Antibiotic Use in Relation with Psychological Profiles of Farmers of a French Pig Cooperative
Abstract
1. Introduction
Aims of This Study
2. Study Design: Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Work Motivation
2.3.2. Attitude towards Medicine
2.3.3. Locus of Control
2.3.4. Indicators for Antimicrobial Consumption
2.4. Data Analysis
3. Results
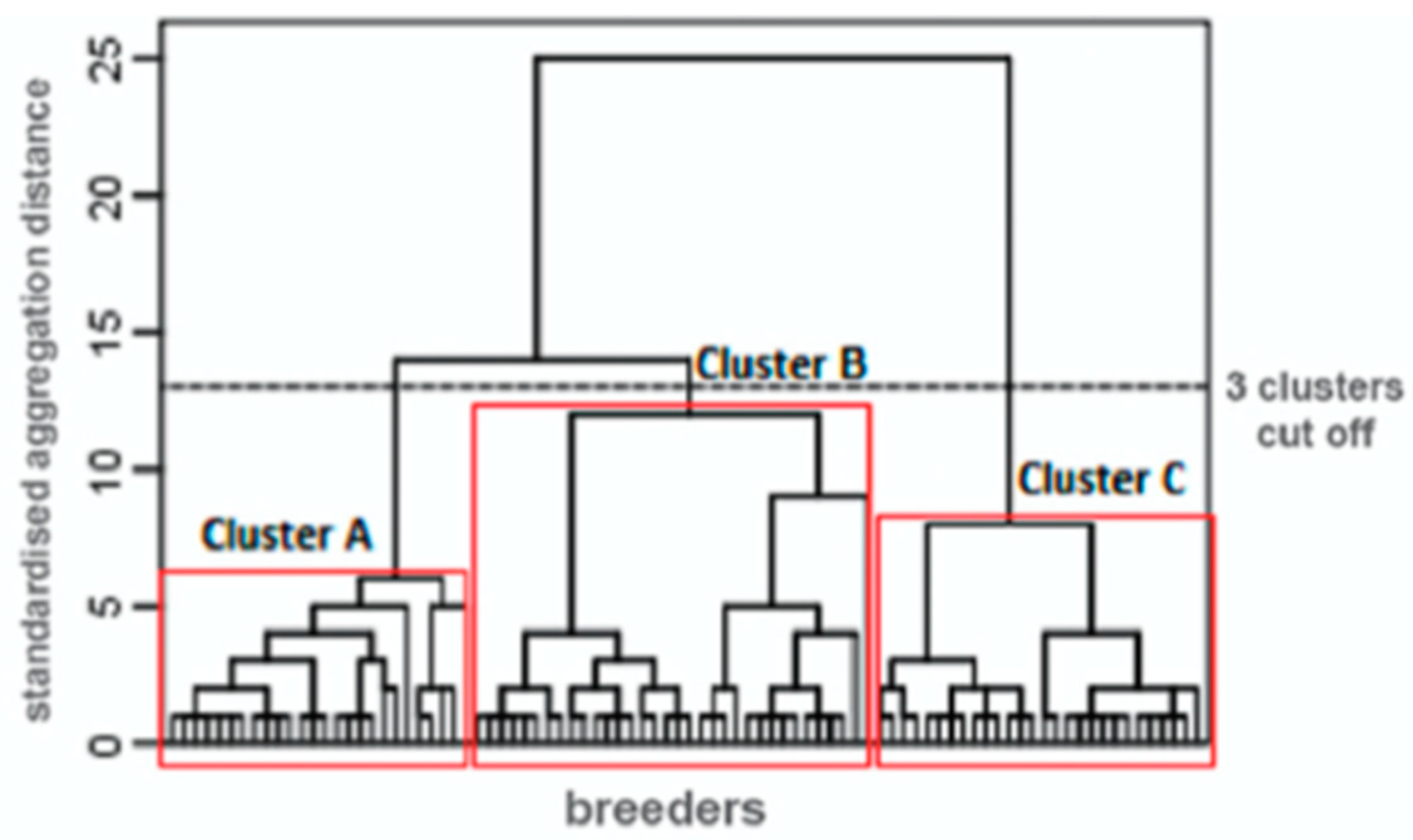
3.1. Objective 1: Identify Participants’ Sub-Profiles According to Their Responses to the Questionnaires
3.2. Objective 2: Identify any Differences in Questionnaire Scores across the Clusters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. WEIMS (Original Version)
| Work motivation Why Do You Do Your Work? | Dimension |
| 1. Because this is the type of work I chose to do to attain a certain lifestyle. | Identified regulation |
| 2. For the income it provides me. | External regulation |
| 3. I ask myself this question, I don’t seem to be able to manage the important tasks related to this work. | Amotivation |
| 4. Because I derive much pleasure from learning new things. | Intrinsic motivation |
| 5. Because it has become a fundamental part of who I am. | Integrated regulation |
| 6. Because I want to succeed at this job;if not I would be very ashamed of myself. | Introjected regulation |
| 7. Because I chose this type of work to attain my career goals. | Identified regulation |
| 8. For the satisfaction I experience from taking on interesting challenges. | Intrinsic motivation |
| 9. Because it allows me to earn money. | External regulation |
| 10. Because it is part of the way in which I have chosen to live my life | Integrated regulation |
| 11. Because I want to be very good at this work, otherwise I would be very disappointed. | Introjected regulation |
| 12. I don’t know why, we are provided with unrealistic working conditions. | Amotivation |
| 13. Because I want to be a “winner” in life | Introjected regulation |
| 14. Because it is the type of work I have chosen to attain certain important objectives. | Identified regulation |
| 15. For the satisfaction I experience when I am successful at doing difficult tasks. | Intrinsic motivation |
| 16. Because this type of work provides me with security. | External regulation |
| 17. I don’t know, too much is expected of us. | Amotivation |
| 18. Because this job is a part of my life. | Integrated regulation |
Appendix B. CACMAS (Original Version)
| Complementary, Alternative, and Conventional Medicine Attitudes Scale | Dimension |
| 1. The health of my body, mind, and spirit are related, and whoever cares for my health should take them into account. | Philosophical congruence with complementary and alternative medicine |
| 2. I have a more equal relationship with my complementary practitioner than with my doctor. | Philosophical congruence with complementary and alternative medicine |
