Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma
Abstract
1. Introduction
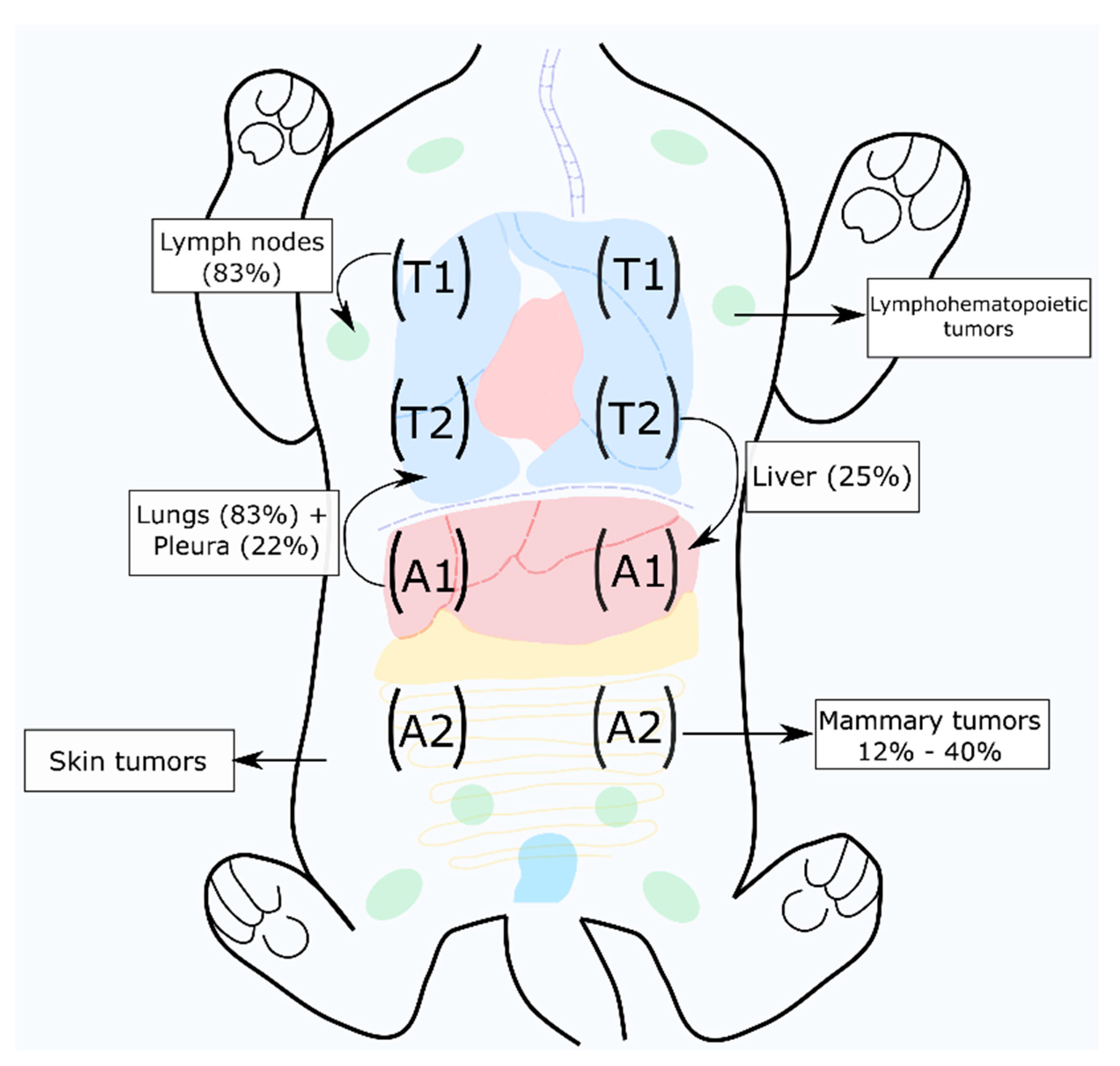
2. Feline Mammary Carcinoma
2.1. Mammary Tumor Diagnosis and Classification
2.2. Feline her2 Mutations Could Be Associated with Tumor Development
2.3. Prognostic Factors for Feline Mammary Carcinoma
3. Feline Mammary Carcinoma Cell-Based Models for Targeted Therapies
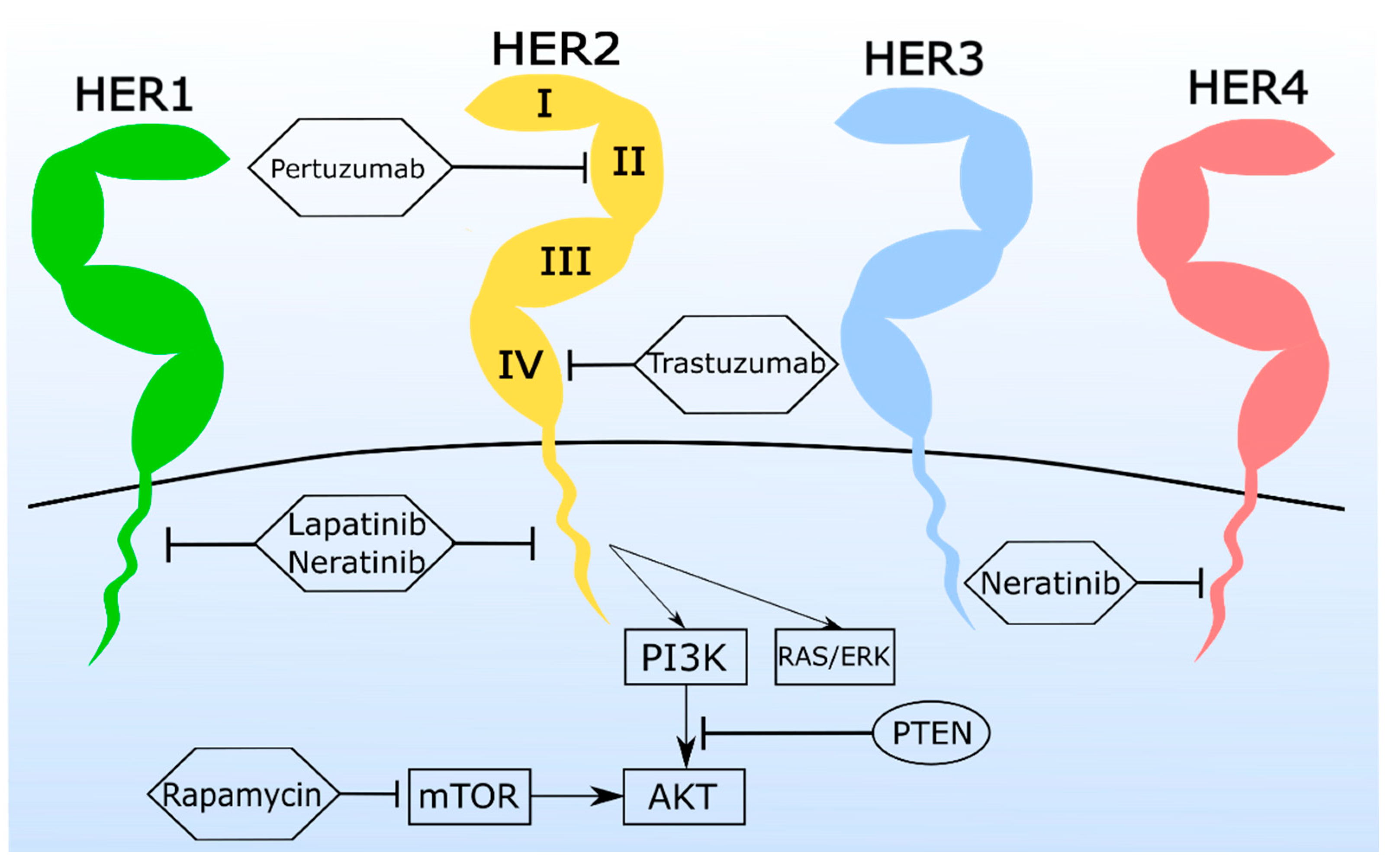
3.1. Monoclonal Antibodies (mAbs) and Antibody-Drug Conjugates (ADC) Are a Promising Tool for the Treatment of Feline Mammary Carcinoma
3.2. Tyrosine Kinase Inhibitors (TKi) Are Valuable in Feline Mammary Carcinoma Therapy
3.3. Combination Therapy Shows Synergistic Antiproliferative Effects in Feline Mammary Carcinoma Cell Lines
3.4. Novel In Vitro Approaches to Feline Mammary Carcinoma Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, J.K.; Gruffydd-Jones, T.J.; Roberts, M.A.; Browne, W.J. Assessing changes in the UK pet cat and dog populations: Numbers and household ownership. Vet. Rec. 2015, 177, 259. [Google Scholar] [CrossRef]
- Hoenig, M.; Hall, G.; Ferguson, D.; Jordan, K.; Henson, M.; Johnson, K.; O’Brien, T. A feline model of experimentally induced islet amyloidosis. Am. J. Pathol. 2000, 157, 2143–2150. [Google Scholar] [CrossRef]
- Henson, M.S.; O’Brien, T.D. Feline models of type 2 diabetes mellitus. ILAR J. 2006, 47, 234–242. [Google Scholar] [CrossRef]
- Zappulli, V.; De Zan, G.; Cardazzo, B.; Bargelloni, L.; Castagnaro, M. Feline mammary tumours in comparative oncology. J. Dairy Res. 2005, 72, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Menotti-Raymond, M.; Deckman, K.H.; David, V.; Myrkalo, J.; O’Brien, S.J.; Narfström, K. Mutation discovered in a feline model of human congenital retinal blinding disease. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- De Maria, R.; Olivero, M.; Iussich, S.; Nakaichi, M.; Murata, T.; Biolatti, B.; Di Renzo, M.F.; Flavia, M.; Renzo, D.; Di Renzo, M.F. Spontaneous feline mammary carcinoma is a model of HER2 overexpressing poor prognosis human breast cancer. Cancer Res. 2005, 65, 907–912. [Google Scholar] [PubMed]
- Burrai, G.P.; Mohammed, S.I.; Miller, M.A.; Marras, V.; Pirino, S.; Addis, M.F.; Uzzau, S.; Antuofermo, E. Spontaneous feline mammary intraepithelial lesions as a model for human estrogen receptor and progesterone receptor-negative breast lesions. BMC Cancer 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Wiese, D.A.; Thaiwong, T.; Yuzbasiyan-Gurkan, V.; Kiupel, M. Feline mammary basal-like adenocarcinomas: A potential model for human triple-negative breast cancer (TNBC) with basal-like subtype. BMC Cancer 2013, 13. [Google Scholar] [CrossRef]
- Almeida, F.; Gameiro, A.; Correia, J.; Ferreira, F. Histone Deacetylase Inhibitors and Microtubule Inhibitors Induce Apoptosis in Feline Luminal Mammary Carcinoma Cells. Animals 2021, 11, 502. [Google Scholar] [CrossRef]
- Gameiro, A.; Almeida, F.; Nascimento, C.; Correia, J. Tyrosine Kinase Inhibitors Are Promising Therapeutic Tools for Cats with HER2-Positive Mammary Carcinoma. Pharmaceutics 2021, 13, 346. [Google Scholar] [CrossRef]
- Gameiro, A.; Nascimento, C.; Correia, J.; Ferreira, F. HER2-Targeted Immunotherapy and Combined Protocols Showed Promising Antiproliferative Effects in Feline Mammary Carcinoma Cell-Based Models. Cancers 2021, 13, 2007. [Google Scholar] [CrossRef]
- Novosad, C.A. Principles of Treatment for Mammary Gland Tumors. Clin. Tech. Small Anim. Pract. 2003, 18, 107–109. [Google Scholar] [CrossRef]
- Panieri, E. Breast cancer screening in developing countries. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 283–290. [Google Scholar] [CrossRef]
- Santos, S.; Baptista, C.S.; Abreu, R.M.V.; Bastos, E.; Amorim, I.; Gut, I.G.; Gärtner, F.; Chaves, R. ERBB2 in cat mammary neoplasias disclosed a positive correlation between RNA and protein low expression levels: A model for erbB-2 negative human breast cancer. PLoS ONE 2013, 8, e83673. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Macewen, E.G. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Investig. 2000, 18, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Correia, J.; Peleteiro, M.C.; Ferreira, F. St Gallen molecular subtypes in feline mammary carcinoma and paired metastases—disease progression and clinical implications from a 3-year follow-up study. Tumor Biol. 2016, 37, 4053–4064. [Google Scholar] [CrossRef]
- Soares, M.; Madeira, S.; Correira, J.; Peleteiro, M.; Cardoso, F.; Ferreira, F.; Correia, J.; Peleteiro, M.; Cardoso, F.; Ferreira, F. Molecular based subtyping of feline mammary carcinomas and clinicopathological characterization. Breast 2016, 27, 44–51. [Google Scholar] [CrossRef]
