The Protective Effect of Snail Secretion Filtrate in an Experimental Model of Excisional Wounds in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Snail Secretion Filtrate (SSF) Collection and Sterilization
2.3. Full-Thickness Excisional Wound Model
2.4. Histopathological and Immunohistochemical Analyses
2.5. ELISA
2.6. Toluidine Blue Staining
2.7. Western Blot
2.8. Statistical Analysis
3. Results
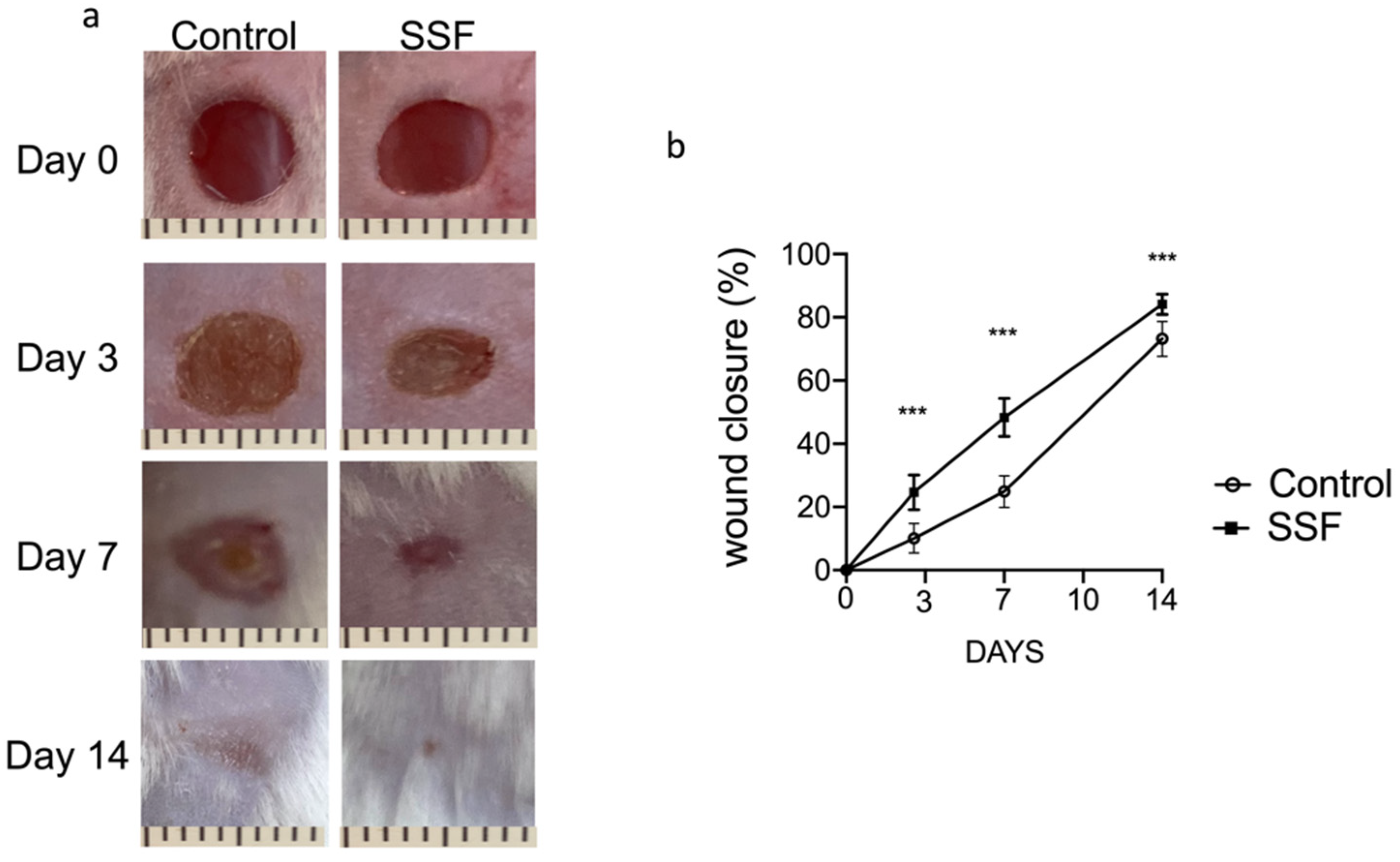
3.1. Snail Secretion Filtrate Accelerated the Rate of Wound Healing
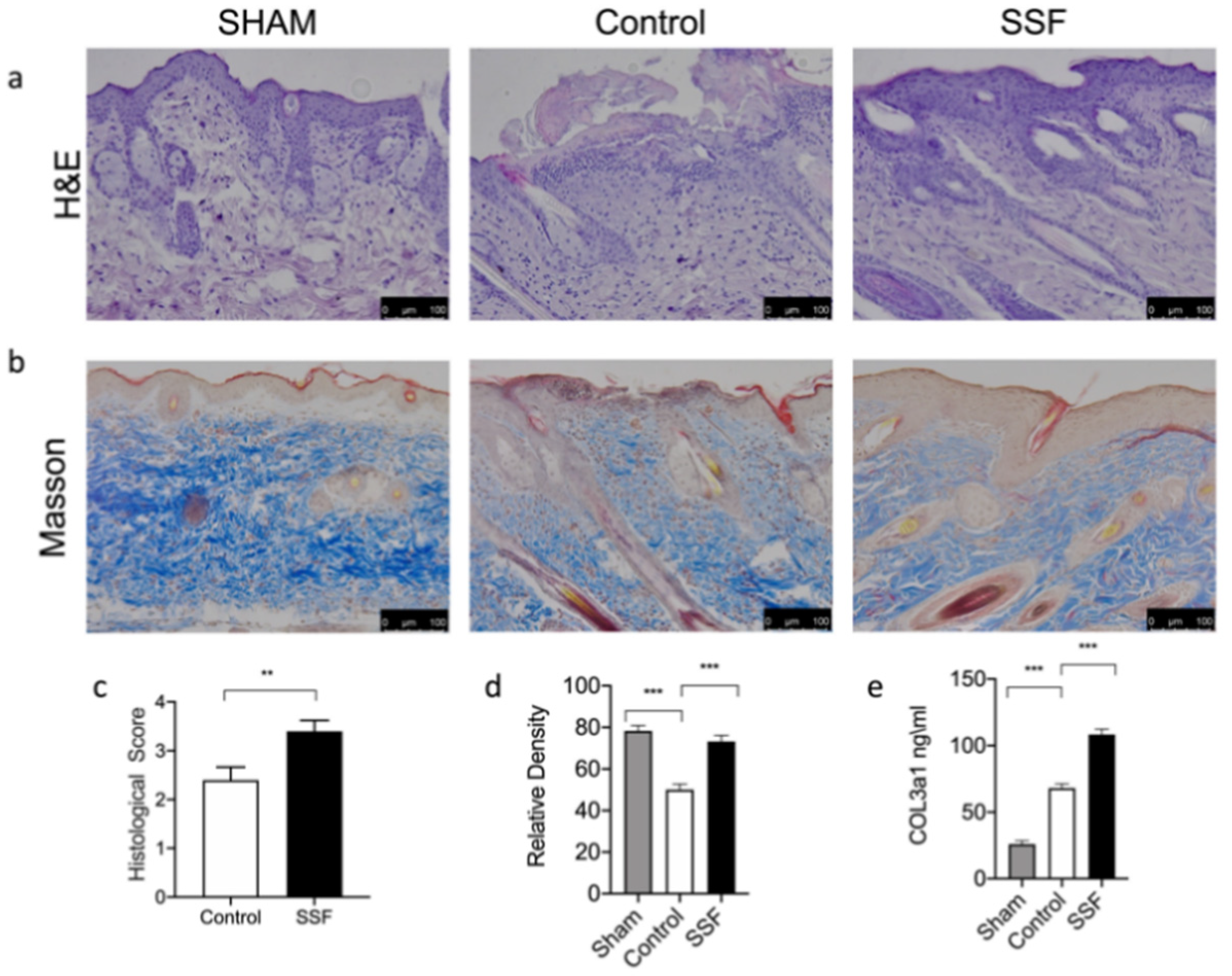
3.2. Effect of SSF on Skin Wound Healing and Collagen Pattern
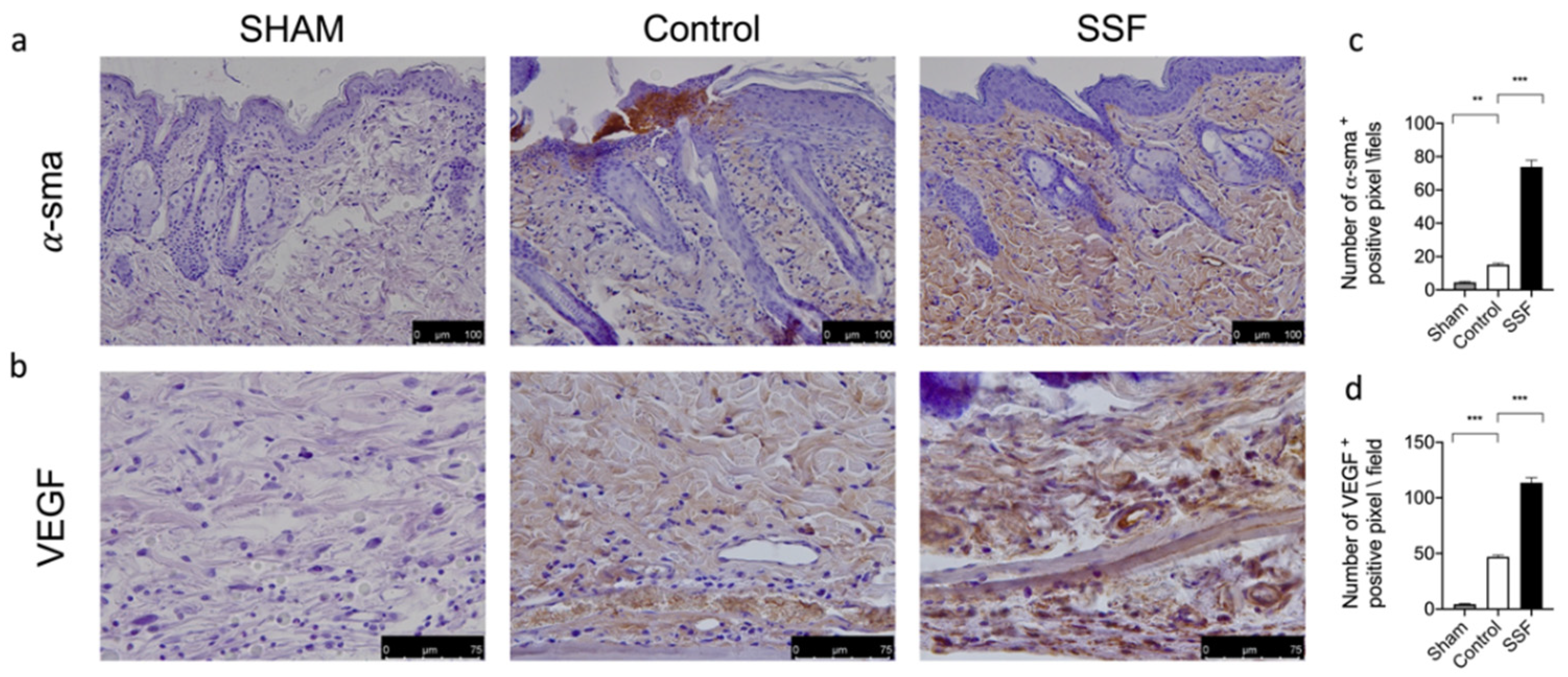
3.3. Effect of SSF on α-sma and VEGF Expression on Wounds
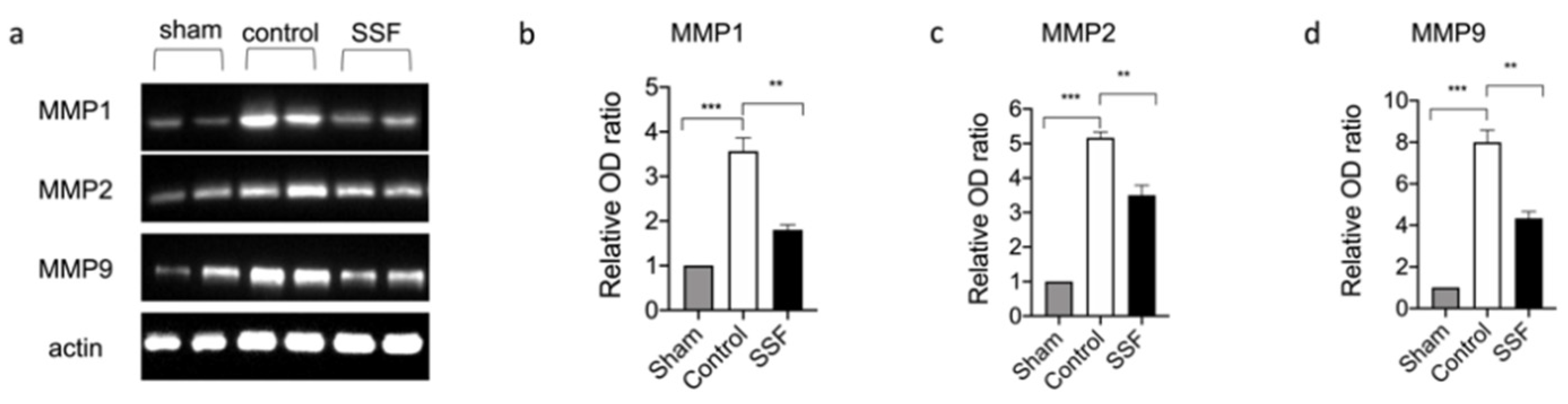
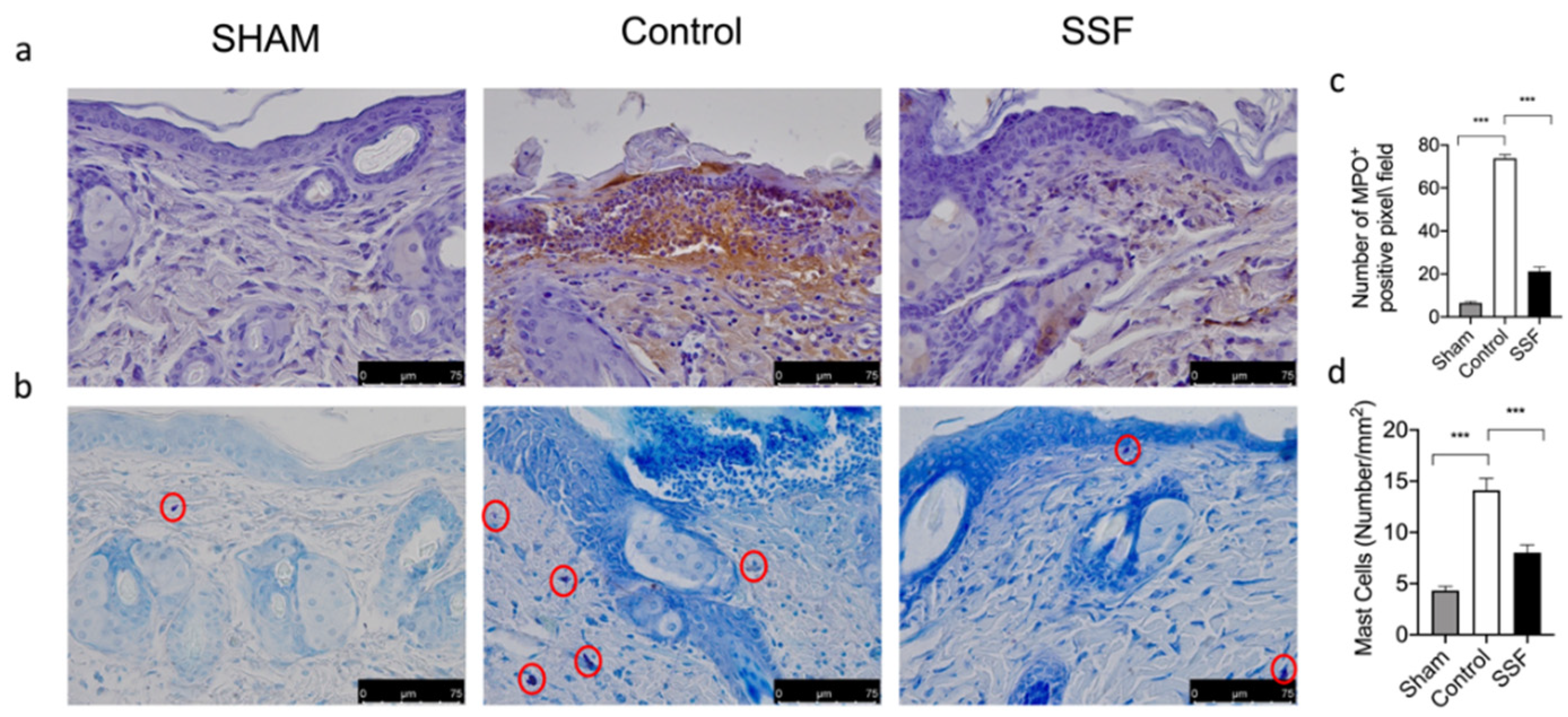
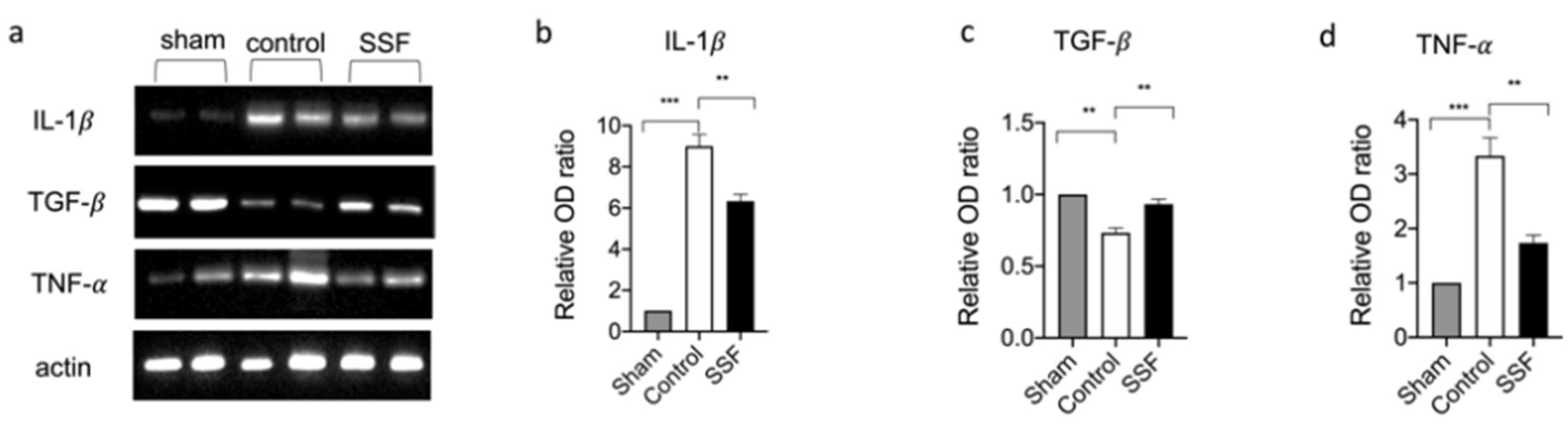
3.4. Effect of SSF on Immune Cell Infiltration and Inflammatory Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsoutsos, D.; Kakagia, D.; Tamparopoulos, K. The efficacy of Helix aspersa Muller extract in the healing of partial thickness burns: A novel treatment for open burn management protocols. J. Dermatol. Treat. 2009, 20, 219–222. [Google Scholar] [CrossRef]