| 3. Effects of complementary therapies are usually the result of a placebo effect. (Reverse scored | Philosophical congruence with complementary and alternative medicine |
| 4. I feel that complementary treatment is a more natural form of healing than orthodox medicine. | Philosophical congruence with complementary and alternative medicine |
| 5. Complementary therapies are a threat to public health. (Reverse scored) | Philosophical congruence with complementary and alternative medicine |
| 6. I feel so relaxed after complementary treatment sessions. | Philosophical congruence with complementary and alternative medicine |
| 7. I believe that complementary medicine enables me to take a more active part in maintaining my health. | Philosophical congruence with complementary and alternative medicine |
| 8. Most complementary therapies stimulate the body’s natural therapeutic powers. | Philosophical congruence with complementary and alternative medicine |
| 9. Complementary therapies include ideas and methods from which conventional medicine could benefit. | Philosophical congruence with complementary and alternative medicine |
| 10. Treatments not tested in a scientifically recognized manner should be discouraged. (Reverse scored) | Philosophical congruence with complementary and alternative medicine |
| 11. I believe that complementary therapy will be more effective for my problem than orthodox medicine. | Philosophical congruence with complementary and alternative medicine |
| 12. The explanation of my illness that I was given by my complementary practitioner made sense. | Philosophical congruence with complementary and alternative medicine |
| 13.I value the emphasis on treating the whole person. | Philosophical congruence with complementary and alternative medicine |
| 14. The last time I went to see a medical doctor, I was very satisfied with the care I received. | Dissatisfaction with conventional medicine |
| 15. The last time I had important questions about my health care and I asked a medical doctor about them, I understood the answer. (Reverse scored) | Dissatisfaction with conventional medicine |
| 16. I have a lot of confidence in the medical doctor I see most often for my health care. (Reverse scored) | Dissatisfaction with conventional medicine |
| 17. I don’t trust doctors and hospitals, so I use them as little as possible. | Dissatisfaction with conventional medicine |
| 18. The last time I saw a medical doctor, he or she did not understand my problem. | Dissatisfaction with conventional medicine |
| 19. The last time I saw a medical doctor, he or she did not give me enough time. | Dissatisfaction with conventional medicine |
Appendix C. Locus of Control Scale (Originale Version)
| Item | Dimension |
| 1. Whether or not I get to be a leader depends mostly on my ability. | Internal |
| 2. To a great extent my life is controlled by accidental happenings. | Chance |
| 3. I feel like what happens in my life is mostly determined by powerful people. | Powerful others |
