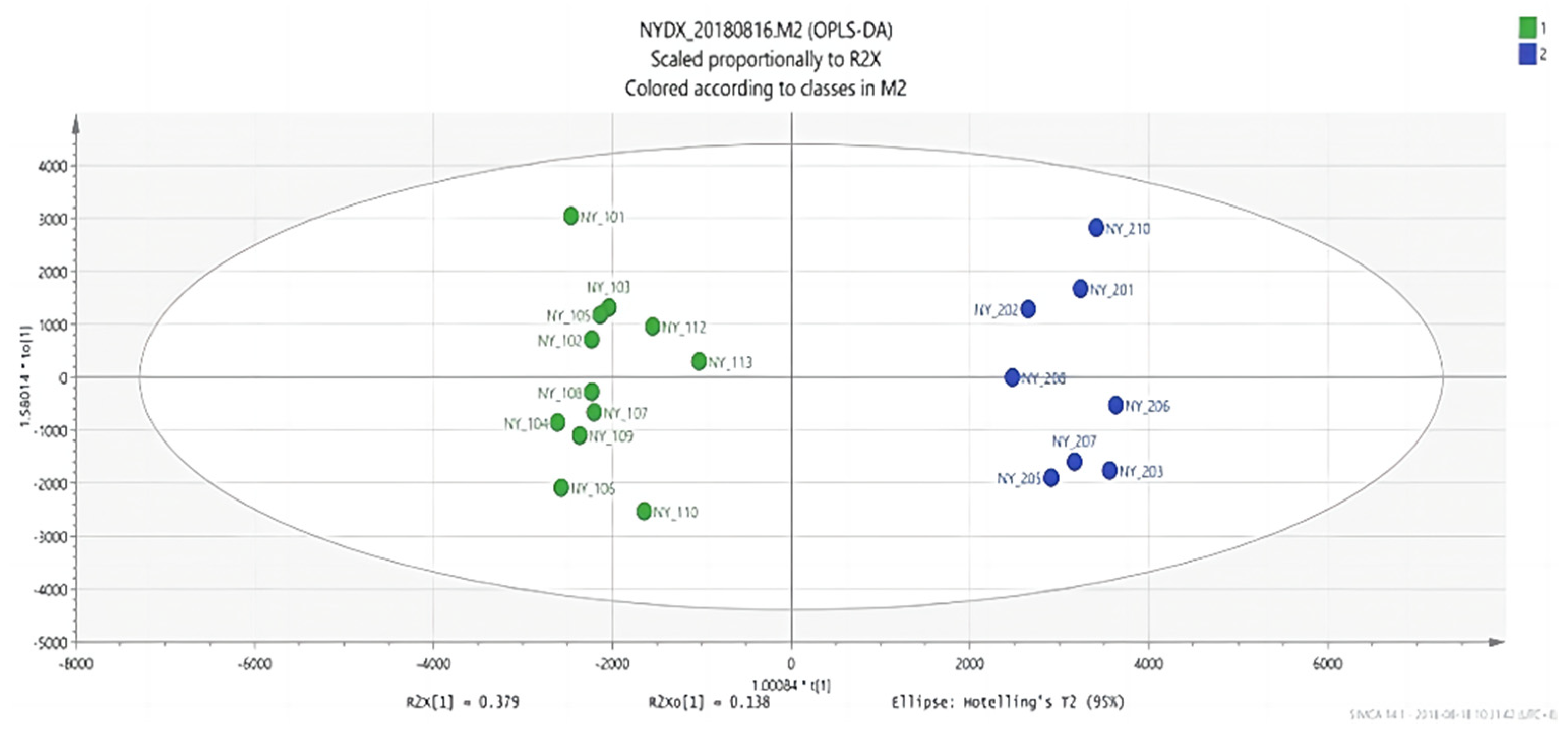
Figure 6.
OPLS-DA score plots between group 1 and group 2.
Figure 6.
OPLS-DA score plots between group 1 and group 2.
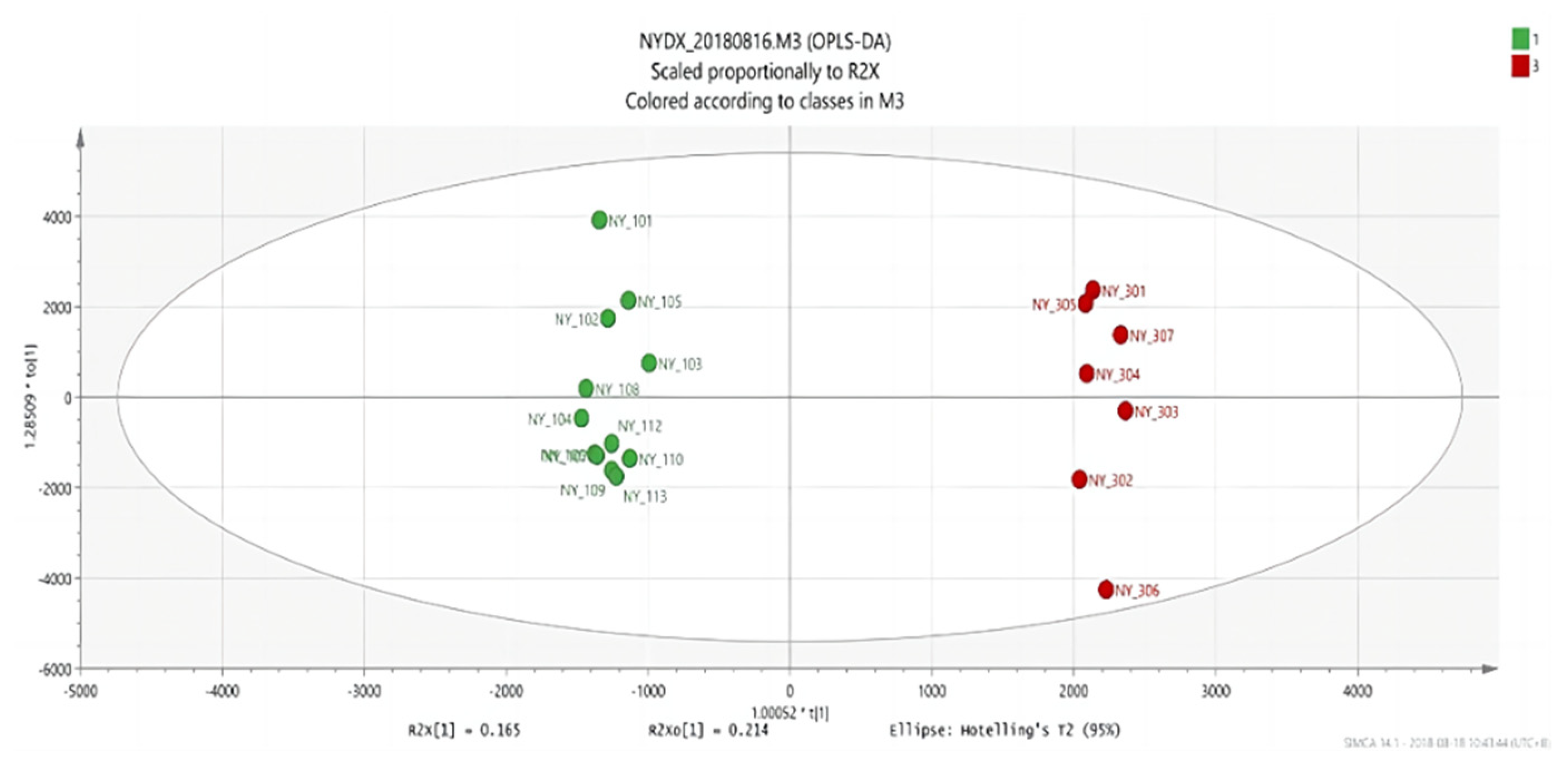
Figure 7.
OPLS-DA score plots between group 2 and group 3.
Figure 7.
OPLS-DA score plots between group 2 and group 3.
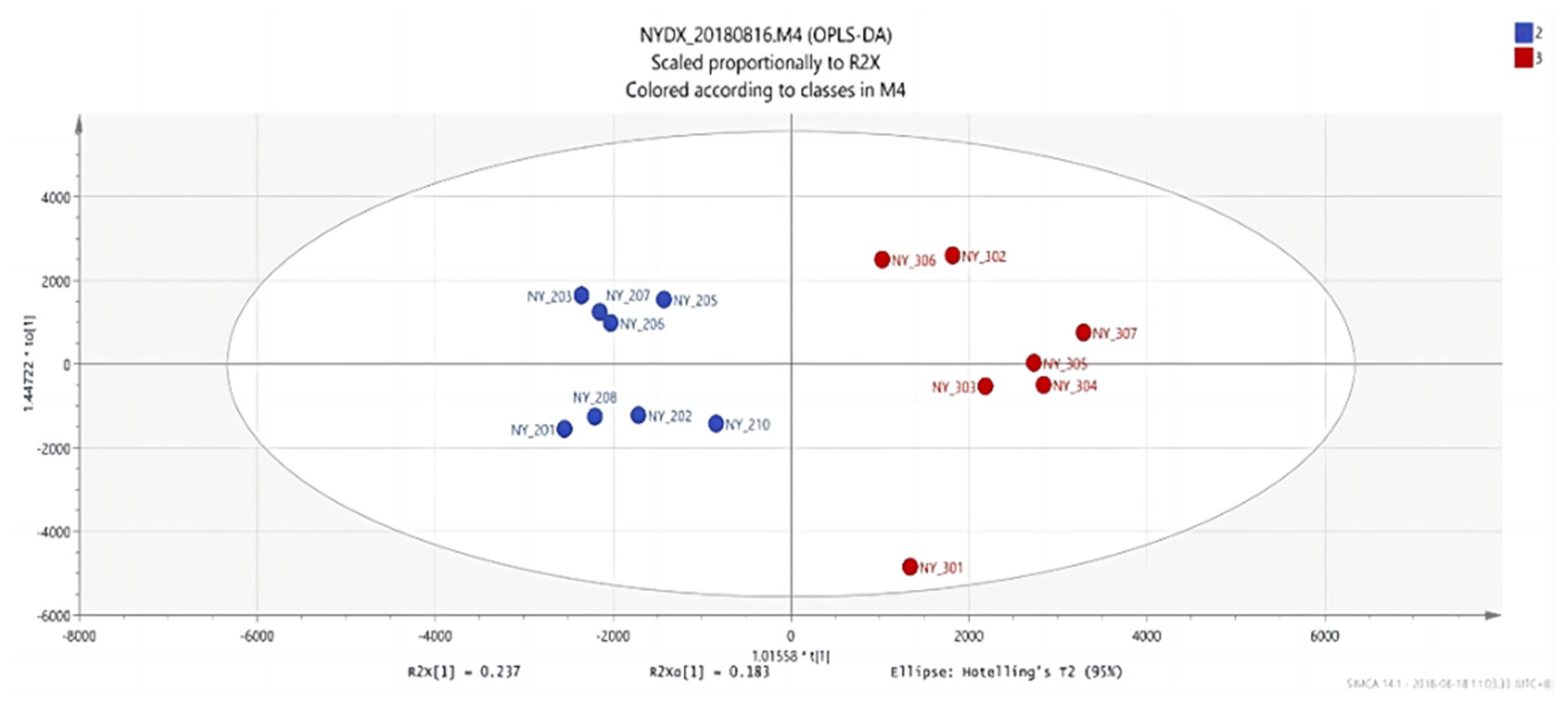
Figure 8.
OPLS-DA score plots between group 1 and group 3.
Figure 8.
OPLS-DA score plots between group 1 and group 3.
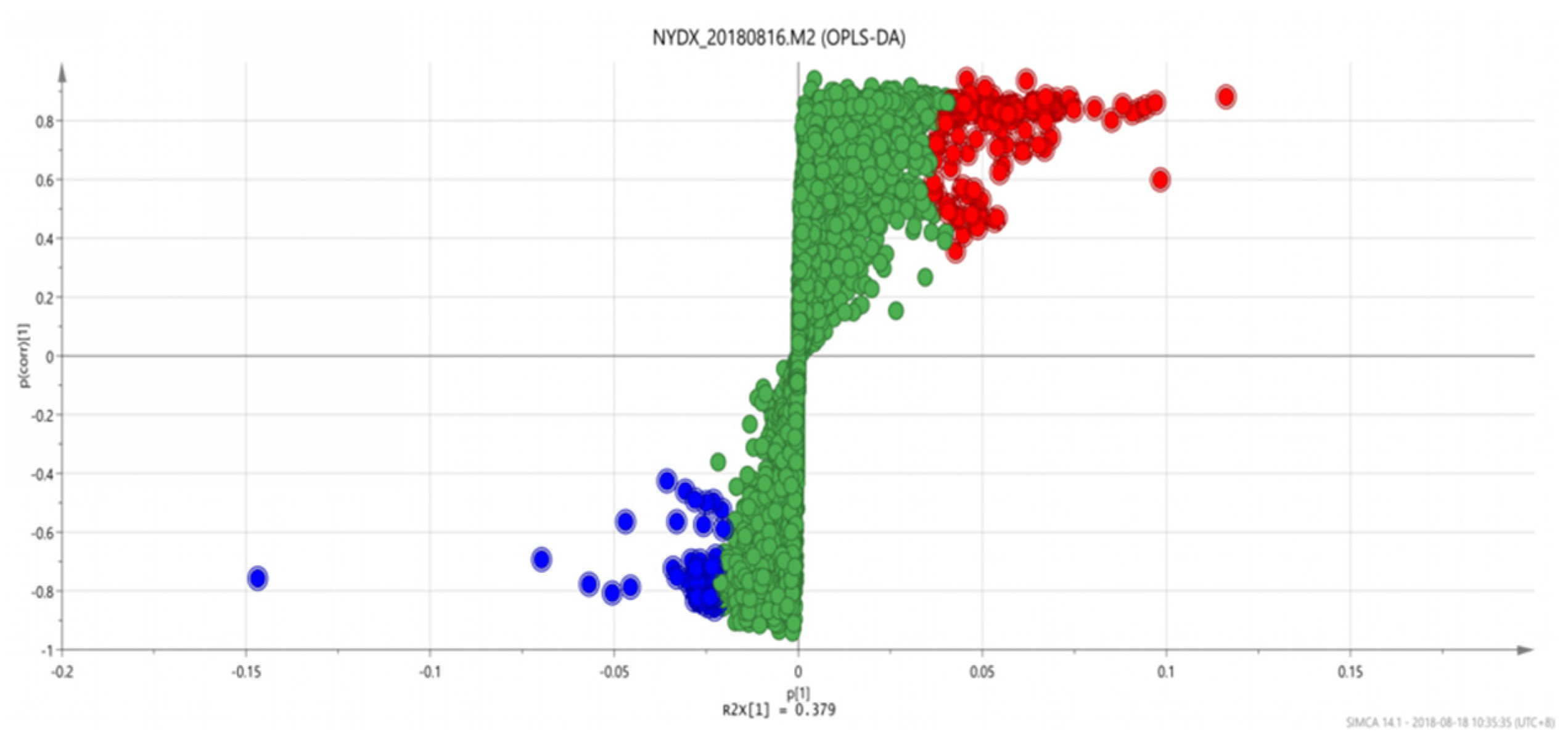
Figure 9.
OPLS-DAS-plot diagram between group 1 and group 3. Note: Different colors indicate different correlations. The same below.
Figure 9.
OPLS-DAS-plot diagram between group 1 and group 3. Note: Different colors indicate different correlations. The same below.