- Cho, H.-S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef]
- Phillips, G.D.L.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Canonici, A.; Gijsen, M.; Mullooly, M.; Bennett, R.; Bouguern, N.; Pedersen, K.; O’Brien, N.A.; Roxanis, I.; Li, J.-L.; Bridge, E.; et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget 2013, 4, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.L.; Stevens, C.L.; Sridhar, J. Small molecule tyrosine kinase inhibitors of ErbB2/HER2/Neu in the treatment of aggressive breast cancer. Molecules 2014, 19, 15196–15212. [Google Scholar] [CrossRef]
- Richard, S.; Selle, F.; Lotz, J.P.; Khalil, A.; Gligorov, J.; Grazziotin-Soares, D. Pertuzumab and trastuzumab: The rationale way to synergy. An. Acad. Bras. Cienc. 2016, 88, 565–577. [Google Scholar] [CrossRef]
- Cocco, E.; Carmona, F.J.; Razavi, P.; Won, H.H.; Cai, Y.; Rossi, V.; Chan, C.; Cownie, J.; Soong, J.; Toska, E.; et al. Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 ( HER2 ). Sci. Signal. 2018, 11, eaat9773. [Google Scholar] [PubMed]
- von Minckwitz, G.; Huang, C.-S.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Munster, P.N.; Troso-Sandoval, T.; Rosen, N.; Rifkind, R.; Marks, P.A.; Richon, V.M. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 2001, 61, 8492–8497. [Google Scholar]
- Huang, L.; Pardee, A.B. Suberoylanilide hydroxamic acid as a potential therapeutic agent for human breast cancer treatment. Mol. Med. 2000, 6, 849–866. [Google Scholar] [CrossRef]
- Risinger, A.L.; Dybdal-Hargreaves, N.F.; Mooberry, S.L. Breast cancer cell lines exhibit differential sensitivities to microtubule-targeting drugs independent of doubling time. Anticancer. Res. 2015, 35, 5845–5850. [Google Scholar]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies To Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Wang, G.; Cai, Q.; Zheng, X.; Zhang, J.; Chen, Q.; Wu, B.; Zhu, X.; Hao, H.; Zhou, F. A promising microtubule inhibitor deoxypodophyllotoxin exhibits better efficacy to multidrug-resistant breast cancer than paclitaxel via avoiding efflux transport. Drug Metab. Dispos. 2018, 46, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Weijer, K.; Head, K.W.; Misdorp, W.; Hampe, J.F. Feline malignant mammary tumors. I. morphology and biology: Some comparisons with human and canine mammary carcinomas1, 2. J. Natl. Cancer Inst. 1972, 49, 1697–1704. [Google Scholar] [CrossRef]
- Giménez, F.; Hecht, S.; Craig, L.E.; Legendre, A.M. Early Detection, Aggressive Therapy. J. Feline Med. Surg. 2010, 12, 214–224. [Google Scholar] [CrossRef]
- Millanta, F.; Calandrella, M.; Citi, S.; della Santa, D.; Poli, A. Overexpression of HER-2 in feline invasive mammary carcinomas: An immunohistochemical survey and evaluation of its prognostic potential. Vet. Pathol. 2005, 42, 30–34. [Google Scholar] [CrossRef]
- Overley, B.; Shofer, F.S.; Goldschmidt, M.H.; Sherer, D.; Sorenmo, K.U. Association between ovarihysterectomy and feline mammary carcinoma. J. Vet. Intern. Med. 2005, 19, 560–563. [Google Scholar] [CrossRef]
- Preziosi, R.; Sarli, G.; Benazzi, C.; Mandrioli, L.; Marcato, P.S. Multiparametric survival analysis of histological stage and proliferative activity in feline mammary carcinomas. Res. Vet. Sci. 2002, 73, 53–60. [Google Scholar] [CrossRef]
- Chocteau, F.; Boulay, M.M.; Besnard, F.; Valeau, G.; Loussouarn, D.; Nguyen, F. Proposal for a Histological Staging System of Mammary Carcinomas in Dogs and Cats. Part 2: Feline Mammary Carcinomas. Front. Vet. Sci. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Mills, S.W.; Musil, K.M.; Davies, J.L.; Hendrick, S.; Duncan, C.; Jackson, M.L.; Kidney, B.; Philibert, H.; Wobeser, B.K.; Simko, E. Prognostic Value of Histologic Grading for Feline Mammary Carcinoma: A Retrospective Survival Analysis. Vet. Pathol. 2015, 52, 238–249. [Google Scholar] [CrossRef]
- Soares, M.; Ribeiro, R.; Najmudin, S.; Gameiro, A.; Rodrigues, R.; Cardoso, F.; Ferreira, F. Serum HER2 levels are increased in cats with mammary carcinomas and predict tissue HER2 status. Oncotarget 2016, 7, 17314–17326. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Bastos, E.; Baptista, C.S.; Sá, D.; Caloustian, C.; Guedes-Pinto, H.; Gärtner, F.; Gut, I.G.; Chaves, R. Sequence variants and haplotype analysis of cat ERBB2 gene: A survey on spontaneous cat mammary neoplastic and non-neoplastic lesions. Int. J. Mol. Sci. 2012, 13, 2783–2800. [Google Scholar] [CrossRef] [PubMed]