| 4. My behavior will determine when I am ready to leave the hospital. | Internal |
| 5. When I make plans, I am almost certain to make them work. | Internal |
| 6. Often there is no chance of protecting my personal interests from bad luck happenings. | Chance |
| 7. When I get what I want, it’s usually because I’m lucky. | Chance |
| 8. Even if I were a good leader, I would not be made a leader unless I play up to those in positions of power | Powerful others |
| 9. How many friends I have depends on how nice a person I am. | Internal |
| 10. I have often found that what is going to happen will happen. | Chance |
| 11. My life is chiefly controlled by powerful others | Powerful others |
| 12. It is impossible for anyone to say how long I’ll be in the hospital. | Chance |
| 13. People like myself have very little chance of protecting our personal interests when they conflict with those of powerful other people | Powerful others |
| 14. It’s not always wise for me to plan too far ahead because many things turn out to be a matter of good or bad fortune | Chance |
| 15. Getting what I want means I have to please those people above me. | Powerful others |
| 16. Whether or not I get to be a leader depends on whether I’m lucky enough to be in the right place at the right time. | Chance |
| 17. If important people were to decide they didn’t like me, I probably wouldn’t make many friends. | Powerful others |
| 18. I can pretty much determine what will happen in my life. | Internal |
| 19. I am usually able to protect my personal interests. | Internal |
| 20. How soon I leave the hospital depends on other people who have power over me. | Powerful others |
| 21. When I get what I want, it’s usually because I worked hard for it. | Internal |
| 22. In order to have my plans work, I make sure that they fit in with the desires of people who have power over me. | Powerful others |
| 23. My life is determined by my own actions. | Internal |
| 24. It’s chiefly a matter of fate whether or not I have a few friends or many friends. | Chance |
References
- Fortané, N. Le problème public de l’antibiorésistance en élevage: Essai de généalogie et caractérisation. Quest. Commun. 2016, 29, 49–66. [Google Scholar] [CrossRef][Green Version]
- Schwarz, S.; Kehrenberg, C.; Walsh, T.R. Use of antimicrobial agents in veterinary medicine and food animal production. Int. J. Antimicrob. Agents. 2001, 17, 431–437. [Google Scholar] [CrossRef]
- Meyer, E.; Gastmeier, P.; Deja, M.; Schwab, F. Antibiotic consumption and resistance: Data from Europe and Germany. Int. J. Med. Microbiol. Suppl. 2013, 303, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef]
- Urban, D.; Chevance, A.; Moulin, G. Suivi des ventes de Médicaments Vétérinaires Contenant des Antibiotiques en France en 2019, Anses-ANMV, France. 2020. rapport, 97p. Available online: https://www.anses.fr/fr/system/files/ANMV-Ra-Antibiotiques2019.pdf (accessed on 30 December 2021).