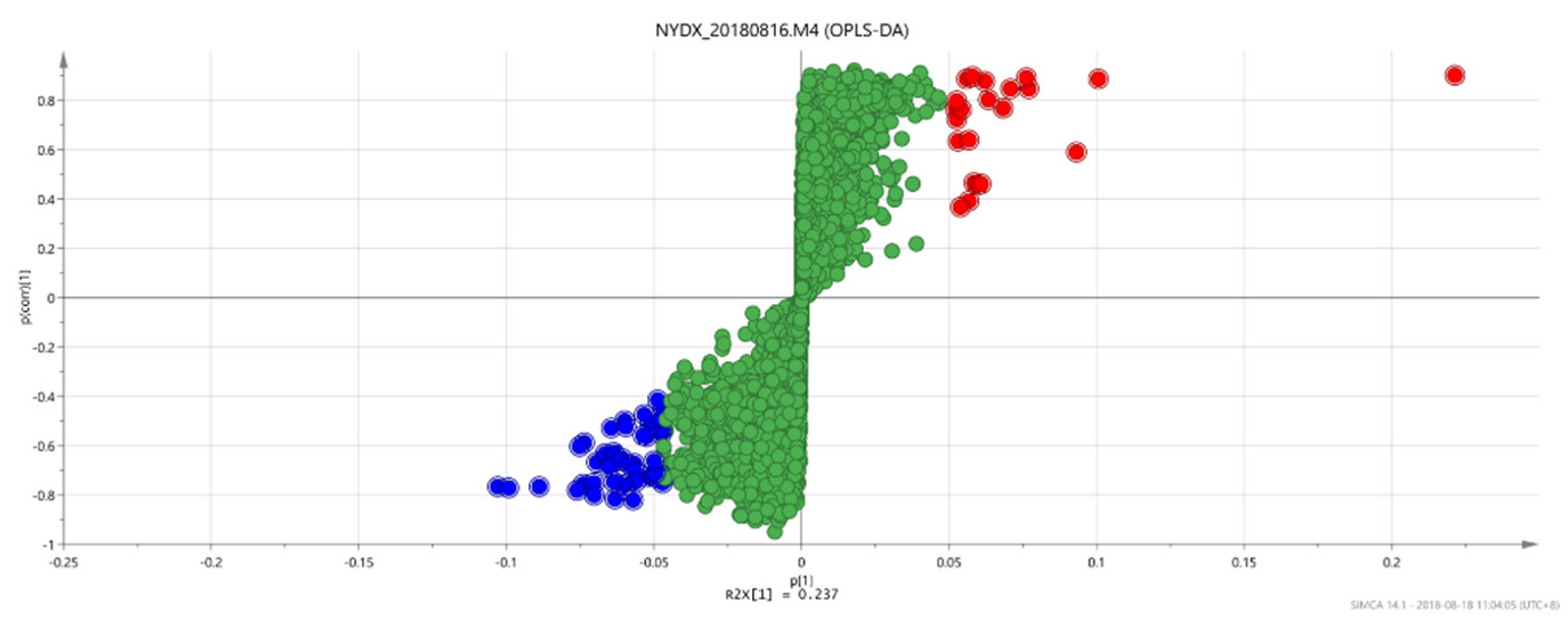
Figure 10.
OPLS-DAS-plot diagram between group 2 and group 3.
Figure 10.
OPLS-DAS-plot diagram between group 2 and group 3.
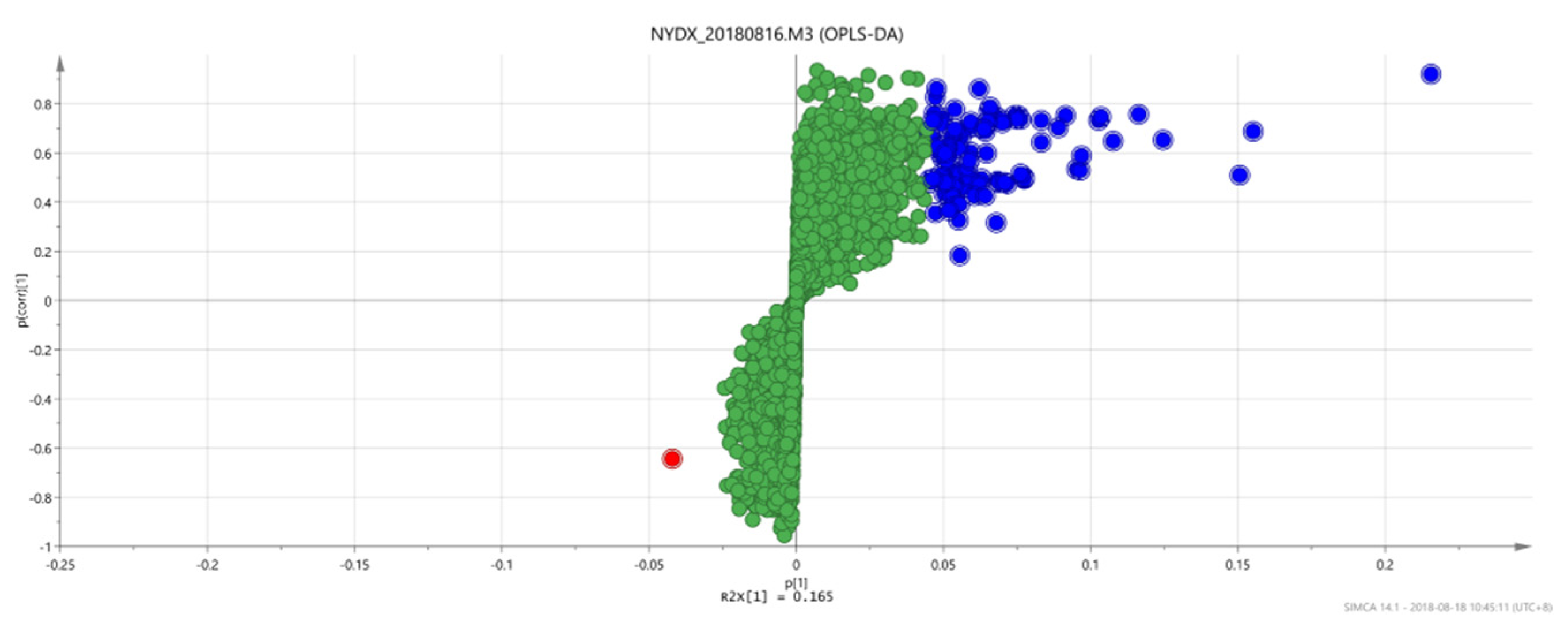
Figure 11.
OPLS-DAS-plot diagram between group 1 and group 2.
Figure 11.
OPLS-DAS-plot diagram between group 1 and group 2.
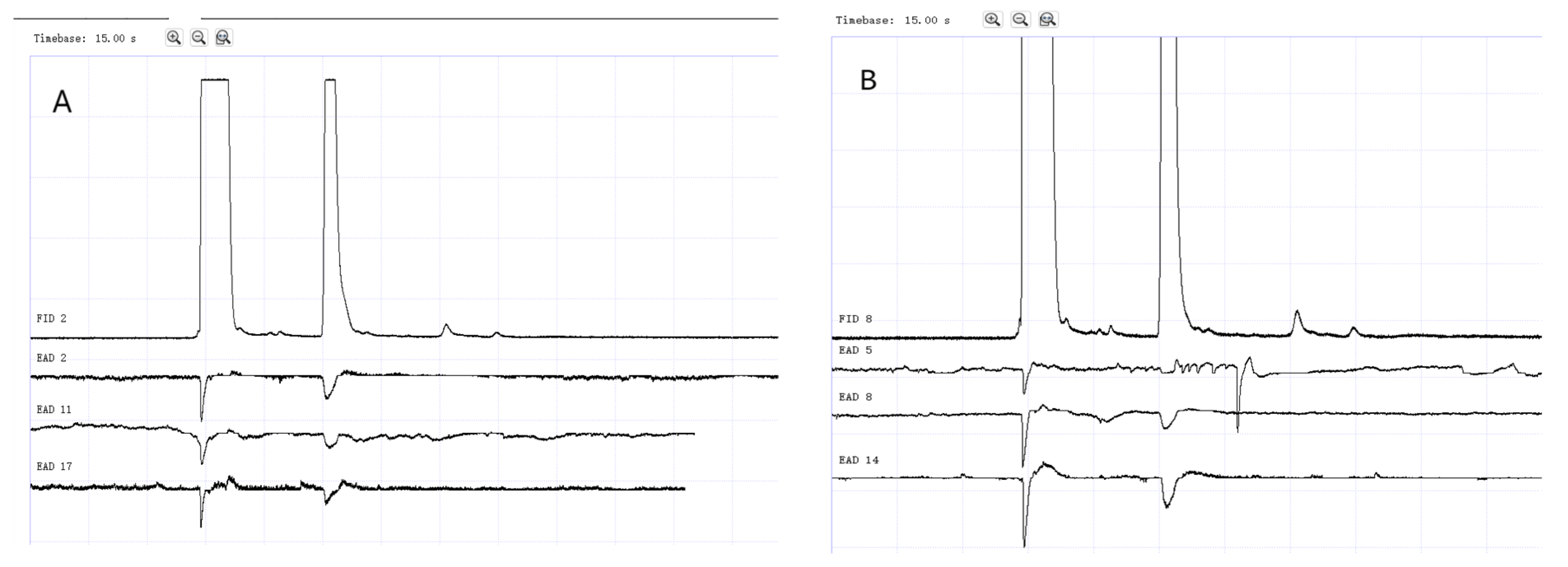
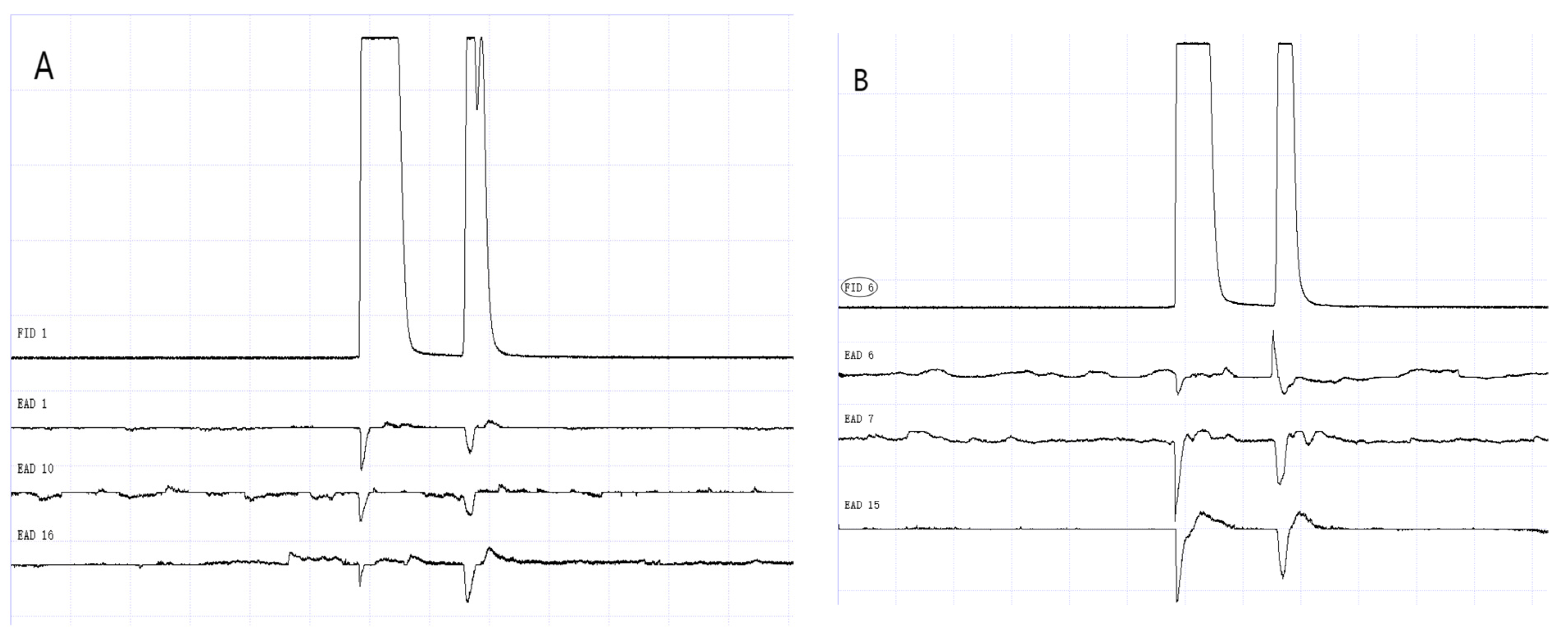
Figure 12.
GC-EAD responses of 1-day-old W. magnifica to methylheptenone. (A) Male. (B) Female.
Figure 12.
GC-EAD responses of 1-day-old W. magnifica to methylheptenone. (A) Male. (B) Female.
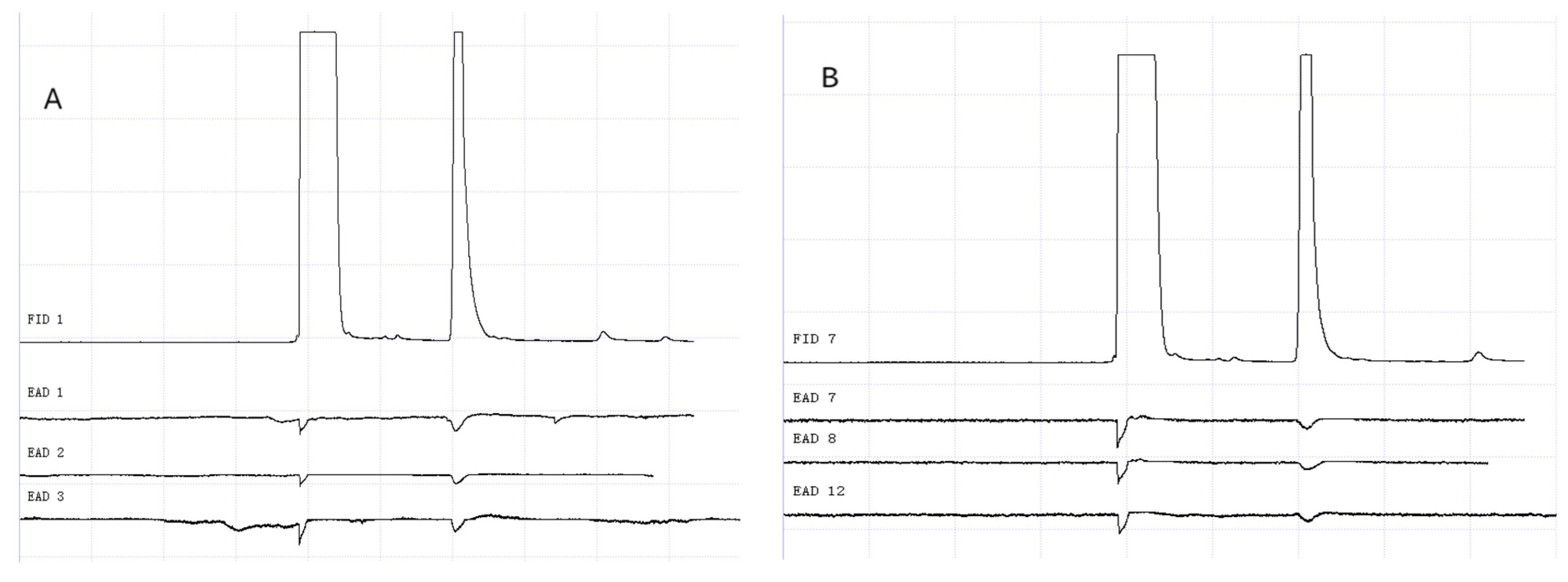
Figure 13.