- Witton, C.J.; Reeves, J.R.; Going, J.J.; Cooke, T.G.; Barlett, J.M.S. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J. Pathol. 2003, 200, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Feng, X.; Qu, J.; Han, W.; Liu, Z.; Li, X.; Zou, M.; Zhen, Y.; Zhu, J. Expression and Characterization of the Extracellular Domain of Human HER2 from Escherichia Coli, and Production of Polyclonal Antibodies Against the Recombinant Proteins. Appl. Biochem. Biotechnol. 2015, 176, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Tripathy, D.; Shete, S.; Ashfaq, R.; Haley, B.; Perkins, S.; Beitsch, P.; Khan, A.; Euhus, D.; Osborne, C.; et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc. Natl. Acad. Sci. USA 2004, 101, 9393–9398. [Google Scholar] [CrossRef]
- Jiang, H.; Bai, X.; Zhang, C.; Zhang, X. Evaluation of HER2 gene amplification in breast cancer using nuclei microarray in Situ hybridization. Int. J. Mol. Sci. 2012, 13, 5519–5527. [Google Scholar] [CrossRef] [PubMed]
- Vicario, R.; Peg, V.; Morancho, B.; Zacarias-Fluck, M.; Zhang, J.; Martínez-Barriocanal, Á.; Jiménez, A.N.; Aura, C.; Burgues, O.; Lluch, A.; et al. Patterns of HER2 gene amplification and response to anti-HER2 therapies. PLoS ONE 2015, 10, e0129876. [Google Scholar] [CrossRef]
- Soares, M.; Correia, J.; Rodrigues, P.; Simões, M.; de Matos, A.; Ferreira, F. Feline HER2 Protein Expression Levels and Gene Status in Feline Mammary Carcinoma: Optimization of Immunohistochemistry (IHC) and In Situ Hybridization (ISH) Techniques. Microsc. Microanal. 2013, 19, 876–882. [Google Scholar] [CrossRef]
- Ferreira, D.; Soares, M.; Correia, J.; Adega, F.; Ferreira, F.; Chaves, R. Assessment of ERBB2 and TOP2agene statusand expression profilein feline mammary tumors: Findings and guidelines. Aging 2019, 11, 4688. [Google Scholar] [CrossRef]
- Muscatello, L.V.; Di Oto, E.; Sarli, G.; Monti, V.; Foschini, M.P.; Benazzi, C.; Brunetti, B. HER2 Amplification Status in Feline Mammary Carcinoma: A Tissue Microarray–Fluorescence In Situ Hydridization–Based Study. Vet. Pathol. 2019, 56, 230–238. [Google Scholar] [CrossRef]
- Hanker, A.B.; Brewer, M.R.; Sheehan, J.H.; Koch, J.P.; Sliwoski, G.R.; Nagy, R.; Lanman, R.; Berger, M.F.; Hyman, D.M.; Solit, D.B.; et al. An acquired HER2T798Igatekeeper mutation induces resistance to neratinib in a patient with HER2 mutant-driven breast cancer. Cancer Discov. 2017, 7, 575–585. [Google Scholar] [CrossRef]
- Rockberg, J.; Schwenk, J.M.; Uhlén, M. Discovery of epitopes for targeting the human epidermal growth factor receptor 2 (HER2) with antibodies. Mol. Oncol. 2009, 3, 238–247. [Google Scholar] [CrossRef]
- Kanthala, S.; Mill, C.P.; Riese, D.J.; Jaiswal, M.; Jois, S. Expression and purification of HER2 extracellular domain proteins in Schneider2 insect cells. Protein Expr. Purif. 2016, 125, 26–33. [Google Scholar] [CrossRef] [PubMed]
- McNeill, C.J.; Sorenmo, K.U.; Shofer, F.S.; Gibeon, L.; Durham, A.C.; Barber, L.G.; Baez, J.L.; Overley, B. Evaluation of Adjuvant Doxorubicin-Based Chemotherapy for the Treatment of Feline Mammary Carcinoma. J. Chem. Inf. Model. 2009, 53, 1689–1699. [Google Scholar] [CrossRef]
- Ito, T.; Kadosawa, T.; Mochizuki, M.; Matsunaga, S.; Nishimura, R.; Sasaki, N. Prognosis of malignant mammary tumor in 53 cats. J. Vet. Med. Sci. 1996, 58, 723–726. [Google Scholar] [CrossRef]
- Soares, M.; Ribeiro, R.; Carvalho, S.; Peleteiro, M.; Correia, J.; Ferreira, F. Ki-67 as a Prognostic Factor in Feline Mammary Carcinoma: What Is the Optimal Cutoff Value? Vet. Pathol. 2016, 53, 37–43. [Google Scholar] [CrossRef]
- Maniscalco, L.; Iussich, S.; de Las Mulas, J.M.; Millán, Y.; Biolatti, B.; Sasaki, N.; Nakagawa, T.; De Maria, R.; Martín de las Mulas, J.; Millán, Y.; et al. Activation of AKT in feline mammary carcinoma: A new prognostic factor for feline mammary tumours. Vet. J. 2012, 191, 65–71. [Google Scholar] [CrossRef]
- Watanabe, R.; Wei, L.; Huang, J. mTOR Signaling, Function, Novel Inhibitors, and Therapeutic Targets. J. Nucl. Med. 2011, 52, 497–501. [Google Scholar] [CrossRef]
- Maniscalco, L.; Millan, Y.; Iussich, S.; Denina, M.; Sanchez-Cespedes, R.; Gattino, F.; Biolatti, B.; Sasaki, N.; Nakagawa, T.; Di Renzo, M.F.; et al. Activation of mammalian target of rapamycin (mTOR) in triple negative feline mammary carcinomas. BMC Vet. Res. 2013, 9, 1–9. [Google Scholar] [CrossRef]
- Murakami, Y.; Tateyama, S.; Rungsipipat, A.; Uchida, K.; Yamaguchi, R. Immunohistochemical Analysis of Cyclin A, Cyclin D1 and P53 in Mammary Tumors, Squamous Cell Carcinomas and Basal Cell Tumors of Dogs and Cats. J. Vet. Med. Sci. 2000, 62, 743–750. [Google Scholar] [CrossRef]
- Nakano, M.; Wu, H.; Taura, Y.; Inoue, M. Immunohistochemical detection of Mdm2 and p53 in feline mammary gland tumors. J. Vet. Med. Sci. 2006, 68, 421–425. [Google Scholar] [CrossRef][Green Version]