- Van der Fels-Klerx, H.J.; Puister-Jansen, L.F.; van Asselt, E.D.; Burgers, S.L.G.E. Farm factors associated with the use of antibiotics in pig production1. J. Anim. Sci. 2011, 89, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P.; Price, S. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Vet. Rec. 2013, 173, 475. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of antibiotic use in global pig production: A systematic review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef]
- Lhermie, G.; Raboisson, D.; Krebs, S.; Dupraz, P. Facteurs déterminants et leviers de réduction de l’usage des antibiotiques en productions animales. Econ. Rural. 2015, 348, 3–22. [Google Scholar] [CrossRef]
- Cernicchiaro, N.; Renter, D.G.; White, B.J.; Babcock, A.H.; Fox, J.T. Associations between weather conditions during the first 45 days after feedlot arrival and daily respiratory disease risks in autumn-placed feeder cattle in the United States1. J. Anim. Sci. 2012, 90, 1328–1337. [Google Scholar] [CrossRef]
- Hémonic, A.; Chauvin, C.; Corrégé, I. Les utilisations d’antibiotiques en élevage de porcs: Motifs et stratégies thérapeutiques associées. Journ. Rech. Porc. 2014, 45, 135–140. [Google Scholar]
- Postma, M.; Backhans, A.; Collineau, L.; Loesken, S.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Stärk, K.D.C.; Dewulf, J.; et al. The biosecurity status and its associations with production and management characteristics in farrow-to-finish pig herds. Animal 2016, 10, 478–489. [Google Scholar] [CrossRef]
- Stygar, A.H.; Chantziaras, I.; Toppari, I.; Maes, D.; Niemi, J.K. High biosecurity and welfare standards in fattening pig farms are associated with reduced antimicrobial use. Animal 2020, 14, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, L.V.; Cipriotti, P.A.; Monterubbianessi, M.; Perfumo, C.; Mateu, E.; Allepuz, A. Network analysis of pig movements in Argentina: Identification of key farms in the spread of infectious diseases and their biosecurity levels. Transbound. Emerg. Dis. 2020, 67, 1152–1163. [Google Scholar] [CrossRef]
- Collineau, L.; Belloc, C.; Hemonic, A.; Guiard, M.; Lehebel, A.; Badouard, B.; Staerk, K. Étude du lien entre niveau de biosécurité et utilisation d’antibiotiques dans les élevages de porcs. Journ. Rech. Porc. 2014, 6, 141–146. [Google Scholar]
- Chauvin, C.; Madec, F.; Sanders, P. Etude de l’usage des antibiotiques en aviculture approche pharmaco-épidémiologique. Bull. Épidémiol. 2010, 37, 5–6. [Google Scholar]
- Assié, S.; Bareille, N.; Beaudeau, F.; Seegers, H. Management- and housing-related risk factors of respiratory disorders in non-weaned French Charolais calves. Prev. Vet. Med. 2009, 91, 218–225. [Google Scholar] [CrossRef]
- Rojo-Gimeno, C.; Postma, M.; Dewulf, J.; Hogeveen, H.; Lauwers, L.; Wauters, E. Farm-economic analysis of reducing antimicrobial use whilst adopting improved management strategies on farrow-to-finish pig farms. Prev. Vet. Med. 2016, 129, 74–87. [Google Scholar] [CrossRef]
- Collineau, L.; Rojo-Gimeno, C.; Léger, A.; Backhans, A.; Loesken, S.; Nielsen, E.O.; Postma, M.; Emanuelson, U.; Beilage, E.; Sjölund, M.; et al. Herd-specific interventions to reduce antimicrobial usage in pig production without jeopardising technical and economic performance. Prev. Vet. Med. 2017, 144, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, C.; Croisier, A.; Tazani, F.; Balaine, L.; Eono, F.; Salaun-Huneau, A.; Le Bouquin, S. Utilisation des antibiotiques en filière cunicole: Enquête en élevages 2009-2010. Journ. Rech. Cunic. 2011, 14, 141–144. [Google Scholar]
- Hémonic, A.; Chauvin, C.; Delzescaux, D.; Verliat, F.; Corrégé, I. Reliable estimation of antimicrobial use and its evolution between 2010 and 2013 in French swine farms. Porc. Health Manag. 2018, 4, 8. [Google Scholar] [CrossRef]