GC-EAD responses of 1-day-old W. magnifica to 1-octen-3-ol. (A) Male. (B) Female.
Figure 13.
GC-EAD responses of 1-day-old W. magnifica to 1-octen-3-ol. (A) Male. (B) Female.
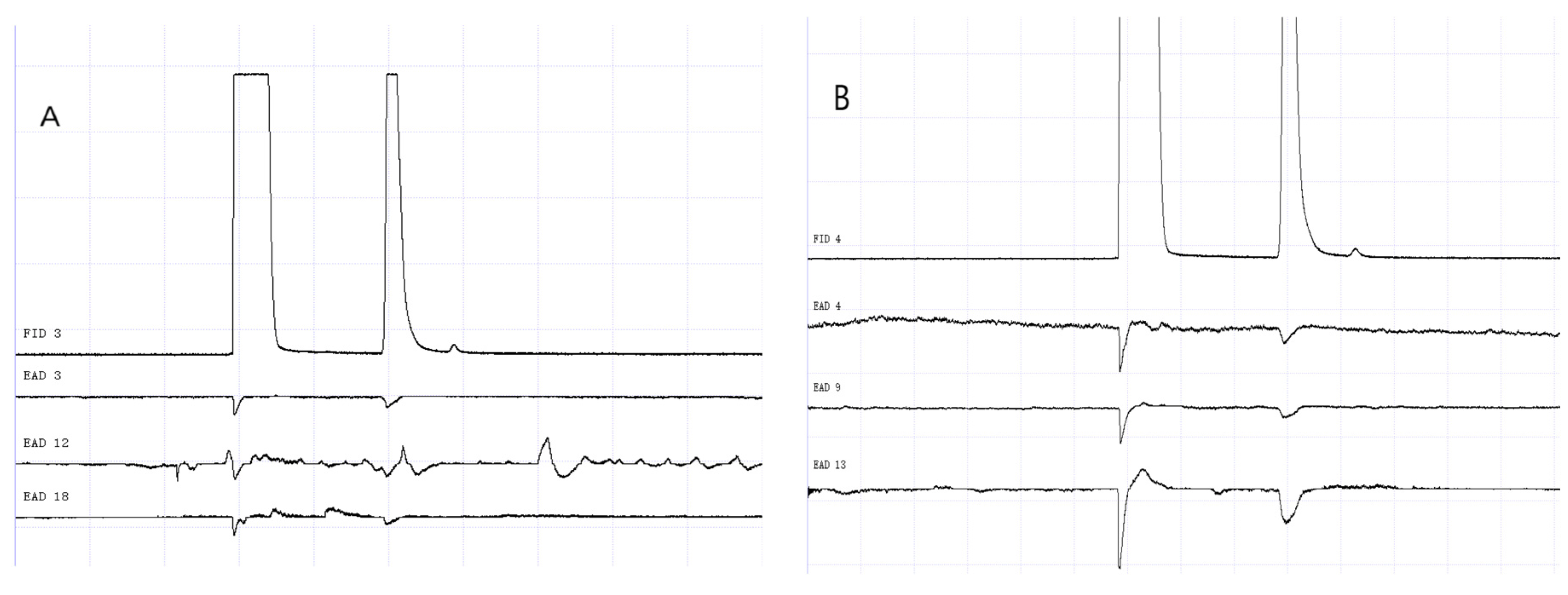
Figure 14.
GC-EAD responses of 1-day-old W. magnifica to propyl butyrate. (A) Male. (B) Female.
Figure 14.
GC-EAD responses of 1-day-old W. magnifica to propyl butyrate. (A) Male. (B) Female.
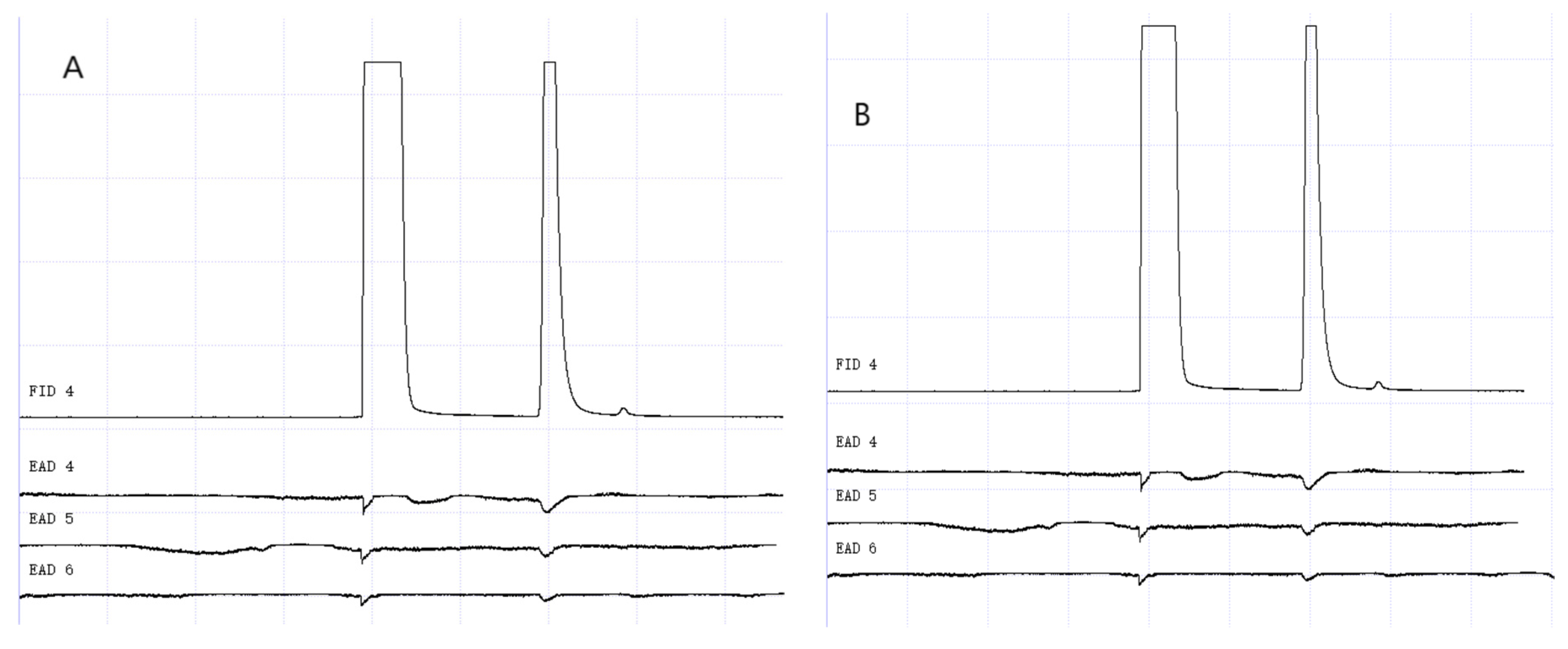
Figure 15.
GC-EAD responses of 7-day-old W. magnifica to methylheptenone. (A) Male. (B) Female.
Figure 15.
GC-EAD responses of 7-day-old W. magnifica to methylheptenone. (A) Male. (B) Female.
Figure 16.
GC-EAD responses of 7-day-old W. magnifica to 1-octen-3-ol. (A) Male. (B) Female.
Figure 16.
GC-EAD responses of 7-day-old W. magnifica to 1-octen-3-ol. (A) Male. (B) Female.
Figure 17.
GC-EAD responses of 7-day-old W. magnifica to propyl butyrate. (A) Male. (B) Female.
Figure 17.
GC-EAD responses of 7-day-old W. magnifica to propyl butyrate. (A) Male. (B) Female.
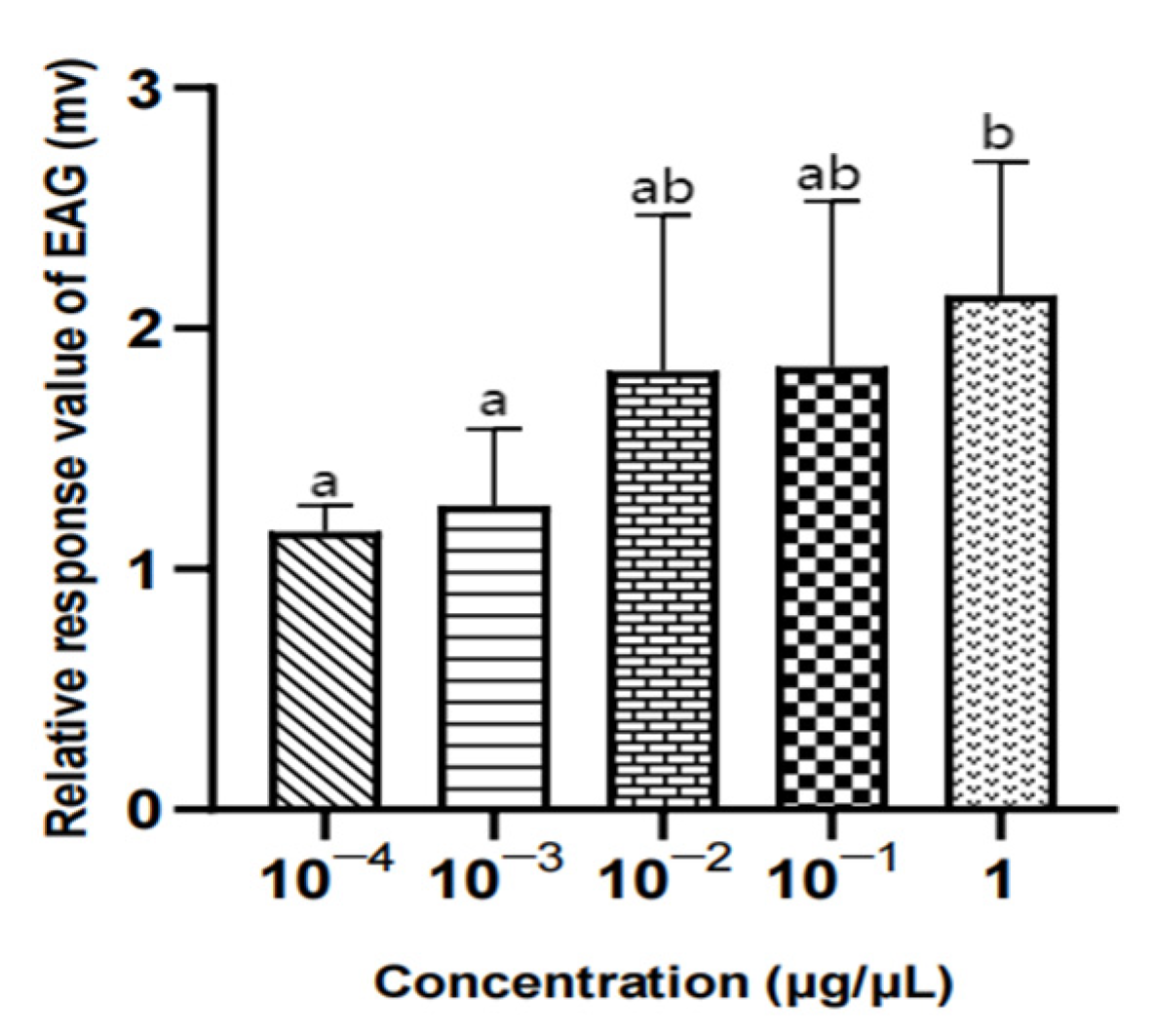
Figure 18.
EAG responses of male W. magnifica to different concentrations of methylheptenone. Note: The same letter indicates no difference (p > 0.05), but different letters indicate significant difference (p < 0.05), so a and b are both indicate no difference, ab indicates significant difference. Different column shapes represent different concentrations of samples. The same below.
Figure 18.
EAG responses of male W. magnifica to different concentrations of methylheptenone. Note: The same letter indicates no difference (p > 0.05), but different letters indicate significant difference (p < 0.05), so a and b are both indicate no difference, ab indicates significant difference. Different column shapes represent different concentrations of samples. The same below.
Figure 19.
EAG responses of male W. magnifica to different concentrations of 1-octen-3-ol.
Figure 19.
EAG responses of male W. magnifica to different concentrations of 1-octen-3-ol.