- Morris, J.S.; Nixon, C.; Bruck, A.; Nasir, L.; Morgan, I.M.; Philbey, A.W. Immunohistochemical expression of TopBP1 in feline mammary neoplasia in relation to histological grade, Ki67, ERα and p53. Vet. J. 2008, 175, 218–226. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Marques, C.S.; Santos, A.R.; Gameiro, A.; Correia, J.; Ferreira, F. CXCR4 and its ligand CXCL12 display opposite expression profiles in feline mammary metastatic disease, with the exception of HER2-overexpressing tumors. BMC Cancer 2018, 18, 1–13. [Google Scholar] [CrossRef]
- Liu, F.; Lang, R.; Wei, J.; Fan, Y.; Cui, L.; Gu, F.; Guo, X.; Pringle, G.A.; Zhang, X.; Fu, L. Increased expression of SDF-1/CXCR4 is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Histopathology 2009, 54, 741–750. [Google Scholar] [CrossRef]
- Marques, C.S.; Soares, M.; Santos, A.; Correia, J.; Ferreira, F. Serum SDF-1 levels are a reliable diagnostic marker of feline mammary carcinoma, discriminating HER2-overexpressing tumors from other subtypes. Oncotarget 2017, 8, 105775–105789. [Google Scholar] [CrossRef]
- Nascimento, C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. Diagnostic Value of VEGF-A, VEGFR-1 and VEGFR-2 in Feline Mammary Carcinoma. Cancers 2021, 13, 117. [Google Scholar] [CrossRef]
- Nascimento, C.; Urbano, A.C.; Gameiro, A.; Correia, J.; Ferreira, F. Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes. Cancers 2020, 12, 1386. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Koutsopoulos, A.V.; Tsoulfas, P.G.; Lagoudaki, E.; Aggouraki, D.; Monastirioti, A.; Koutoulaki, C.; Apostolopoulou, C.A.; Merodoulaki, A.C.; Papadaki, C.; et al. Clinical relevance of immune checkpoints on circulating tumor cells in breast cancer. Cancers 2020, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, A.; Nascimento, C.; Urbano, A.C.; Correia, J.; Ferreira, F. Serum levels and tumour expression of leptin and leptin receptor as promising clinical biomarkers of specific feline mammary carcinoma subtypes. Front. Vet. Sci. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Hosney, M.; Sabet, S.; Shinawi, M.E.L. Leptin is overexpressed in the tumor microenvironment of obese patients with estrogen receptor positive breast cancer. Exp. Ther. Med. 2017, 13, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Deng, L.-L.; Cui, J.-Q.; Shi, L.; Yang, Y.-C.; Luo, J.-H.; Qin, D.; Wang, L. Association between serum leptin levels and breast cancer risk: An updated systematic review and meta-analysis. Medicine 2018, 97, e11345. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Sasaki, N.; Honjoh, T.; Ohishi, I.; Takiguchi, M.; Ishioka, K.; Ahmed, M.; Soliman, M.; Kimura, K.; Saito, M. Feline Leptin: Immunogenic and Biological Activities of the Recombinant Protein, and Its Measurement by ELISA. J. Vet. Med. Sci. 2003, 65, 1207–1211. [Google Scholar] [CrossRef]
- Georgiou, G.P.; Provatopoulou, X.; Kalogera, E.; Siasos, G.; Menenakos, E.; Zografos, G.C.; Gounaris, A. Serum resistin is inversely related to breast cancer risk in premenopausal women. Breast 2016, 29, 163–169. [Google Scholar] [CrossRef]
- Saxena, N.; Taliaferro-Smith, L.; Knight, B.B.; Merlin, D.; Anania, F.A.; O’Regan, R.M.; Sharma, D. Bidirectional Crosstalk between Leptin and Insulin-Like Growth Factor-1 Signaling Promotes Invasion and Migration of Breast Cancer Cells via Transactivation of Epidermal Growth Factor Receptor. Cancer Res. 2008, 68, 9712–9722. [Google Scholar] [CrossRef]
- Liang, X.; Wang, S.; Wang, X.; Zhang, L.; Zhao, H.; Zhang, L. Leptin promotes the growth of breast cancer by upregulating the wnt/β-catenin pathway. Exp. Ther. Med. 2018, 16, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell. Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lim, J.H.; Choi, S.W.; Kim, M.; Kim, S.T.; Kim, M.S.; Cho, Y.S.; Chun, E.; Lee, K.Y. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem. Biophys. Res. Commun. 2010, 394, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Funahashi, T.; Tanaka, S.; Taguchi, T.; Tamaki, Y.; Shimomura, I.; Noguchi, S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int. J. Cancer 2006, 118, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kitayama, J.; Nagawa, H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin. Cancer Res. 2004, 10, 4325–4331. [Google Scholar] [CrossRef]
- Urbano, A.C.; Nascimento, C.; Soares, M.; Correia, J.; Ferreira, F. Clinical Relevance of the serum CTLA-4 in Cats with Mammary Carcinoma. Sci. Rep. 2020, 10, 3822. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Jiang, J. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer: A systematic review and meta-analysis. Medicine 2019, 98, e15201. [Google Scholar] [CrossRef]