- Visschers, V.H.M.; Backhans, A.; Collineau, L.; Iten, D.; Loesken, S.; Postma, M.; Belloc, C.; Dewulf, J.; Emanuelson, U.; Beilage, E.; et al. Perceptions of antimicrobial usage, antimicrobial resistance and policy measures to reduce antimicrobial usage in convenient samples of Belgian, French, German, Swedish and Swiss pig farmers. Prev. Vet. Med. 2015, 119, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Visschers, V.H.M.; Postma, M.; Sjölund, M.; Backhans, A.; Collineau, L.; Loesken, S.; Belloc, C.; Dewulf, J.; Emanuelson, U.; Beilage, E.G.; et al. Higher perceived risks of antimicrobial use are related to lower usage among pig farmers in four European countries. Vet. Rec. 2016, 179, 490. [Google Scholar] [CrossRef]
- Bouquin, S.; Rouxel, G.; Mihoc, E.; Chauveau, V.; Terrade, F.; Chauvin, C. Facteurs humains et usages des antibiotiques en filière cunicole: Étude de quelques déterminants psychologiques. In Proceedings of the 15émes Journées de la Recherche Cunicole, Le Mans, France, 19–20 November 2013; p. 6. [Google Scholar]
- Willock, J.; Deary, I.J.; McGregor, M.M.; Sutherland, A.; Edwards-Jones, G.; Morgan, O.; Austin, E. Farmers’ Attitudes, Objectives, Behaviors, and Personality Traits: The Edinburgh Study of Decision Making on Farms. J. Vocat. Behav. 1999, 54, 5–36. [Google Scholar] [CrossRef]
- Kuvaas, B.; Buch, R.; Weibel, A.; Dysvik, A.; Nerstad, C.G.L. Do intrinsic and extrinsic motivation relate differently to employee outcomes? J. Econ. Psychol. 2017, 61, 244–258. [Google Scholar] [CrossRef]
- Garforth, C.J.; Bailey, A.P.; Tranter, R.B. Farmers’ attitudes to disease risk management in England: A comparative analysis of sheep and pig farmers. Prev. Vet. Med. 2013, 110, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Wieland, B.; Mateus, A.L.P.; Dewberry, C. Pig farmers’ perceptions, attitudes, influences and management of information in the decision-making process for disease control. Prev. Vet. Med. 2014, 116, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, M.; Cobb-Clark, D.A.; Uhlendorff, A. Locus of Control and Job Search Strategies. Rev. Econ. Stat. 2015, 97, 88–103. [Google Scholar] [CrossRef]
- Tremblay, M.A.; Blanchard, C.M.; Taylor, S.; Pelletier, L.G.; Villeneuve, M. “Work Extrinsic and Intrinsic Motivation Scale: Its value for organizational psychology research”: Correction to Tremblay et al. (2009). Can. J. Behav. Sci. 2010, 42, 70. [Google Scholar] [CrossRef]
- Deci, E.L.; Ryan, R.M. Self-determination theory: A macrotheory of human motivation, development, and health. Can. Psychol. 2008, 49, 182–185. [Google Scholar] [CrossRef]
- McFadden, K.L.; Hernández, T.D.; Ito, T.A. Attitudes toward Complementary and Alternative Medicine Influence Its Use. Explore 2010, 6, 380–388. [Google Scholar] [CrossRef]
- Levenson, H. Multidimensional locus of control in psychiatric patients. J. Consult. Clin. Psychol. 1973, 41, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Loas, G.; Dardennes, R.; Dhee-Perot, P.; Leclerc, V.; Fremaux, D. Operationalization of the “locus of control” concept: Translation and first validation study of the Levenson control scale (IPC: The internal powerful others and chance scale). Ann. Med. Psychol. 1994, 152, 466–469. [Google Scholar]
- EMA. “Defined Daily Doses for Animals (DDDvet) and Defined Course Doses for Animals (DCDvet)”. EMA/224954/2016, 29 p. Available online: https://www.ema.europa.eu/en/documents/other/defined-daily-doses-animals-dddvet-defined-course-doses-animals-dcdvet-european-surveillance_en.pdf (accessed on 30 December 2021).
- IBM Corp. IBM SPSS Statistics for Windows [Internet]; IBM Corp: Armonk, NY, USA, 2017; Available online: https://hadoop.apache.org (accessed on 30 December 2021).