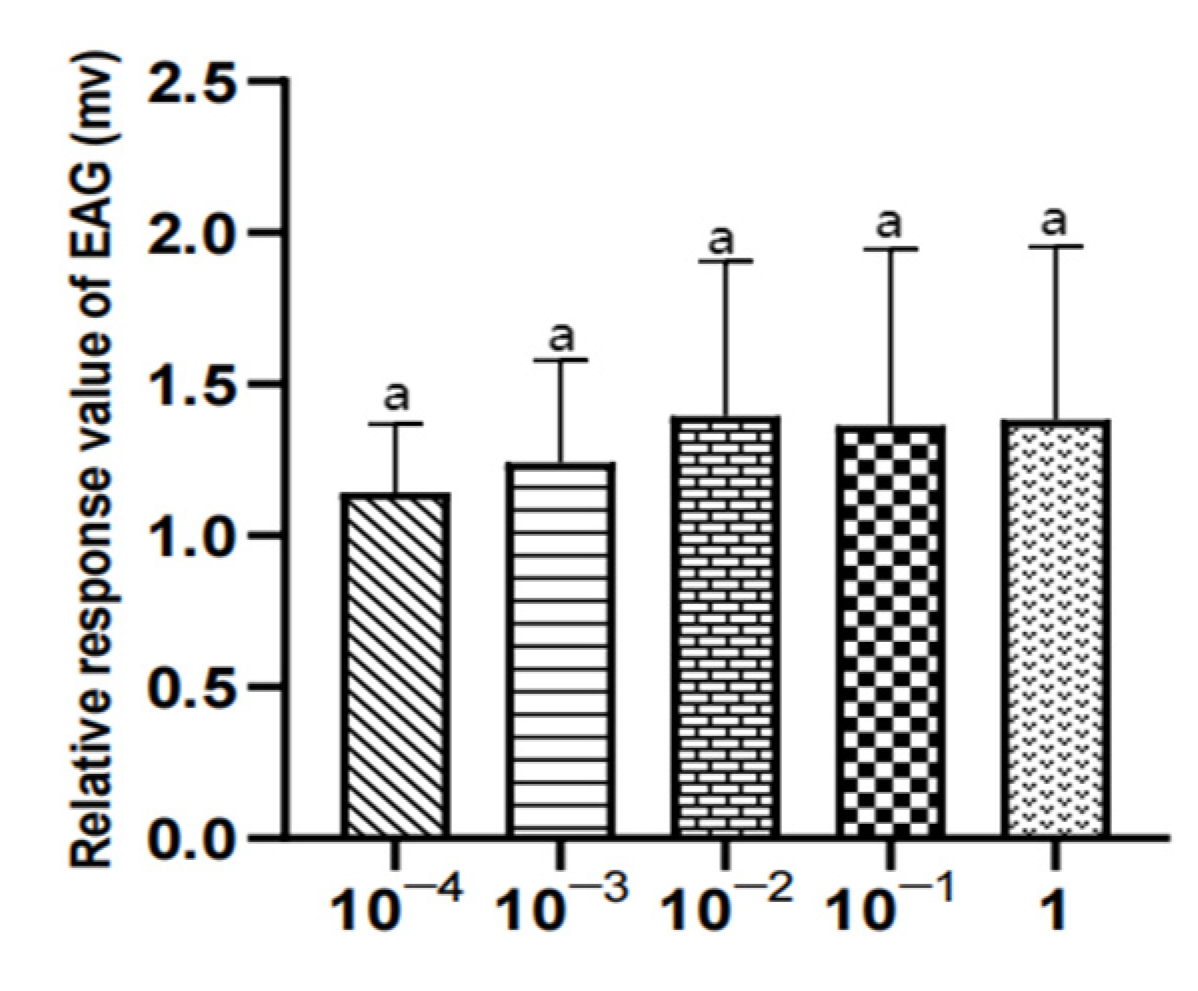
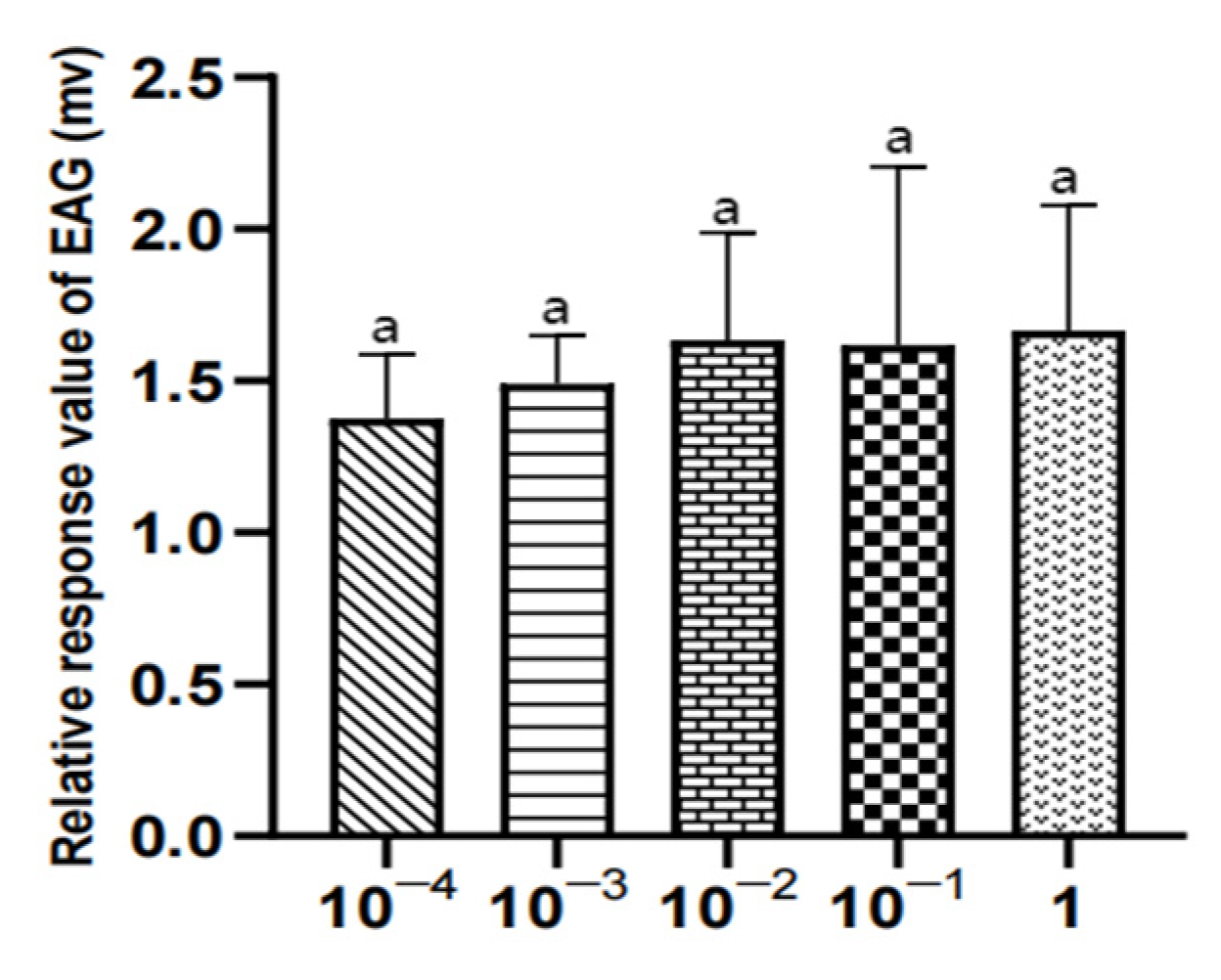
Figure 20.
EAG responses of male W. magnifica to different concentrations of propyl butyrate.
Figure 20.
EAG responses of male W. magnifica to different concentrations of propyl butyrate.
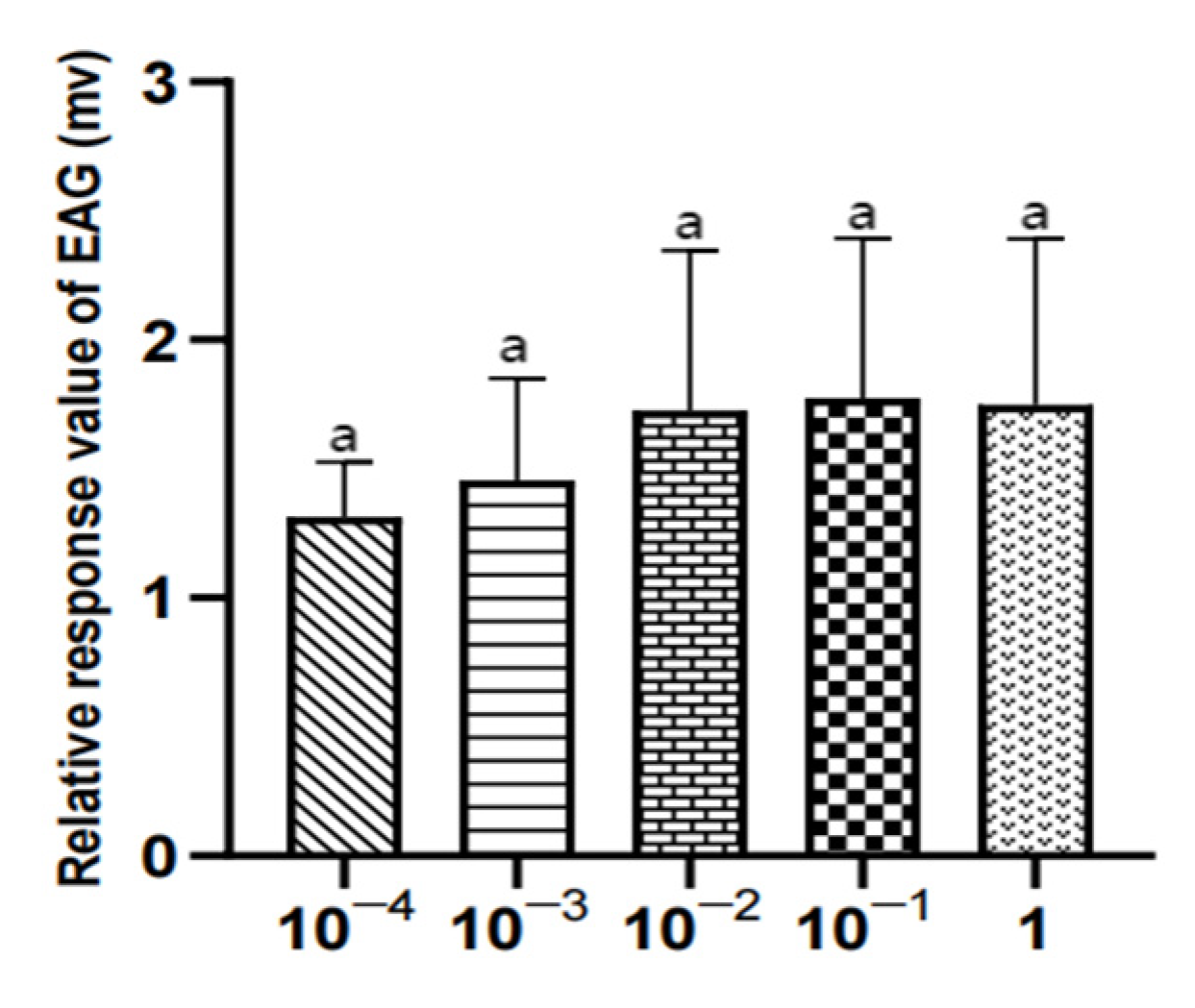
Figure 21.
EAG responses of female W. magnifica to different concentrations of methylheptenone.
Figure 21.
EAG responses of female W. magnifica to different concentrations of methylheptenone.
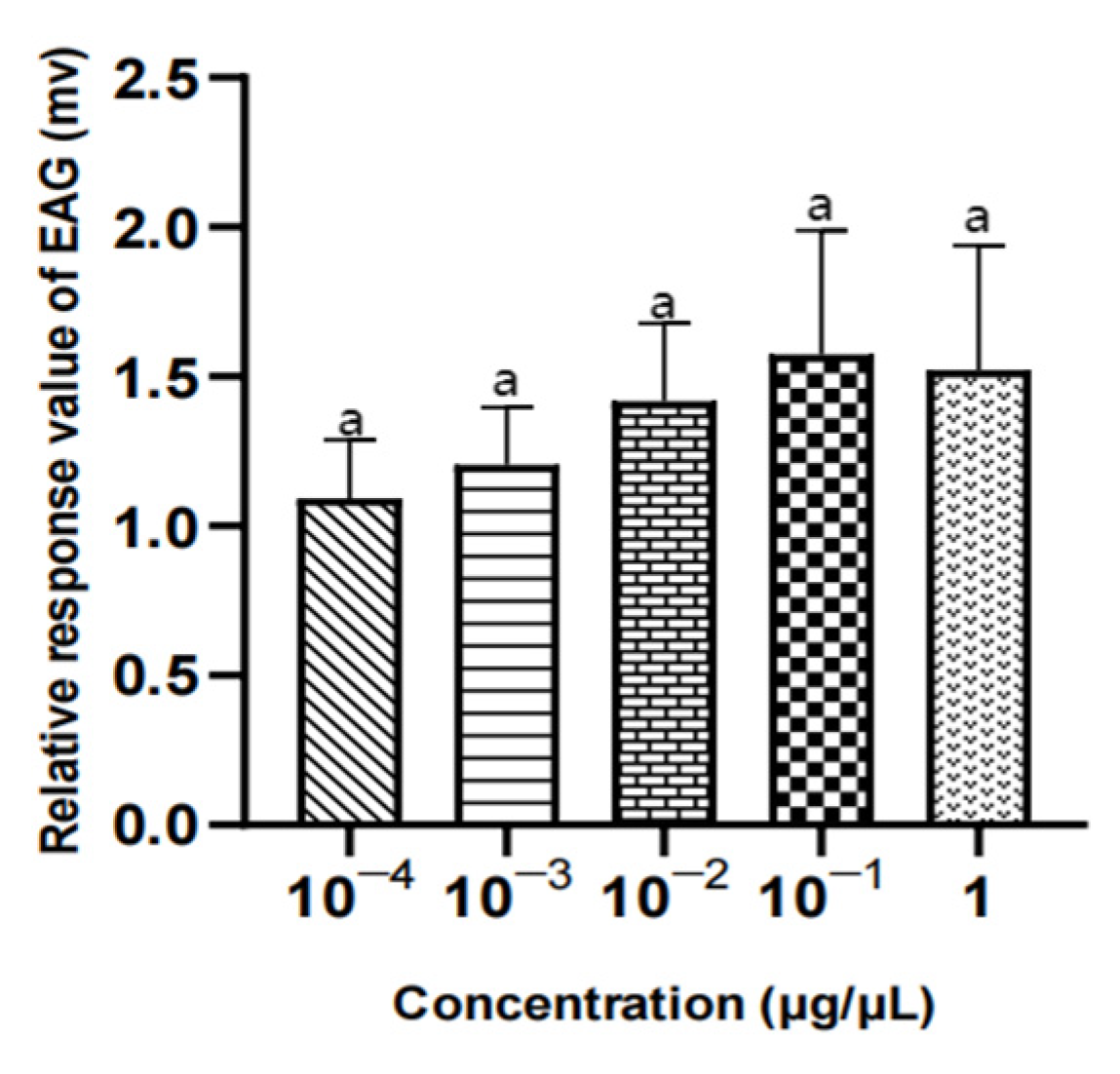
Figure 22.
EAG responses of female W. magnifica to different concentrations of 1-octen-3-ol.
Figure 22.
EAG responses of female W. magnifica to different concentrations of 1-octen-3-ol.
Figure 23.
EAG responses of female W. magnifica to different concentrations of propyl butyrate.
Figure 23.
EAG responses of female W. magnifica to different concentrations of propyl butyrate.
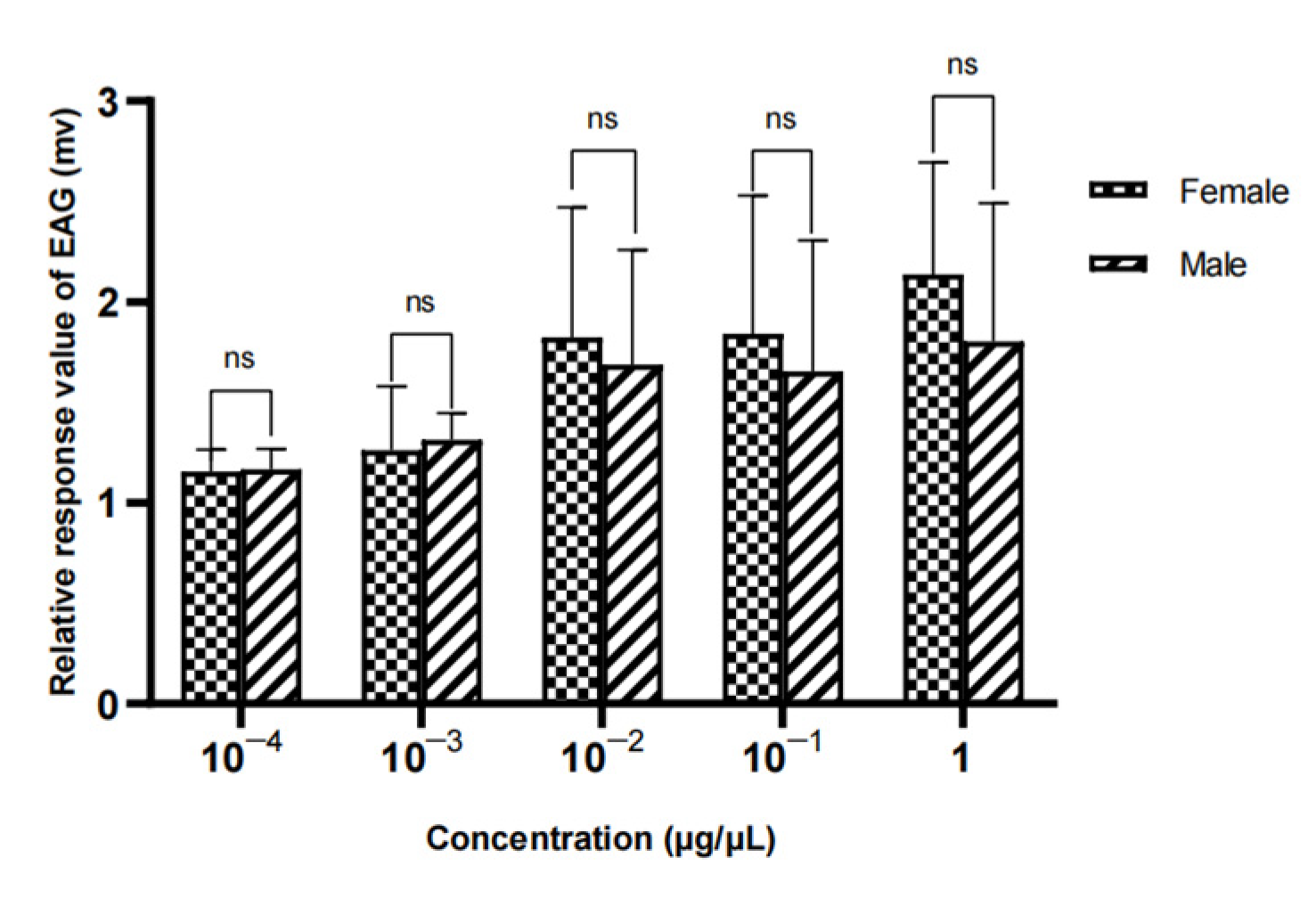
Figure 24.