- Li, Y.; Cui, X.; Yang, Y.J.; Chen, Q.Q.; Zhong, L.; Zhang, T.; Cai, R.L.; Miao, J.Y.; Yu, S.C.; Zhang, F. Serum sPD-1 and sPD-L1 as Biomarkers for Evaluating the Efficacy of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients. Clin. Breast Cancer 2019, 19, 326–332.e1. [Google Scholar] [CrossRef]
- Michishita, M.; Ohtsuka, A.; Nakahira, R.; Tajima, T.; Nakagawa, T.; Sasaki, N.; Arai, T.; Takahashi, K. Anti-tumor effect of bevacizumab on a xenograft model of feline mammary carcinoma. J. Vet. Med. Sci. 2016, 78, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Jeglum, K.A.; DeGuzman, E.; Young, K.M. Chemotherapy of advanced mammary adenocarcinoma in 14 cats. J. Am. Vet. Med. Assoc. 1985, 187, 157–160. [Google Scholar] [PubMed]
- Uyama, R.; Hong, S.-H.; Nakagawa, T.; Yazawa, M.; Kadosawa, T.; Mochizuki, M.; Tsujimoto, H.; Nishumura, R.; Sasaki, N. Establishment and Characterization of Eight Feline Mammary Adenocarcinoma Cell Lines. J. Vet. Med. Sci. 2005, 67, 1273–1276. [Google Scholar] [CrossRef]
- Chuang, H.; Lin, Y.; Chen, T. Establishment and Characterization of a Feline Mammary Tumor Patient-Derived Xenograft Model Methods. BMC Vet. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Hassan, B.B.; Elshafae, S.M.; Supsavhad, W.; Simmons, J.K.; Dirksen, W.P.; Sokkar, S.M.; Rosol, T.J. Feline Mammary Cancer: Novel Nude Mouse Model and Molecular Characterization of Invasion and Metastasis Genes. Vet. Pathol. 2017, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Figueira, A.C.; Gomes, C.; Mendes, N.; Amorim, I.; de Matos, A.J.F.; Dias-Pereira, P.; Gärtner, F. Catenin Adhesion Complex in a Feline Mammary Carcinoma Cell Line. Clin. Diagn. Pathol. 2016, 1, 1–8. [Google Scholar] [CrossRef][Green Version]
- Subik, K.; Lee, J.-F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.-C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer 2010, 4, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, A.; Weinberg, R.A. Comparative biology of mouse versus human cells: Modelling human cancer in mice. Nat. Rev. Cancer 2003, 3, 952–959. [Google Scholar] [CrossRef]
- De Las Mulas, J.M.; Reymundo, C. Animal models of human breast carcinoma: Canine and feline neoplasms. Clin. Transl. Oncol. 2000, 2, 274–281. [Google Scholar] [CrossRef]
- Yamashita-Kashima, Y.; Shu, S.; Yorozu, K.; Moriya, Y.; Harada, N. Mode of action of pertuzumab in combination with trastuzumab plus docetaxel therapy in a HER2-positive breast cancer xenograft model. Oncol. Lett. 2017, 14, 4197–4205. [Google Scholar] [CrossRef]
- Bonkobara, M. Dysregulation of tyrosine kinases and use of imatinib in small animal practice. Vet. J. 2015, 205, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Agus, D.B.; Akita, R.W.; Fox, W.D.; Lewis, G.D.; Higgins, B.; Pisacane, P.I.; Lofgren, J.A.; Tindell, C.; Evans, D.P.; Maiese, K.; et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002, 2, 127–137. [Google Scholar] [CrossRef]
- Scheuer, W.; Friess, T.; Burtscher, H.; Bossenmaier, B.; Endl, J.; Hasmann, M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009, 69, 9330–9336. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Gelmon, K.A.; Verma, S.; Wardley, A.; Conte, P.F.; Miles, D.; Bianchi, G.; Cortes, J.; McNally, V.A.; Ross, G.A.; et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J. Clin. Oncol. 2010, 28, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Metzger-Filho, O.; Winer, E.P.; Krop, I. Pertuzumab: Optimizing HER2 blockade. Clin. Cancer Res. 2013, 19, 5552–5556. [Google Scholar] [CrossRef]
- Klapper, L.N.; Waterman, H.; Sela, M.; Yarden, Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 2000, 60, 3384–3388. [Google Scholar]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Nahta, R.; Yuan, L.X.H.; Du, Y.; Esteva, F.J. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: Effects on insulin-like growth factor I signaling. Mol. Cancer Ther. 2007, 6, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 364, 225–237. [Google Scholar] [CrossRef]
- Menyhart, O.; Santarpia, L.; Gyorffy, B. A Comprehensive Outline of Trastuzumab Resistance Biomarkers in HER2 Overexpressing Breast Cancer. Curr. Cancer Drug Targets 2015, 15, 665–683. [Google Scholar] [CrossRef]
- Kast, K.; Schoffer, O.; Link, T.; Forberger, A.; Petzold, A.; Niedostatek, A.; Werner, C.; Klug, S.J.; Werner, A.; Gatzweiler, A.; et al. Trastuzumab and survival of patients with metastatic breast cancer. Arch. Gynecol. Obstet. 2017, 296, 303–312. [Google Scholar] [CrossRef]