- Madhulatha, T.S. An overview on clustering methods. IOSR J. Eng. 2012, 2, 719–725. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Gagné, M.; Chemolli, E.; Forest, J.; Koestner, R. A Temporal Analysis of the Relation between Organisational Commitment and Work Motivation. Psychol. Belg. 2008, 48, 219. [Google Scholar] [CrossRef]
- Gagné, M.; Deci, E.L. Self-determination theory and work motivation: Self-determination theory and work motivation. J. Organ. Behav. 2005, 26, 331–362. [Google Scholar] [CrossRef]
- Greiner, R. Motivations and attitudes influence farmers’ willingness to participate in biodiversity conservation contracts. Agric. Syst. 2015, 137, 154–165. [Google Scholar] [CrossRef]
- Vik, J.; McElwee, G. Diversification and the Entrepreneurial Motivations of Farmers in Norway. J. Small Bus. Manag. 2011, 49, 390–410. [Google Scholar] [CrossRef]
- Michelik, F. La relation attitude-comportement: Un état des lieux. Ethics Econ. 2008, 6, 1–11. [Google Scholar]
- Lathers, C.M. Role of Veterinary Medicine in Public Health: Antibiotic Use in Food Animals and Humans and the Effect on Evolution of Antibacterial Resistance. J. Clin. Pharmacol. 2001, 41, 595–599. [Google Scholar] [CrossRef]
- Coyne, L.A.; Pinchbeck, G.L.; Williams, N.J.; Smith, R.F.; Dawson, S.; Pearson, R.B.; Latham, S.M. Understanding antimicrobial use and prescribing behaviours by pig veterinary surgeons and farmers: A qualitative study. Vet. Rec. 2014, 175, 593. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S.; McKernan, C.; Benson, T.; Elliott, C.; Dean, M. Understanding farmers’ and veterinarians’ behavior in relation to antimicrobial use and resistance in dairy cattle: A systematic review. J. Dairy Sci. 2021, 104, 4584–4603. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.M.; Main, D.; Roe, E.; Haase, A.; Whay, H.R.; Reyher, K.K. To change or not to change? Veterinarian and farmer perceptions of relational factors influencing the enactment of veterinary advice on dairy farms in the United Kingdom. J. Dairy Sci. 2019, 102, 10379–10394. [Google Scholar] [CrossRef] [PubMed]
- Joule, R.V.; Girandola, F.; Bernard, F. How Can People Be Induced to Willingly Change Their Behavior? The Path from Persuasive Communication to Binding Communication. Soc. Personal. Psychol. Compass. 2007, 1, 493–505. [Google Scholar] [CrossRef]
| N | Gender | Age | ALEA ª | |
|---|---|---|---|---|
| Cluster A | 28 | 27 men | 46.21 (10.49) b | 0.37 (0.31) |
| Cluster B | 34 | 30 men | 49.52 (9.27) | 0.65 (0.46) |
| Cluster C | 26 | 24 men | 50.31 (8.89) | 0.51 (0.41) |
| IM | A | CCAM | DMC | I | C | OA | |
|---|---|---|---|---|---|---|---|
| Cluster A | 6.02 (0.63) b | 2.23 (0.80) | 4.82 (0.69) | 2.51 (0.76) | 4.81 (0.77) | 3.04 (1.01) | 2.72 (0.86) |
| Cluster B | 5.24 (1.06) | 4.26 (0.90) | 4.66 (0.79) | 3.29 (1.05) | 5.12 (0.86) | 3.68 (1.03) | 4.19 (1.19) |
| Cluster C | 5.47 (0.77) | 4.47 (0.81) | 5.02 (1.10) | 2.52 (0.83) | 4.82 (1.11) | 2.65 (0.74) | 2.77 (0.94) |
| F-Statistic | p-Value | η·p | Differences between Groups (LSD) | |
|---|---|---|---|---|
| IM | 6.65 | 0.002 | 0.14 | A > B, C |
| A | 61.32 | <0.001 | 0.59 | A < B, C |
| CCAM | 1.24 | 0.295 | 0.03 | N.S |
| DMC | 6.24 | 0.001 | 0.15 | A, C < B |
| I | 1.91 | 0.324 | 0.03 | N.S |
| C | 16.40 | <0.001 | 0.18 | A, C < B |
| OA | 43.64 | <0.001 | 0.33 | A, C < B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, J.-C.; Buchet, A.; Sialelli, J.-N.; Delouvée, S. Antibiotic Use in Relation with Psychological Profiles of Farmers of a French Pig Cooperative. Vet. Sci. 2022, 9, 14. https://doi.org/10.3390/vetsci9010014
David J-C, Buchet A, Sialelli J-N, Delouvée S. Antibiotic Use in Relation with Psychological Profiles of Farmers of a French Pig Cooperative. Veterinary Sciences. 2022; 9(1):14. https://doi.org/10.3390/vetsci9010014
Chicago/Turabian StyleDavid, Jean-Charles, Arnaud Buchet, Jean-Noël Sialelli, and Sylvain Delouvée. 2022. "Antibiotic Use in Relation with Psychological Profiles of Farmers of a French Pig Cooperative" Veterinary Sciences 9, no. 1: 14. https://doi.org/10.3390/vetsci9010014
APA StyleDavid, J.-C., Buchet, A., Sialelli, J.-N., & Delouvée, S. (2022). Antibiotic Use in Relation with Psychological Profiles of Farmers of a French Pig Cooperative. Veterinary Sciences, 9(1), 14. https://doi.org/10.3390/vetsci9010014