Comparison of EAG responses of W. magnifica to methylheptenone.
Figure 24.
Comparison of EAG responses of W. magnifica to methylheptenone.
Figure 25.
Comparison of EAG responses of W. magnifica to 1-octen-3-ol.
Figure 25.
Comparison of EAG responses of W. magnifica to 1-octen-3-ol.
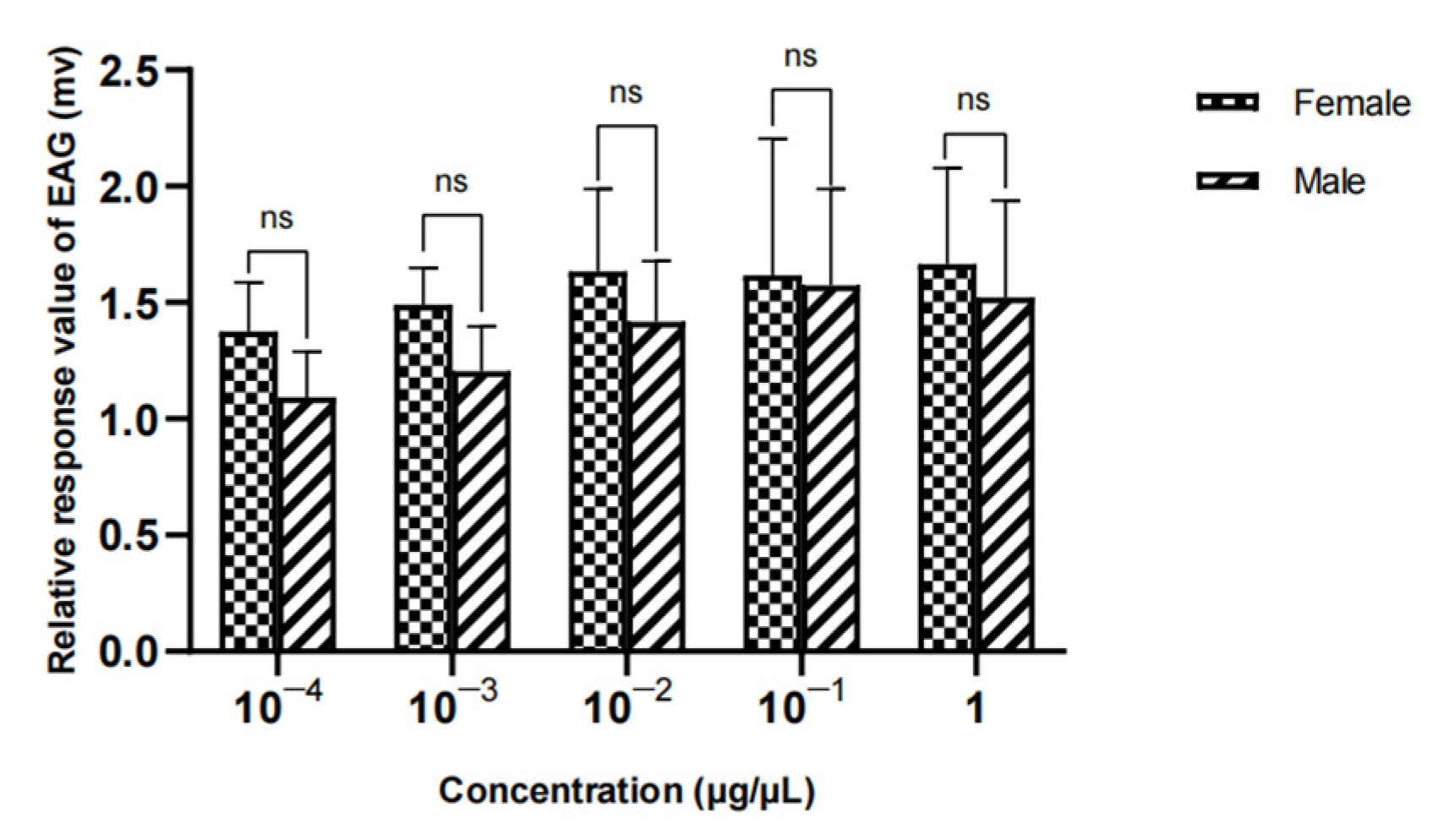
Figure 26.
Comparison of EAG responses of W. magnifica to propyl butyrate.
Figure 26.
Comparison of EAG responses of W. magnifica to propyl butyrate.
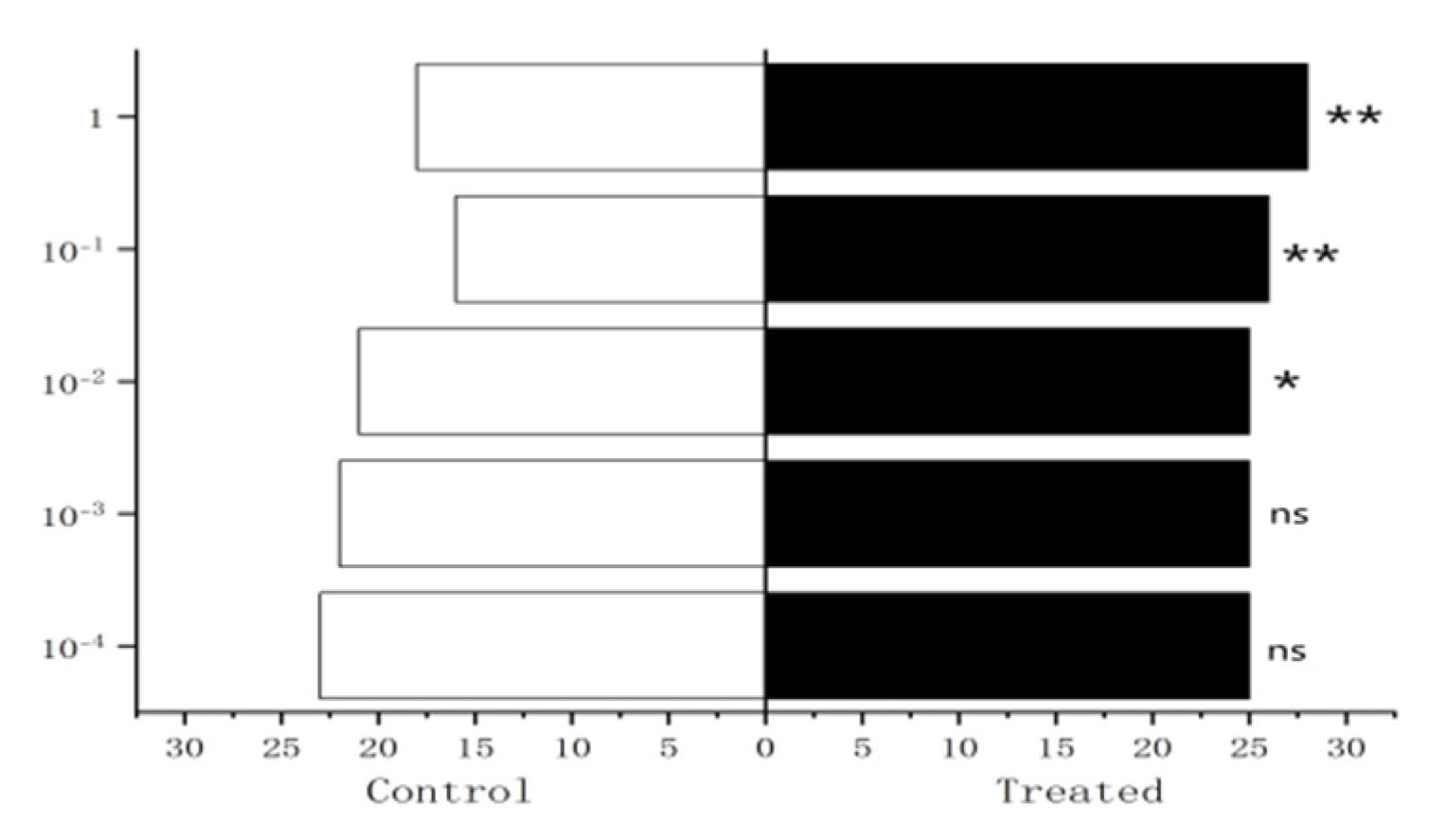
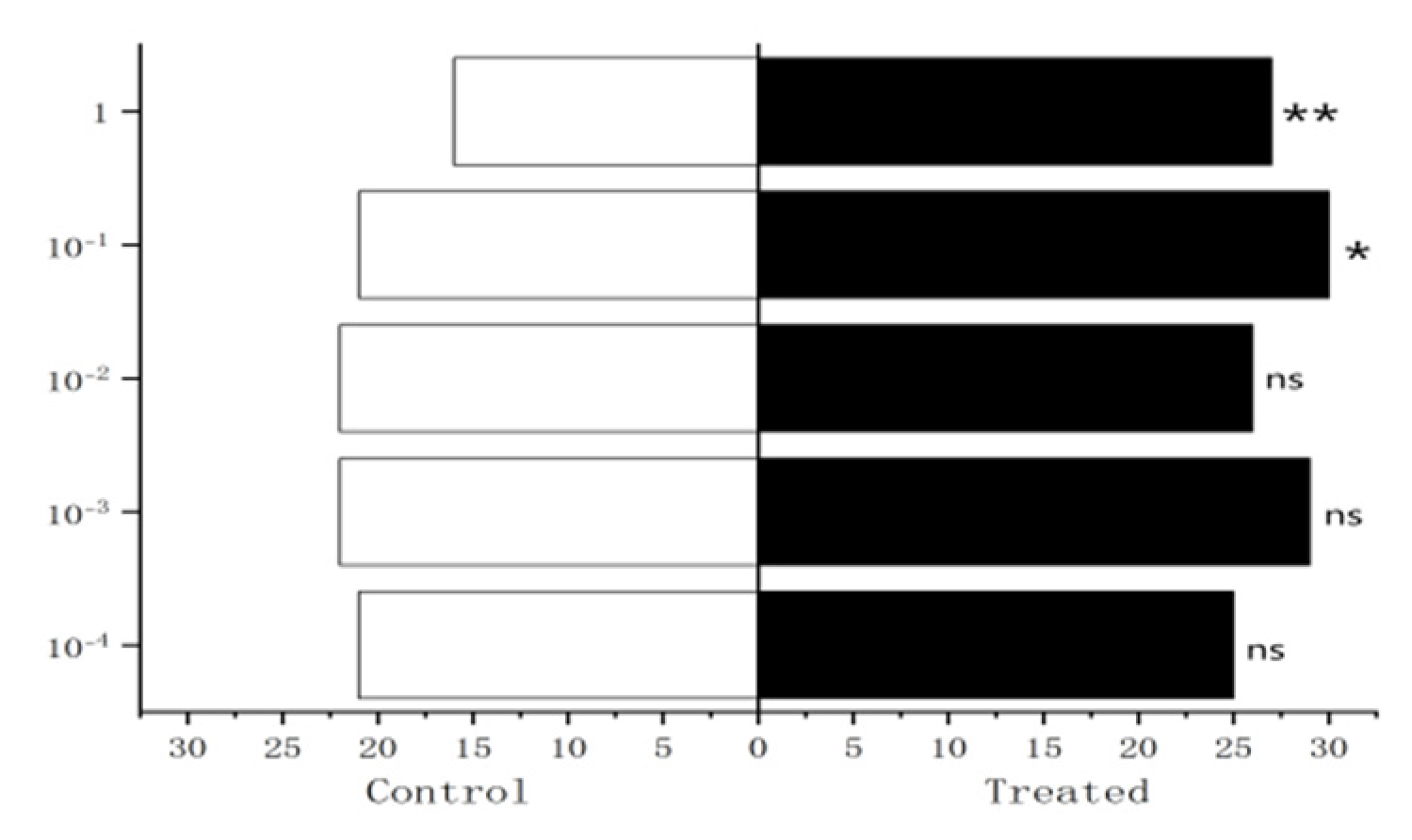
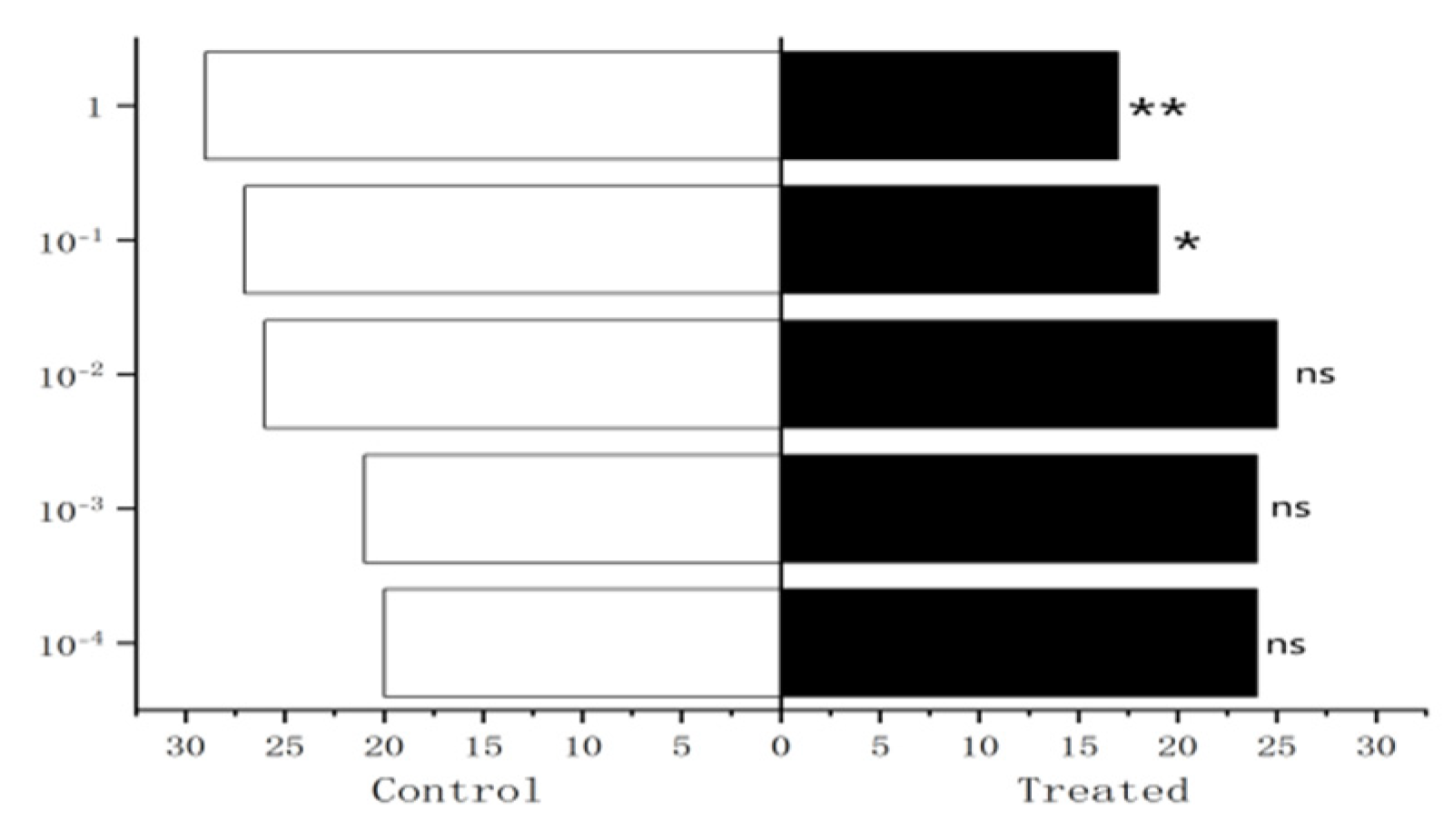
Figure 27.