- Ellijimi, C.; Ben Hammouda, M.; Othman, H.; Moslah, W.; Jebali, J.; Mabrouk, H.B.; Morjen, M.; Haoues, M.; Luis, J.; Marrakchi, N.; et al. Helix aspersa maxima mucus exhibits antimelanogenic and antitumoral effects against melanoma cells. Biomed. Pharmacother. 2018, 101, 871–880. [Google Scholar] [CrossRef]
- Pitt, S.J.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Trapella, C.; Rizzo, R.; Gallo, S.; Alogna, A.; Bortolotti, D.; Casciano, F.; Zauli, G.; Secchiero, P.; Voltan, R. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci. Rep. 2018, 8, 17665. [Google Scholar] [CrossRef]
- Greistorfer, S.; Klepal, W.; Cyran, N.; Gugumuck, A.; Rudoll, L.; Suppan, J.; von Byern, J. Snail mucus—Glandular origin and composition in Helix pomatia. Zoology (Jena) 2017, 122, 126–138. [Google Scholar] [CrossRef]
- Gentili, V.; Bortolotti, D.; Benedusi, M.; Alogna, A.; Fantinati, A.; Guiotto, A.; Turrin, G.; Cervellati, C.; Trapella, C.; Rizzo, R.; et al. HelixComplex snail mucus as a potential technology against O3 induced skin damage. PLoS ONE 2020, 15, e0229613. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, E.