- Lambert, J.M.; Chari, R.V.J. Ado-trastuzumab emtansine (T-DM1): An antibody-drug conjugate (ADC) for HER2-positive breast cancer. J. Med. Chem. 2014, 57, 6949–6964. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, V.; Beaudoin, S.; Jean, S.; Leyton, J.V. A Novel Proteomic Method Reveals NLS Tagging of T-DM1 Contravenes Classical Nuclear Transport in a Model of HER2-Positive Breast Cancer. Mol. Ther. Methods Clin. Dev. 2020, 19, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Fan, J.; Wang, Z.; Zai, W.; Song, P.; Li, Y.; Ju, D. The role of autophagy in the cytotoxicity induced by trastuzumab emtansine (T-DM1) in HER2-positive breast cancer cells. AMB Express 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; De Vos, A.M.; Sliwkowski, M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Diermeier-Daucher, S.; Breindl, S.; Buchholz, S.; Ortmann, O.; Brockhoff, G. Modular anti-EGFR and anti-Her2 targeting of SK-BR-3 and BT474 breast cancer cell lines in the presence of ErbB receptor-specific growth factors. Cytom. Part A 2011, 79, 684–693. [Google Scholar] [CrossRef]
- Li, J.; Ma, M.; Yang, X.; Zhang, M.; Luo, J.; Zhou, H.; Huang, N.; Xiao, F.; Lai, B.; Lv, W.; et al. Circular HER2 RNA positive triple negative breast cancer is sensitive to Pertuzumab. Mol. Cancer 2020, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yokote, H.; Murakami-murofushi, K.; Tamura, T.; Saijo, N.; Nishio, K. Pertuzumab, a novel HER dimerization inhibitor, inhibits the growth of human lung cancer cells mediated by the HER3 signaling pathway. Cancer Sci. 2007, 98, 1498–1503. [Google Scholar] [CrossRef]
- Burguin, A.; Furrer, D.; Ouellette, G.; Jacob, S.; Diorio, C.; Durocher, F. Trastuzumab effects depend on HER2 phosphorylation in HER2-negative breast cancer cell lines. PLoS ONE 2020, 15, e0234991. [Google Scholar] [CrossRef]
- Endo, Y.; Takeda, K.; Mohan, N.; Shen, Y.; Jiang, J.; Rotstein, D.; Wu, W.J. Payload of T-DM1 binds to cell surface cytoskeleton-associated protein 5 to mediate cytotoxicity of hepatocytes. Oncotarget 2018, 9, 37200–37215. [Google Scholar] [CrossRef]
- Weigelt, B.; Lo, A.T.; Park, C.C.; Gray, J.W.; Bissell, M.J. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res. Treat. 2010, 122, 35–43. [Google Scholar] [CrossRef]
- Tatara, T.; Mukohara, T.; Tanaka, R.; Shimono, Y.; Funakoshi, Y.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Hirai, M.; Kakeji, Y.; et al. 3D Culture Represents Apoptosis Induced by Trastuzumab Better than 2D Monolayer Culture. Anticancer. Res. 2018, 38, 2831–2839. [Google Scholar] [CrossRef] [PubMed]
- Matkar, S.; An, C.; Hua, X. Kinase inhibitors of HER2/AKT pathway induce ERK phosphorylation via a FOXO-dependent feedback loop. Am. J. Cancer Res. 2017, 7, 1476–1485. [Google Scholar]
- Faber, A.C.; Li, D.; Song, Y.C.; Liang, M.C.; Yeap, B.Y.; Bronson, R.T.; Lifshits, E.; Chen, Z.; Maira, S.M.; García-Echeverría, C.; et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc. Natl. Acad. Sci. USA 2009, 106, 19503–19508. [Google Scholar] [CrossRef]
- O’Brien, N.A.; Browne, B.C.; Chow, L.; Wang, Y.; Ginther, C.; Arboleda, J.; Duffy, M.J.; Crown, J.; O’Donovan, N.; Slamon, D.J. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol. Cancer Ther. 2010, 9, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Frenel, J.S.; Bourbouloux, E.; Berton-Rigaud, D.; Sadot-Lebouvier, S.; Zanetti, A.; Campone, M. Lapatinib in metastatic breast cancer. Women’s Heal. 2009, 5, 603–612. [Google Scholar] [CrossRef]
- Opdam, F.L.; Guchelaar, H.; Beijnen, J.H.; Schellens, J.H.M. Lapatinib for Advanced or Metastatic Breast Cancer. Oncologist 2012, 17, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, W.; Zhi, Q.; Jiang, M. Lapatinib resistance in HER2+ cancers: Latest findings and new concepts on molecular mechanisms. Tumor Biol. 2016, 37, 15411–15431. [Google Scholar] [CrossRef]
- Stanley, A.; Ashrafi, G.H.; Seddon, A.M.; Modjtahedi, H. Synergistic effects of various Her inhibitors in combination with IGF-1R, C-MET and Src targeting agents in breast cancer cell lines. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Tiwari, S.R.; Mishra, P.; Abraham, J. Neratinib, A Novel HER2-Targeted Tyrosine Kinase Inhibitor. Clin. Breast Cancer 2015, 16, 344–348. [Google Scholar] [CrossRef]
- Carmona, F.J.; Montemurro, F.; Kannan, S.; Rossi, V.; Verma, C.; Baselga, J.; Scaltriti, M. AKT signaling in ERBB2-amplified breast cancer F. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Kidger, A.M.; Sipthorp, J.; Cook, S.J. ERK1/2 inhibitors: New weapons to inhibit the RAS-regulated RAF-MEK1/2-ERK1/2 pathway. Pharmacol. Ther. 2018, 187, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Yu, Z. EGFR promotes the development of triple negative breast cancer through JAK/STAT3 signaling. Cancer Manag. Res. 2020, 12, 703–717. [Google Scholar] [CrossRef]