Behavioral responses to methylheptenone in male W. magnifica. Note: ns: no significant difference (p > 0.05); *: significant difference (0.01 < p < 0.05); **: extremely significant difference (p < 0.01); x-axis: sample grouping; y-axis: sample concentration. The same below.
Figure 27.
Behavioral responses to methylheptenone in male W. magnifica. Note: ns: no significant difference (p > 0.05); *: significant difference (0.01 < p < 0.05); **: extremely significant difference (p < 0.01); x-axis: sample grouping; y-axis: sample concentration. The same below.
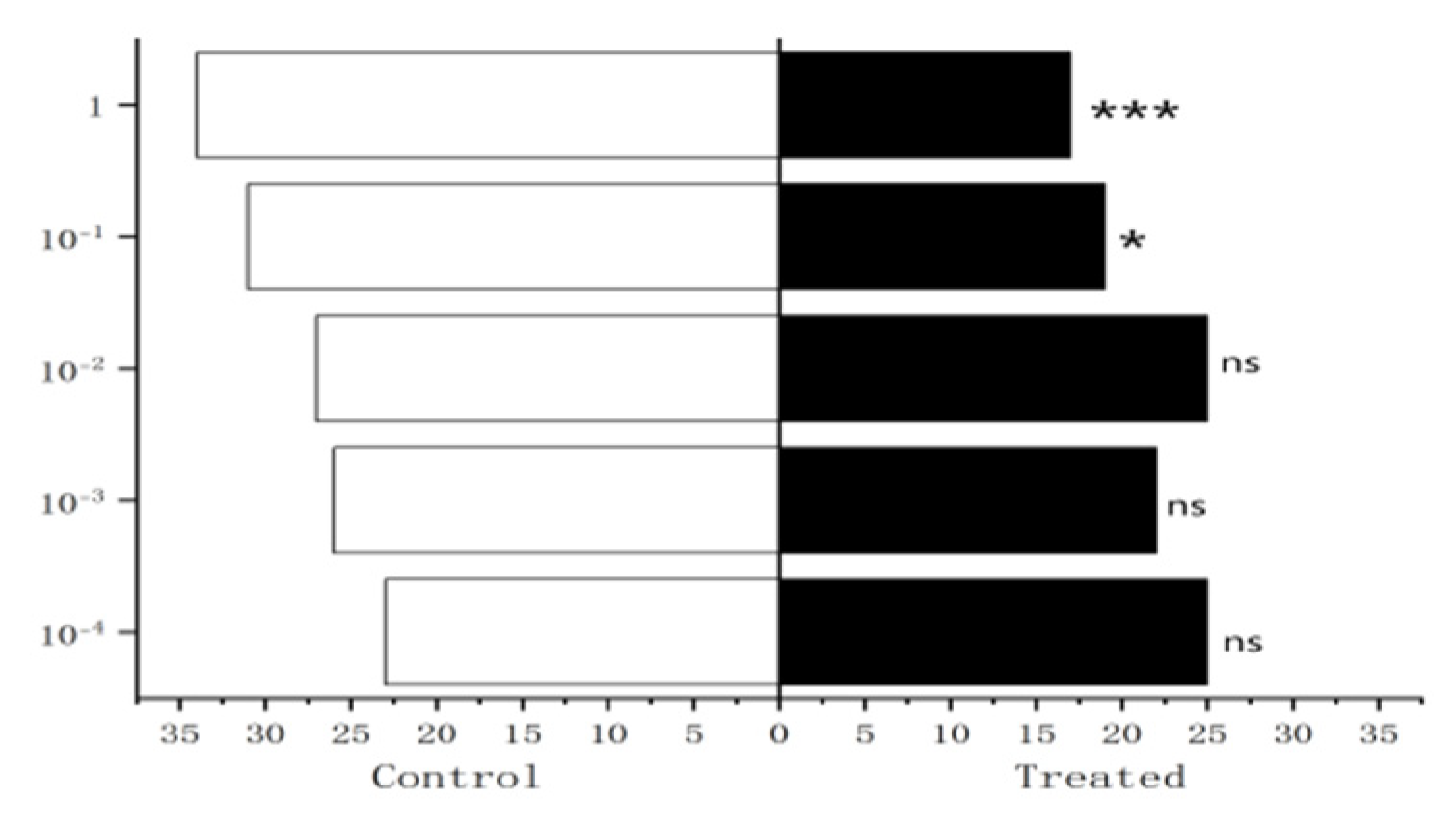
Figure 28.
Behavioral responses to 1-octen-3-ol in male W. magnifica.
Figure 28.
Behavioral responses to 1-octen-3-ol in male W. magnifica.
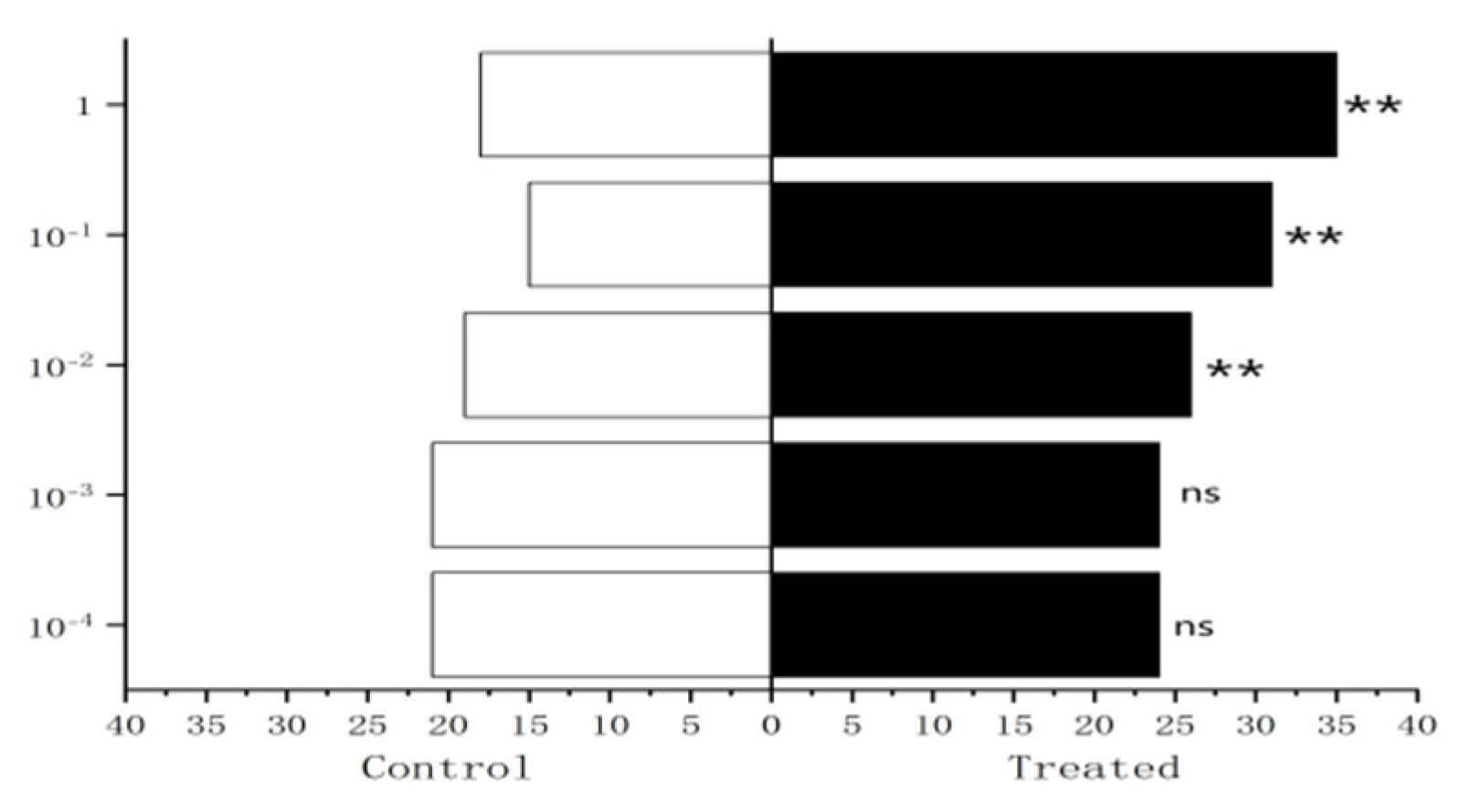
Figure 29.
Behavioral responses to propyl butyrate in male W. magnifica.
Figure 29.
Behavioral responses to propyl butyrate in male W. magnifica.
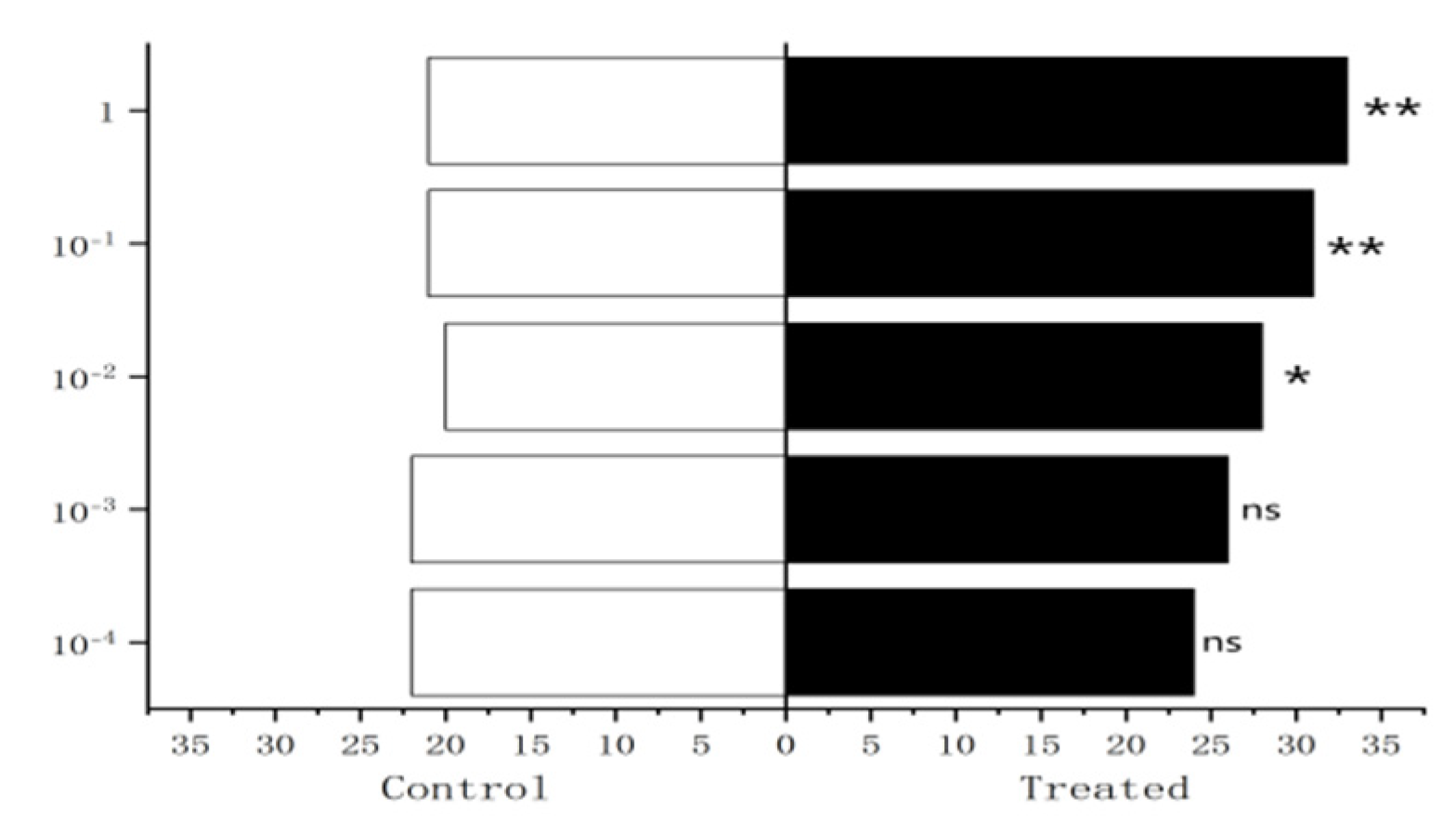
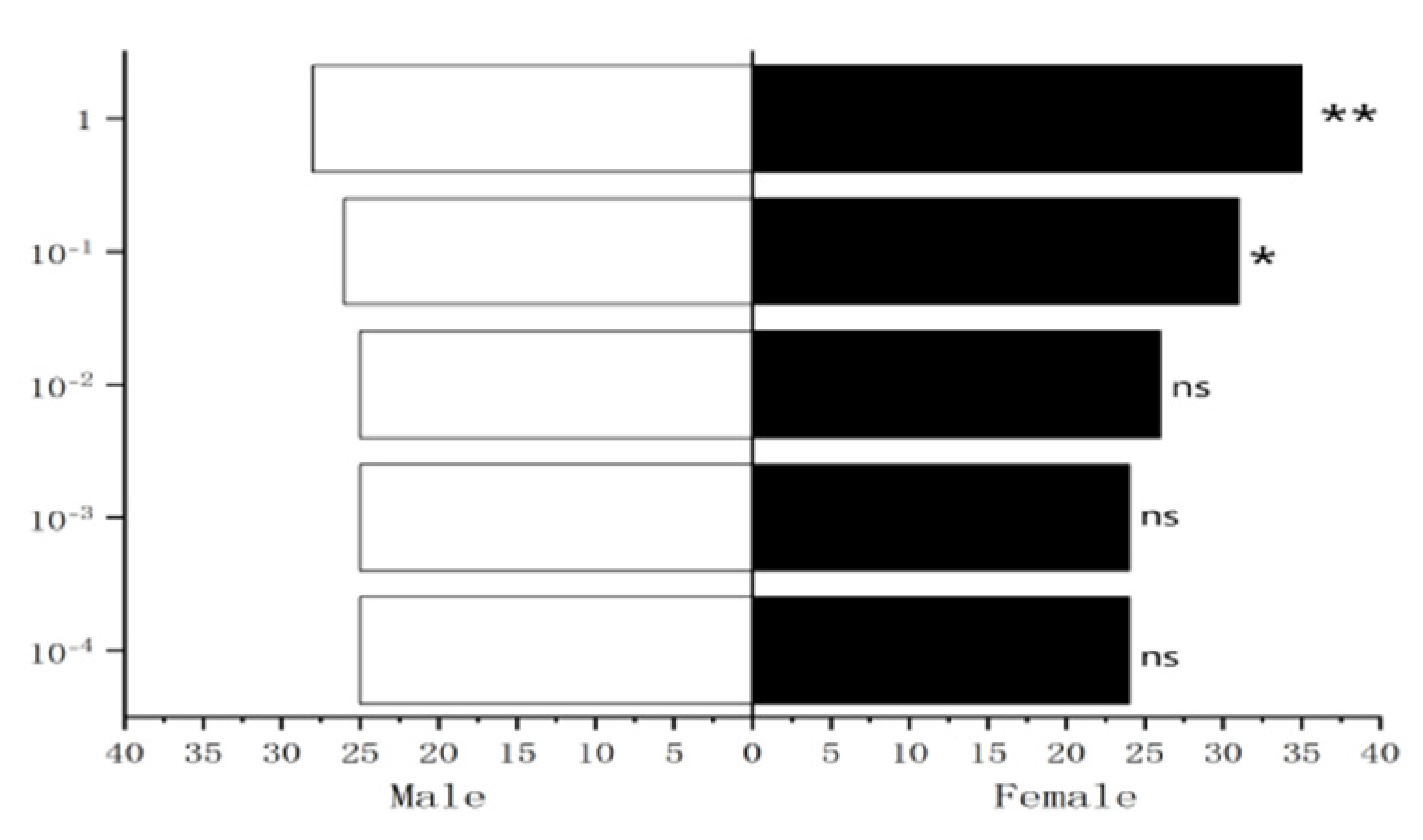
Figure 30.