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Siracusa, R.; Genovese, T.; D’Amico, R.; Impellizzeri, D.; Di Paola, R.; Cuzzocrea, S.; et al. Protective effect of snail secretion filtrate against ethanol-induced gastric ulcer in mice. Sci. Rep. 2021, 11, 3638. [Google Scholar] [CrossRef]
- Hu, C.; Chu, C.; Liu, L.; Wang, C.; Jin, S.; Yang, R.; Rung, S.; Li, J.; Qu, Y.; Man, Y. Dissecting the microenvironment around biosynthetic scaffolds in murine skin wound healing. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Di Paola, R.; Cordaro, M.; Gugliandolo, E.; Casili, G.; Morittu, V.M.; Britti, D.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 2016, 119, 27–41. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Campolo, M.; Latteri, S.; Carughi, A.; Mandalari, G.; Cuzzocrea, S. The Antioxidant Activity of Pistachios Reduces Cardiac Tissue Injury of Acute Ischemia/Reperfusion (I/R) in Diabetic Streptozotocin (STZ)-Induced Hyperglycaemic Rats. Front. Pharmacol. 2018, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.R.; Xu, X.J.; Yao, S.K. Increased intestinal mucosal leptin levels in patients with diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2018, 24, 46–57. [Google Scholar] [CrossRef]