- Chen, Y.J.; Yeh, M.H.; Yu, M.C.; Wei, Y.L.; Chen, W.S.; Chen, J.Y.; Shih, C.Y.; Tu, C.Y.; Chen, C.H.; Hsia, T.C.; et al. Lapatinib-induced NF-kappaB activation sensitizes triple-negative breast cancer cells to proteasome inhibitors. Breast Cancer Res. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Hu, M.H.; Hsu, C.J.; Huang, C.T.; Wang, D.S.; Tsai, W.C.; Chen, Y.T.; Lee, C.H.; Chu, P.Y.; Hsu, C.C.; et al. Lapatinib inhibits CIP2A/PP2A/p-Akt signaling and induces apoptosis in triple negative breast cancer cells. Oncotarget 2016, 7, 9135–9149. [Google Scholar] [CrossRef]
- Scaltriti, M.; Verma, C.; Guzman, M.; Jimenez, J.; Parra, J.L.; Pedersen, K.; Landolfi, S.; Ramon y Cajal, S.; Arribas, J.; Baselga, J. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 2009, 28, 803–814. [Google Scholar] [CrossRef]
- McGowan, P.M.; Mullooly, M.; Caiazza, F.; Sukor, S.; Madden, S.F.; Maguire, A.A.; Pierce, A.; McDermott, E.W.; Crown, J.; O’Donovan, N.; et al. ADAM-17: A novel therapeutic target for triple negative breast cancer. Ann. Oncol. 2013, 24, 362–369. [Google Scholar] [CrossRef]
- Nagpal, A.; Redvers, R.P.; Ling, X.; Ayton, S.; Fuentes, M.; Tavancheh, E.; Diala, I.; Lalani, A.; Loi, S.; David, S.; et al. Neoadjuvant neratinib promotes ferroptosis and inhibits brain metastasis in a novel syngeneic model of spontaneous HER2+ve breast cancer metastasis. Breast Cancer Res. 2019, 21, 1–19. [Google Scholar] [CrossRef]
- Breslin, S.; Lowry, M.C.; O’Driscoll, L. Neratinib resistance and cross-resistance to other HER2-targeted drugs due to increased activity of metabolism enzyme cytochrome P4503A4. Br. J. Cancer 2017, 116, 620–625. [Google Scholar] [CrossRef]
- Rani, S.; Corcoran, C.; Shiels, L.; Germano, S.; Breslin, S.; Madden, S.; McDermott, M.S.; Browne, B.C.; O’Donovan, N.; Crown, J.; et al. Neuromedin U: A candidate biomarker and therapeutic target to predict and overcome resistance to HER-tyrosine kinase inhibitors. Cancer Res. 2014, 74, 3821–3833. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Szöőr, Á.; Simon, L.; Yarden, Y.; Szöllősi, J.; Vereb, G. The combination of trastuzumab and pertuzumab administered at approved doses may delay development of trastuzumab resistance by additively enhancing antibody-dependent cell-mediated cytotoxicity. MAbs 2016, 8, 1361–1370. [Google Scholar] [CrossRef]
- Nahta, R.; Hung, M.C.; Esteva, F.J. The HER-2-Targeting Antibodies Trastuzumab and Pertuzumab Synergistically Inhibit the Survival of Breast Cancer Cells. Cancer Res. 2004, 64, 2343–2346. [Google Scholar] [CrossRef]
- Okita, R.; Shimizu, K.; Nojima, Y.; Yukawa, T.; Maeda, A.; Saisho, S.; Nakata, M. Lapatinib enhances trastuzumab-mediated antibody-dependent cellular cytotoxicity via upregulation of HER2 in malignant mesothelioma cells. Oncol. Rep. 2015, 34, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.S.; Dane, M.; Chin, K.; Tatarova, Z.; Liu, M.; Liby, T.; Thompson, W.; Smith, R.; Nederlof, M.; Bucher, E.; et al. Microenvironment-Mediated Mechanisms of Resistance to HER2 Inhibitors Differ between HER2+ Breast Cancer Subtypes. Cell Syst. 2018, 6, 329–342.e6. [Google Scholar] [CrossRef]
- Canonici, A.; Ivers, L.; Conlon, N.T.; Pedersen, K.; Gaynor, N.; Browne, B.C.; O’Brien, N.A.; Gullo, G.; Collins, D.M.; O’Donovan, N.; et al. HER-targeted tyrosine kinase inhibitors enhance response to trastuzumab and pertuzumab in HER2-positive breast cancer. Investig. New Drugs 2019, 37, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yacoub, R.; Taliaferro-Smith, L.D.; Sun, S.-Y.; Graham, T.R.; Dolan, R.; Lobo, C.; Tighiouart, M.; Yang, L.; Adams, A.; et al. Combinatorial Effects of Lapatinib and Rapamycin in Triple-Negative Breast Cancer Cells. Mol. Cancer Ther. 2011, 10, 1460–1469. [Google Scholar] [CrossRef]
- Mallon, R.; Feldberg, L.R.; Lucas, J.; Chaudhary, I.; Dehnhardt, C.; Delos Santos, E.; Chen, Z.; Dos Santos, O.; Ayral-Kaloustian, S.; Venkatesan, A.; et al. Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin. Cancer Res. 2011, 17, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, L.; Lindquist, D. Dual HER2 Suppression with Lapatinib plus Trastuzumab for Metastatic Inflammatory Breast Cancer: A Case Report of Prolonged Stable Disease. Case Rep. Oncol. 2018, 11, 855–860. [Google Scholar] [CrossRef]
- Noh, W.; Mondesire, W.H.; Peng, J.; Jian, W.; Zhang, H. Determinants of Rapamycin Sensitivity in Breast Cancer Cells. Clin. Cancer Res. 2004, 10, 1013–1023. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019, 72, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Flanagan, L.; Quinn, C.; Evoy, D.; McDermott, E.W.; Pierce, A.; Duffy, M.J. MTOR in breast cancer: Differential expression in triple-negative and non-triple-negative tumors. Breast 2012, 21, 178–182. [Google Scholar] [CrossRef]