Behavioral responses to methylheptenone in female W. magnifica.
Figure 30.
Behavioral responses to methylheptenone in female W. magnifica.
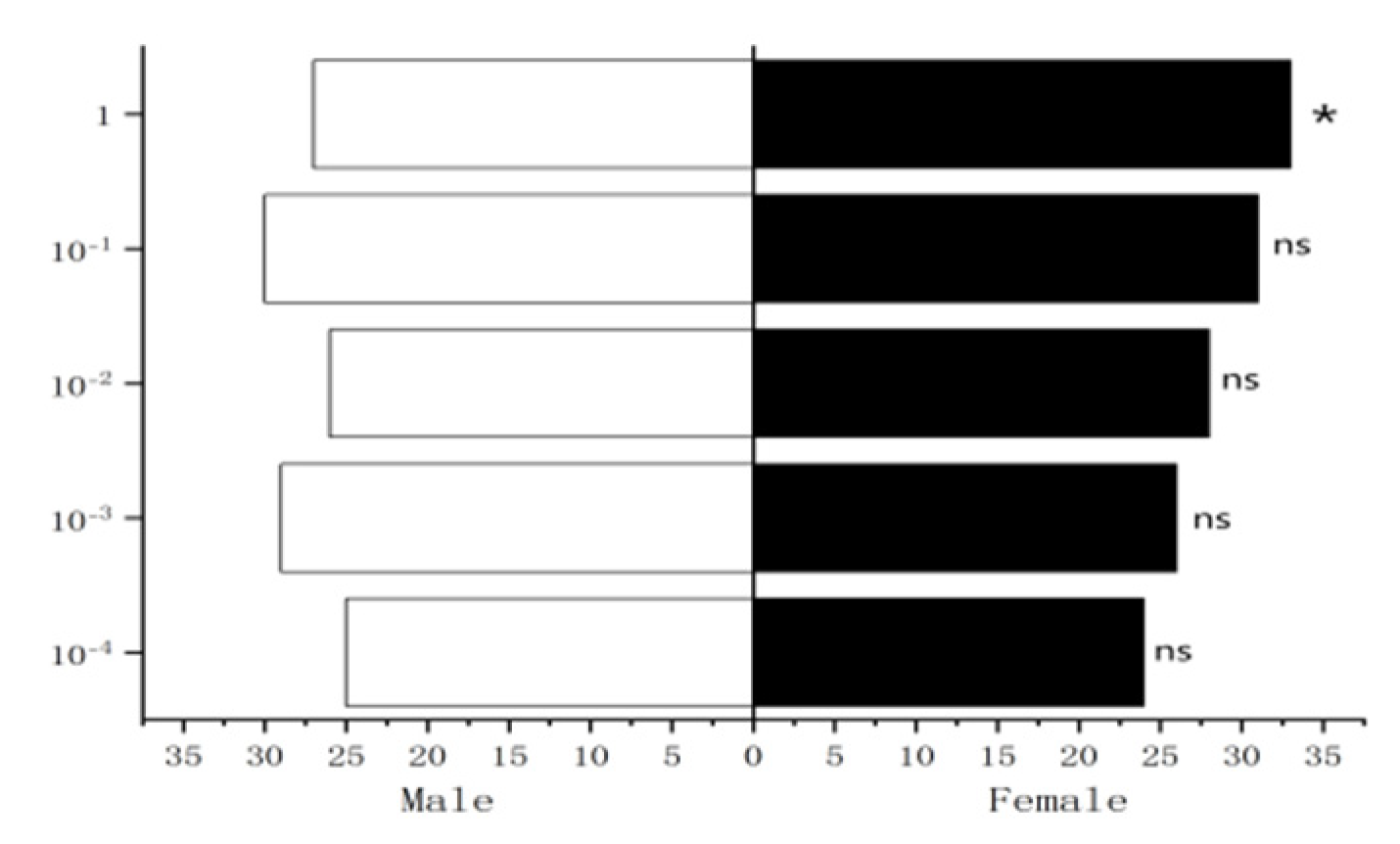
Figure 31.
Behavioral responses to 1-octen-3-ol in female W. magnifica.
Figure 31.
Behavioral responses to 1-octen-3-ol in female W. magnifica.
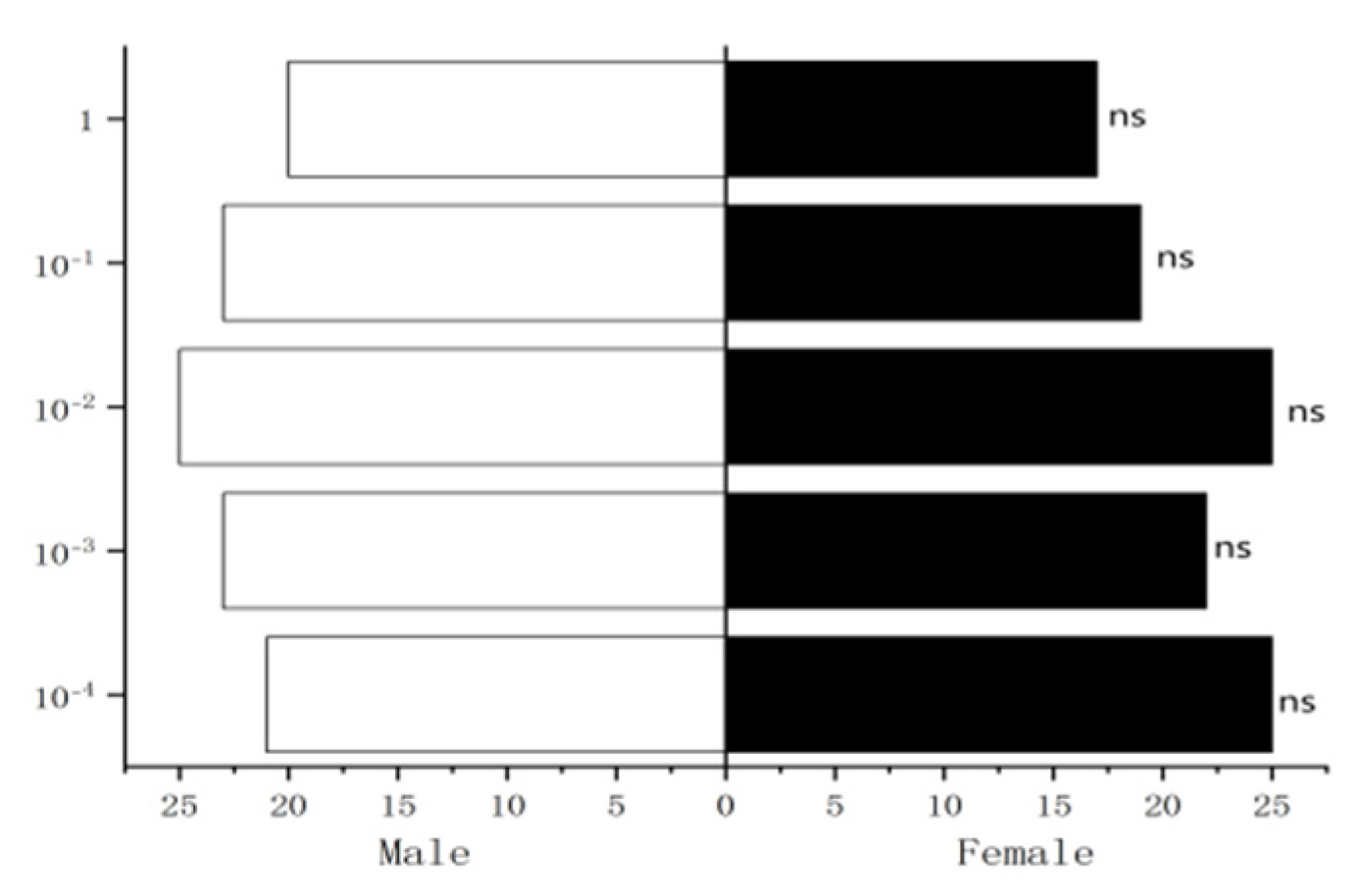
Figure 32.
Behavioral responses to propyl butyrate in female W. magnifica.
Figure 32.
Behavioral responses to propyl butyrate in female W. magnifica.
Figure 33.
Comparison of behavioral responses of W. magnifica to methylheptenone.
Figure 33.
Comparison of behavioral responses of W. magnifica to methylheptenone.
Figure 34.
Comparison of behavioral responses of W. magnifica to 1-octen-3-ol.
Figure 34.
Comparison of behavioral responses of W. magnifica to 1-octen-3-ol.
Figure 35.
Comparison of behavioral responses of W. magnifica to propyl butyrate.
Figure 35.
Comparison of behavioral responses of W. magnifica to propyl butyrate.
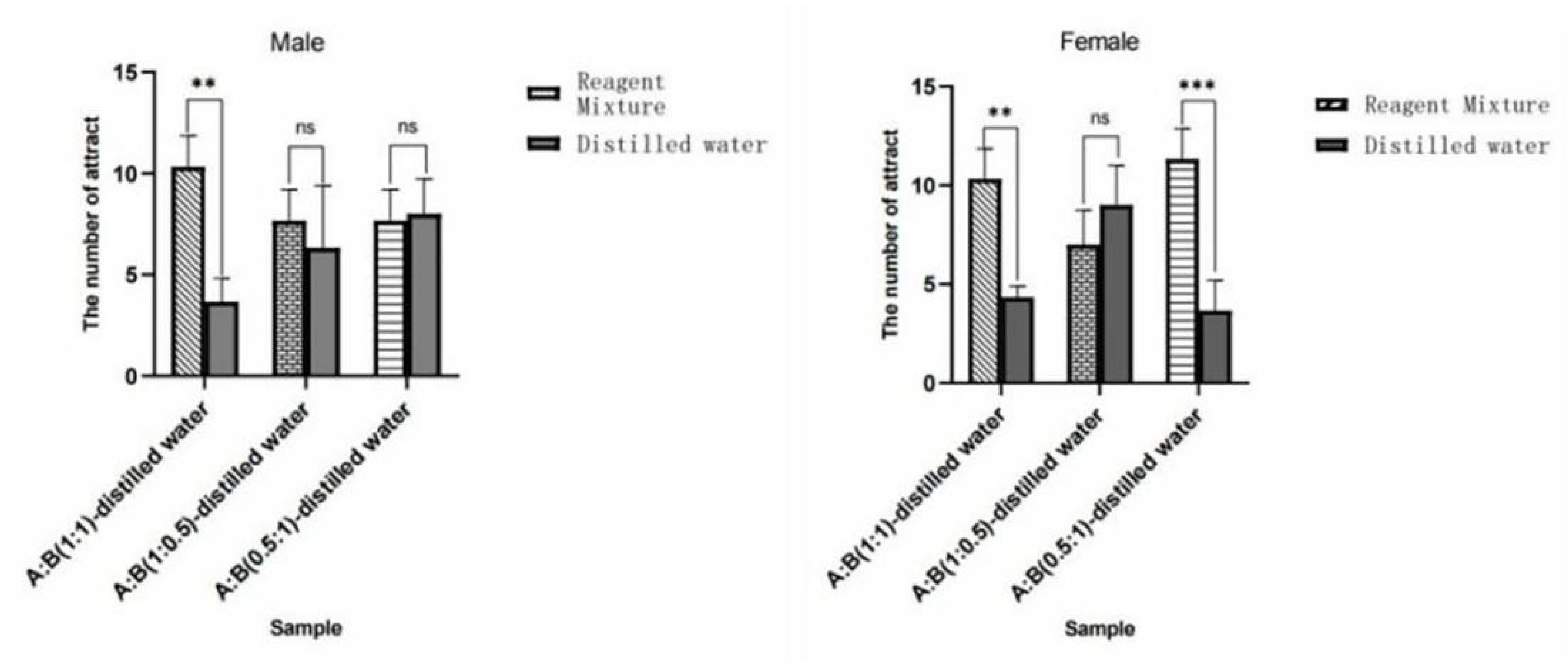
Figure 36.
Behavioral responses of W. magnifica by mixing methylheptenone and 1-octen-3-ol in different proportions. Note: A: methylheptenone; B: 1-octen-3-ol; ns: no significant difference (p > 0.05); ** and ***: extremely significant difference (p < 0.01). The same below.
Figure 36.
Behavioral responses of W. magnifica by mixing methylheptenone and 1-octen-3-ol in different proportions. Note: A: methylheptenone; B: 1-octen-3-ol; ns: no significant difference (p > 0.05); ** and ***: extremely significant difference (p < 0.01). The same below.
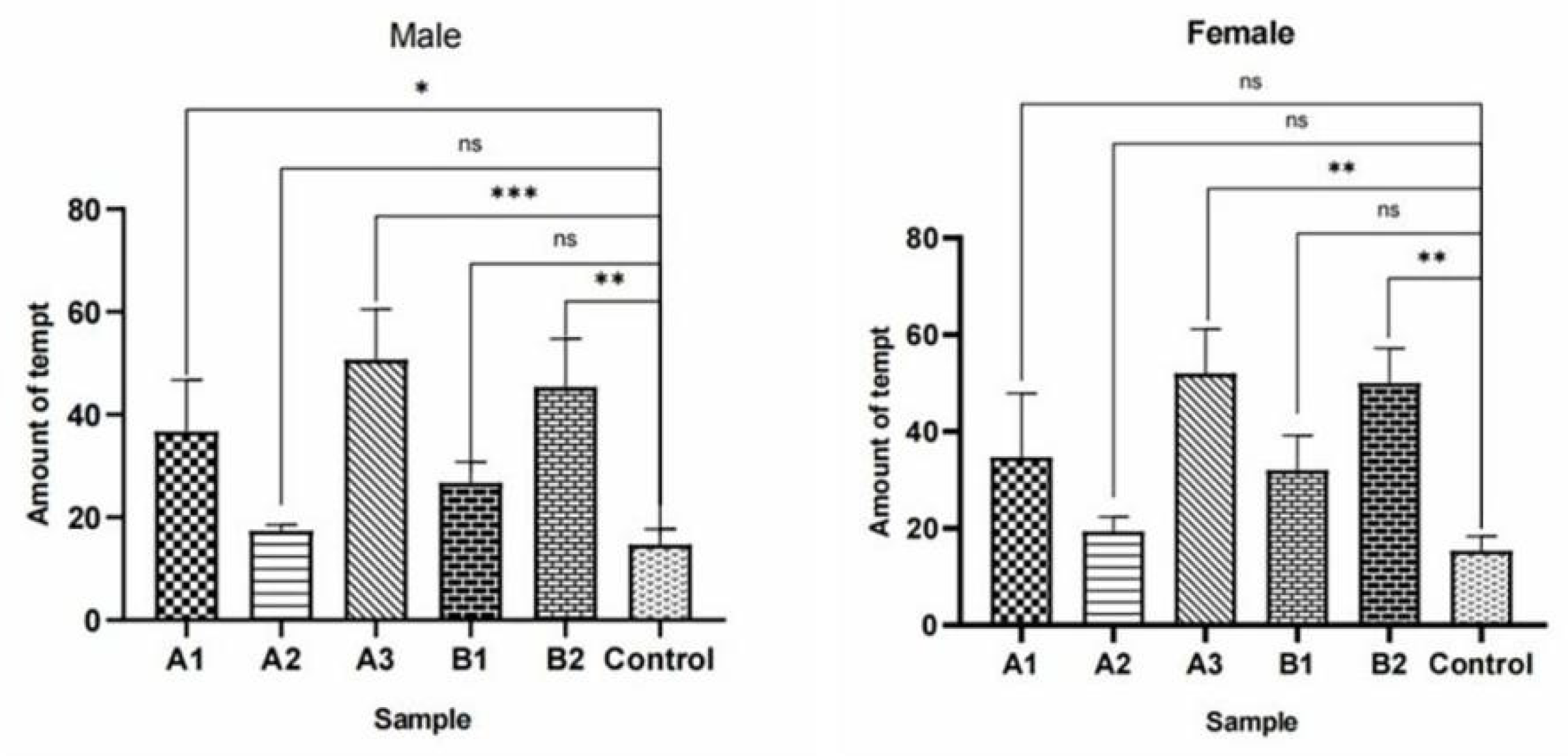
Figure 37.