- Esposito, E.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S. A new co-micronized composite containing palmitoylethanolamide and polydatin shows superior oral efficacy compared to their association in a rat paw model of carrageenan-induced inflammation. Eur. J. Pharmacol. 2016, 782, 107–118. [Google Scholar] [CrossRef]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A Receptor by PDRN Reduces Neuronal Damage and Stimulates WNT/beta-CATENIN Driven Neurogenesis in Spinal Cord Injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Impellizzeri, D.; D’Amico, R.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Fusco, R.; Di Paola, R.; Schievano, C.; et al. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. Ther. 2019, 21, 254. [Google Scholar] [CrossRef] [Green Version]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Lopez Angulo, D.E.; do Amaral Sobral, P.J. Characterization of gelatin/chitosan scaffold blended with aloe vera and snail mucus for biomedical purpose. Int. J. Biol. Macromol. 2016, 92, 645–653. [Google Scholar] [CrossRef]
- Fleck, C.A.; Simman, R. Modern collagen wound dressings: Function and purpose. J. Am. Coll. Certif. Wound Spec. 2010, 2, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Chattopadhyay, P.; Goyary, D.; Mitra Mazumder, P.; Veer, V. Ixora coccinea Enhances Cutaneous Wound Healing by Upregulating the Expression of Collagen and Basic Fibroblast Growth Factor. ISRN Pharmacol. 2014, 2014, 751824. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Chattopadhyay, P.; Goyary, D.; Mazumder, P.M.; Veer, V. Eleutherine indica L. accelerates in vivo cutaneous wound healing by stimulating Smad-mediated collagen production. J. Ethnopharmacol. 2013, 146, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Yager, D.R.; Nwomeh, B.C. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999, 7, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Fray, M.J.; Dickinson, R.P.; Huggins, J.P.; Occleston, N.L. A potent, selective inhibitor of matrix metalloproteinase-3 for the topical treatment of chronic dermal ulcers. J. Med. Chem. 2003, 46, 3514–3525. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, B.; Tuft, S.J.; Khaw, P.T. Matrix metalloproteinase distribution during early corneal wound healing. Eye (Lond.) 2005, 19, 584–588. [Google Scholar] [CrossRef]