Attractive effects of different concentrations of methylheptenone and 1-octen-3-ol on W. magnifica. Note: A1: 10−1 µg/µL methylheptenone; A2: 10−2 µg/µL methylheptenone; A3: 1 µg/µL methylheptenone; B1: 10−1 µg/µL 1-octen-3-ol; B2: 1 µg/µL 1-octen-3-ol; ns: no significant difference (p > 0.05); *: significant difference (0.01 < p < 0.05); ** and ***: extremely significant difference (p < 0.01). The same below.
Figure 37.
Attractive effects of different concentrations of methylheptenone and 1-octen-3-ol on W. magnifica. Note: A1: 10−1 µg/µL methylheptenone; A2: 10−2 µg/µL methylheptenone; A3: 1 µg/µL methylheptenone; B1: 10−1 µg/µL 1-octen-3-ol; B2: 1 µg/µL 1-octen-3-ol; ns: no significant difference (p > 0.05); *: significant difference (0.01 < p < 0.05); ** and ***: extremely significant difference (p < 0.01). The same below.
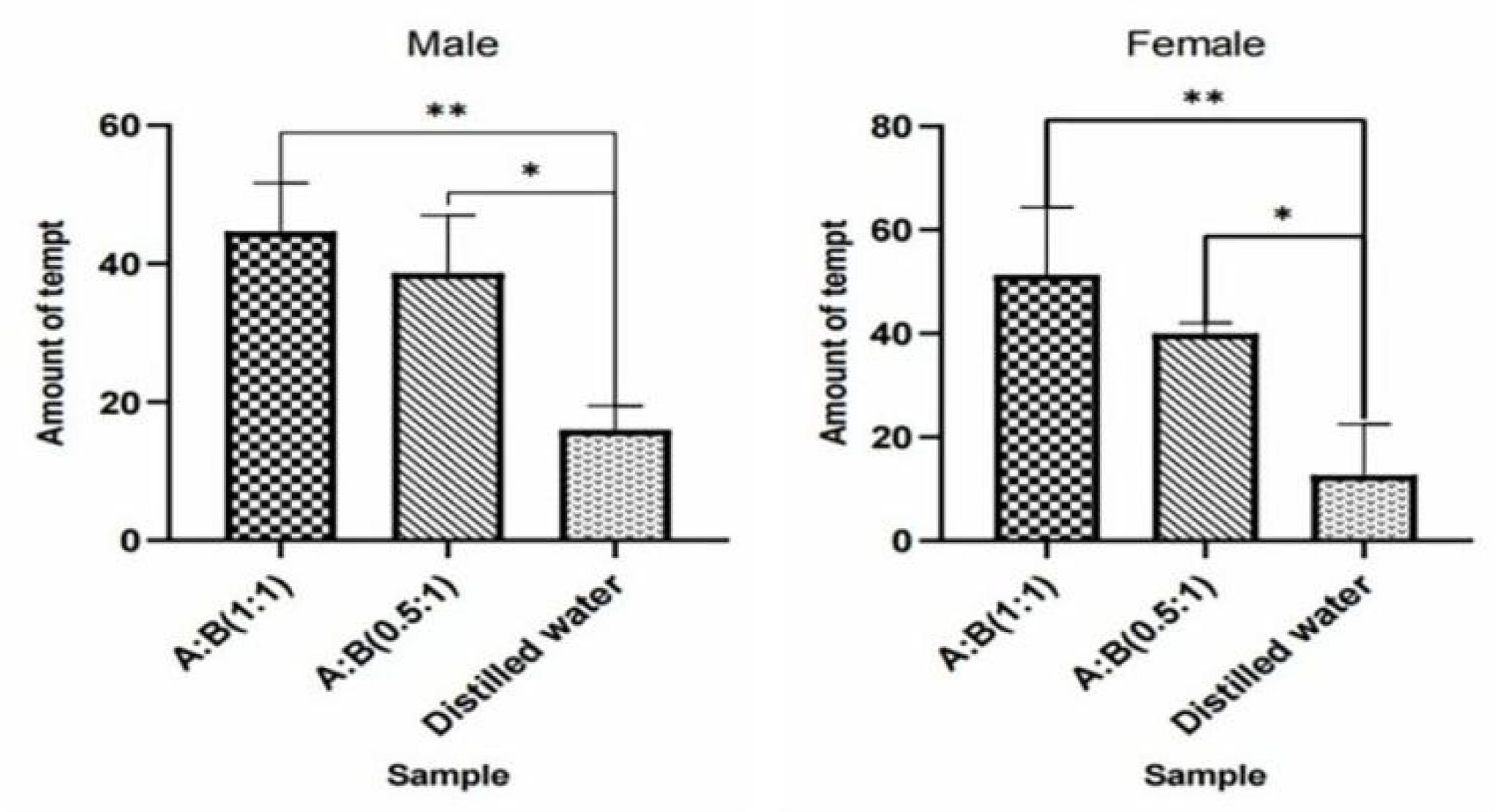
Figure 38.
Attractive effects of W. magnifica by mixing methylheptenone and 1-octen-3-ol in different proportions.
Figure 38.
Attractive effects of W. magnifica by mixing methylheptenone and 1-octen-3-ol in different proportions.
Table 1.
Gradient mobile phase for Ultra Performance Liquid Chromatograph.
Table 1.
Gradient mobile phase for Ultra Performance Liquid Chromatograph.
| Time | Flow Rate (mL/min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|
| 0 | 0.6 | 70 | 30 |
| 12 | 0.6 | 20 | 80 |
| 15 | 0.6 | 0 | 100 |
| 20 | 0.6 | 70 | 30 |
Table 2.
Mass spectrometry condition.
Table 2.
Mass spectrometry condition.
| Project | Conditions |
|---|
| Collection quality range | 50–1200 Da |
| Sweep time | 0.1 s |
| Acquisition mode | ESI+, MSE |
| Lock mass | Leucine enkephalin (LE) 1 ppm (scanning time: 0.3 s; interval: 15 s) |
| A tube voltage | 3 KV |
| Taper hole voltage | 100 V |
| Collision energy (eV) | Low CE: 6/High CE: 20–50 |
| Ionization source temperature | 100 °C |
| Desolvation temperature | 300 °C |
| Cone hole gas flow rate | 100 L/h |
| Desolvation gas flow rate | 800 L/h |
| Date acquisition time | 20 min |
Table 3.
The proportion of the mixture.
Table 3.
The proportion of the mixture.
| Reagent | Mixing Ratio |
|---|
| Methylheptenone:1-octen-3-ol | 1:1 |
| 1:0.5 |
| 0.5:1 |
Table 4.
EAG relative response value of male W. magnifica to different concentrations of three compounds.
Table 4.
EAG relative response value of male W. magnifica to different concentrations of three compounds.
| Sample Concentration | 10−4 µg/µL | 10−3 µg/µL | 10−2 µg/µL | 10−1 µg/µL | 1 µg/µL |
|---|
| methylheptenone | 1.167 mv | 1.313 mv | 1.688 mv | 1.654 mv | 1.803 mv |
| 1-octen-3-ol | 1.143 mv | 1.240 mv | 1.396 mv | 1.362 mv | 1.382 mv |
| Propyl butyrate | 1.092 mv | 1.204 mv | 1.417 mv | 1.573 mv | 1.521 mv |
Table 5.
EAG relative response value of female W. magnifica to different concentrations of three compounds.
Table 5.
EAG relative response value of female W. magnifica to different concentrations of three compounds.
| Sample Concentration | 10−4 µg/µL | 10−3 µg/µL | 10−2 µg/µL | 10−1 µg/µL | 1 µg/µL |
|---|
| methylheptenone | 1.157 mv | 1.263 mv | 1.824 mv | 1.841 mv | 2.318 mv |
| 1-octen-3-ol | 1.313 mv | 1.456 mv | 1.726 mv | 1.775 mv | 1.749 mv |
| Propyl butyrate | 1.375 mv | 1.490 mv | 1.635 mv | 1.618 mv | 1.666 mv |
Table 6.
Statistical table of the trapping experiment for female W. magnifica with different concentrations of methylheptenone and 1-octen-3-ol.
Table 6.
Statistical table of the trapping experiment for female W. magnifica with different concentrations of methylheptenone and 1-octen-3-ol.
| Time | 2 h | 4 h | 6 h | 8 h | 10 h | 12 h |
|---|
| Concentration of Reagent (µg/µL) | Number of Experiments |
|---|
| methylheptenone | 10−1 | 1 | 2 | 8 | 20 | 30 | 30 | 38 |
| 2 | 2 | 4 | 10 | 14 | 18 | 20 |
| 3 | 4 | 6 | 12 | 24 | 34 | 46 |
| 10−2 | 1 | 2 | 6 | 14 | 18 | 20 | 22 |
| 2 | 0 | 2 | 2 | 10 | 12 | 16 |
| 3 | 2 | 2 | 8 | 16 | 16 | 20 |
| 1 | 1 | 4 | 8 | 16 | 24 | 34 | 44 |
| 2 | 4 | 14 | 26 | 38 | 52 | 62 |
| 3 | 2 | 2 | 8 | 16 | 16 | 20 |
| 1-octen-3-ol | 10−1 | 1 | 0 | 4 | 10 | 20 | 24 | 26 |
| 2 | 4 | 10 | 16 | 24 | 30 | 40 |
| 3 | 2 | 8 | 10 | 14 | 24 | 30 |
| 1 | 1 | 4 | 12 | 20 | 38 | 38 | 48 |
| 2 | 8 | 16 | 28 | 40 | 50 | 58 |
| 3 | 4 | 8 | 20 | 28 | 34 | 44 |
| n-hexane | 1 | 0 | 2 | 6 | 6 | 14 | 16 |
| 2 | 2 | 4 | 4 | 4 | 8 | 18 |
| 3 | 2 | 2 | 6 | 10 | 12 | 12 |
Table 7.
Statistical table of the trapping for male W. magnifica with different concentrations of methylheptenone and 1-octen-3-ol.
Table 7.
Statistical table of the trapping for male W. magnifica with different concentrations of methylheptenone and 1-octen-3-ol.
| Time | 2 h | 4 h | 6 h | 8 h | 10 h | 12 h |
|---|
| Concentration of Reagent (µg/µL) | Number of Experiments |
|---|
| methylheptenone | 10−1 | 1 | 2 | 2 | 4 | 10 | 28 | 46 |
| 2 | 2 | 6 | 12 | 16 | 18 | 26 |
| 3 | 6 | 6 | 8 | 18 | 24 | 38 |
| 10−2 | 1 | 4 | 6 | 10 | 16 | 18 | 18 |
| 2 | 2 | 8 | 10 | 12 | 14 | 18 |
| 3 | 0 | 2 | 2 | 6 | 8 | 16 |
| 1 | 1 | 2 | 6 | 12 | 24 | 34 | 46 |
| 2 | 2 | 10 | 20 | 30 | 44 | 62 |
| 3 | 4 | 8 | 8 | 20 | 30 | 44 |
| 1-octen-3-ol | 10−1 | 1 | 2 | 2 | 6 | 12 | 20 | 22 |
| 2 | 2 | 6 | 10 | 16 | 26 | 28 |
| 3 | 4 | 4 | 12 | 20 | 24 | 30 |
| 1 | 1 | 0 | 6 | 8 | 18 | 28 | 38 |
| 2 | 6 | 10 | 20 | 34 | 48 | 56 |
| 3 | 2 | 8 | 10 | 24 | 34 | 42 |
| n-hexane | 1 | 0 | 2 | 6 | 8 | 10 | 14 |
| 2 | 4 | 4 | 4 | 6 | 8 | 12 |
| 3 | 0 | 2 | 6 | 6 | 12 | 18 |
Table 8.
Statistical table of the trapping for female W. magnifica by mixing methylheptenone and 1-octen-3-ol in different proportions.
Table 8.
Statistical table of the trapping for female W. magnifica by mixing methylheptenone and 1-octen-3-ol in different proportions.
| Time | 2 h | 4 h | 6 h | 8 h | 10 h | 12 h |
|---|
| Mixing Ratio of Methylheptenone and 1-octen-3-ol | Number of Experiments |
|---|
| 1:1 | 1 | 4 | 4 | 12 | 28 | 52 | 64 |
| 2 | 8 | 10 | 10 | 26 | 38 | 52 |
| 3 | 2 | 6 | 8 | 8 | 24 | 38 |
| 0.5:1 | 1 | 4 | 8 | 8 | 15 | 27 | 40 |
| 2 | 4 | 4 | 8 | 16 | 26 | 38 |
| 3 | 8 | 10 | 10 | 20 | 28 | 42 |
| Distilled water | 1 | 8 | 8 | 12 | 20 | 24 | 24 |
| 2 | 4 | 4 | 4 | 6 | 6 | 6 |
| 3 | 0 | 2 | 2 | 6 | 8 | 8 |
Table 9.
Statistical table of the trapping for male W. magnifica by mixing methylheptenone and 1-octen-3-ol in different proportions.
Table 9.
Statistical table of the trapping for male W. magnifica by mixing methylheptenone and 1-octen-3-ol in different proportions.
| Time | 2 h | 4 h | 6 h | 8 h | 10 h | 12 h |
|---|
| Mixing Ratio of Methylheptenone and 1-octen-3-ol | Number of Experiments |
|---|
| 1:1 | 1 | 4 | 4 | 12 | 20 | 30 | 44 |
| 2 | 6 | 10 | 10 | 24 | 28 | 52 |
| 3 | 4 | 6 | 16 | 26 | 34 | 38 |
| 0.5:1 | 1 | 4 | 8 | 16 | 20 | 44 | 48 |
| 2 | 0 | 4 | 4 | 8 | 18 | 32 |
| 3 | 4 | 8 | 10 | 16 | 24 | 36 |
| Distilled water | 1 | 12 | 12 | 20 | 20 | 20 | 20 |
| 2 | 6 | 8 | 8 | 10 | 14 | 14 |
| 3 | 0 | 4 | 4 | 6 | 12 | 14 |