- Bonte, F.; Dumas, M.; Chaudagne, C.; Meybeck, A. Influence of asiatic acid, madecassic acid, and asiaticoside on human collagen I synthesis. Planta Med. 1994, 60, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [Green Version]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar] [PubMed]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen 2015, 23, 874–877. [Google Scholar] [CrossRef] [Green Version]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [Green Version]
- Mayhew, T.M. The new stereological methods for interpreting functional morphology from slices of cells and organs. Exp. Physiol. 1991, 76, 639–665. [Google Scholar] [CrossRef] [Green Version]
- Damsgaard, T.E.; Olesen, A.B.; Sorensen, F.B.; Thestrup-Pedersen, K.; Schiotz, P.O. Mast cells and atopic dermatitis. Stereological quantification of mast cells in atopic dermatitis and normal human skin. Arch. Dermatol. Res. 1997, 289, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet. Med. 2006, 23, 594–608. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugliandolo, E.; Macrì, F.; Fusco, R.; Siracusa, R.; D’Amico, R.; Cordaro, M.; Peritore, A.F.; Impellizzeri, D.; Genovese, T.; Cuzzocrea, S.; et al. The Protective Effect of Snail Secretion Filtrate in an Experimental Model of Excisional Wounds in Mice. Vet. Sci. 2021, 8, 167. https://doi.org/10.3390/vetsci8080167
Gugliandolo E, Macrì F, Fusco R, Siracusa R, D’Amico R, Cordaro M, Peritore AF, Impellizzeri D, Genovese T, Cuzzocrea S, et al. The Protective Effect of Snail Secretion Filtrate in an Experimental Model of Excisional Wounds in Mice. Veterinary Sciences. 2021; 8(8):167. https://doi.org/10.3390/vetsci8080167
Chicago/Turabian StyleGugliandolo, Enrico, Francesco Macrì, Roberta Fusco, Rosalba Siracusa, Ramona D’Amico, Marika Cordaro, Alessio Filippo Peritore, Daniela Impellizzeri, Tiziana Genovese, Salvatore Cuzzocrea, and et al. 2021. "The Protective Effect of Snail Secretion Filtrate in an Experimental Model of Excisional Wounds in Mice" Veterinary Sciences 8, no. 8: 167. https://doi.org/10.3390/vetsci8080167
APA StyleGugliandolo, E., Macrì, F., Fusco, R., Siracusa, R., D’Amico, R., Cordaro, M., Peritore, A. F., Impellizzeri, D., Genovese, T., Cuzzocrea, S., Paola, R. D., Licata, P., & Crupi, R. (2021). The Protective Effect of Snail Secretion Filtrate in an Experimental Model of Excisional Wounds in Mice. Veterinary Sciences, 8(8), 167. https://doi.org/10.3390/vetsci8080167