- Crown, J.; O’Shaughnessy, J.; Gullo, G. Emerging targeted therapies in triple-negative breast cancer. Ann. Oncol. 2012, 23, vi56–vi65. [Google Scholar] [CrossRef]
- Gandhi, L.; Bahleda, R.; Tolaney, S.M.; Kwak, E.L.; Cleary, J.M.; Pandya, S.S.; Hollebecque, A.; Abbas, R.; Ananthakrishnan, R.; Berkenblit, A.; et al. Phase i study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J. Clin. Oncol. 2014, 32, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Mcdonnel, S.J.; Tell, L.A.; Murphy, B.G. Pharmacokinetics and pharmacodynamics of suberoylanilide hydroxamic acid in cats. J. Vet. Pharmacol. Ther. 2013, 37, 196–200. [Google Scholar] [CrossRef]
- Samantha, J.M.; Liepnieks, M.L.; Murphy, B.G. Treatment of chronically FIV-infected cats with suberoylanilide hydroxamic acid. Antivir. Res. 2014, 23, 1–7. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Emerging role of histone deacetylase inhibitors as anti-breast-cancer agents. Drug Discov. Today 2019, 24, 685–702. [Google Scholar] [CrossRef]
- Villanueva, C.B.; Bazan, F.F.; Pivot, X.B. New microtubule inhibitors in breast cancer. Curr. Breast Cancer Rep. 2013, 5, 1–10. [Google Scholar] [CrossRef]
- Xu, W.S.; Parmigiani, R.B.; Marks, P.A. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene 2007, 26, 5541–5552. [Google Scholar] [CrossRef]
- Chun, P. Histone deacetylase inhibitors in hematological malignancies and solid tumors. Arch. Pharm. Res. 2015, 38, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Kamarulzaman, N.S.; Dewadas, H.D.; Leow, C.Y.; Yaacob, N.S.; Mokhtar, N.F. The role of REST and HDAC2 in epigenetic dysregulation of Nav1.5 and nNav1.5 expression in breast cancer. Cancer Cell Int. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Xie, M.; Wu, Z.; Shi, Y. Relationship between histone deacetylase 3 (HDAC3) and breast cancer. Med. Sci. Monit. 2018, 24, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.I.; Shimada, M.; Harimoto, N.; Rikimaru, T.; Shirabe, K.; Tanaka, S.; Sugimachi, K. Histone deacetylase inhibitor trichostatin a induces cell-cycle arrest/apoptosis and hepatocyte differentiation in human hepatoma cells. Int. J. Cancer 2003, 103, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Rene Gonzalez, R.; Watters, A.; Xu, Y.; Singh, U.P.; Mann, D.R.; Rueda, B.R.; Penichet, M.L. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009, 11. [Google Scholar] [CrossRef] [PubMed]
- Cimino-Mathews, A.; Thompson, E.; Taube, J.M.; Ye, X.; Lu, Y.; Meeker, A.; Xu, H.; Sharma, R.; Lecksell, K.; Cornish, T.C.; et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 2016, 25, 1032–1057. [Google Scholar] [CrossRef]
- Sun, Z.; Lan, X.; Xu, S.; Li, S.; Xi, Y. Efficacy of bevacizumab combined with chemotherapy in the treatment of HER2-negative metastatic breast cancer: A network meta-analysis. BMC Cancer 2020, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]

| Tumor Classification of Feline Mammary Carcinomas | |||
|---|---|---|---|
| Tumor Clinical Stage * | |||
| Stage | Tumor size (T) | Lymph node status (N) | Metastasis (M) |
| 1 | T1 (<2 cm) | N0 | M0 |
| 2 | T2 (2–3 cm) | N0 | M0 |
| T1 | N1 | M0 | |
| 3 | T2 | N1 | M0 |
| T3 (>3 cm) | N0/N1 | M0 | |
| 4 | Any | N0/N1 | M1 |
| Histological Grade (EE System) | |||
| Histologic feature | Score | Sum of the scores 3–5 6–7 8–9 | Grade I II III |
| Tubule formation | |||
| >75% | 1 | ||
| 10–75% | 2 | ||
| <10% | 3 | ||
| Nuclear pleomorphism | |||
| Mild | 1 | ||
| Moderate | 2 | ||
| Marked | 3 | ||
| Mitotic count (per 10 microscopic fields) | |||
| 0–5 | 1 | ||
| 6–10 | 2 | ||
| >11 | 3 | ||
| Cell Line | Tumor Classification | ER (%) | PR (%) | Ki-67 (%) | Ck5/6 (%) | HER2 |
|---|---|---|---|---|---|---|
| CAT-M | Mammary Adenocarcinoma | 10 | 80 | 50.2 | <1 | 2+ |
| FMCp | Primary breast tumor | 60 | Negative | 57.4 | <1 | 0 |
| FMCm | Metastatic lymph node | 2 | Negative | 68.5 | <1 | 1+ |
| mAb | Target | Mechanism of Action | Breast Cancer Clinical Application | References | FMC In Vitro System | |||
|---|---|---|---|---|---|---|---|---|
| Cell Line | HER2 Status | Concentration (µg/mL) | Cytotoxicity (%) | |||||
| Pertuzumab | HER2 ECD II | Prevents HER2 heterodimerization; Inhibits EGFR downstream pathways; Stimulates ADCC and apoptosis | HER2-overexpressing and metastatic tumors | Agus et al., 2002 [91]; Scheuer et al., 2009 [92]; Baselga et al., 2010 [93]; Metzger-Filho et al., 2013 [94]; Richard et al., 2016 [22]; and Yamashita-Kashima et al., 2017 [89] | CAT-M | 2+ | 10,000 (EC50 = 2837.92 µg/mL ± 1.50) | 60.2 |
| FMCp | 0 | 10,000 (EC50 = 928.97 µg/mL ± 1.11) | 52.1 | |||||
| FMCm | 1+ | 10,000 (EC50 = 1205.04 µg/mL ± 1.23) | 61.8 | |||||
| Trastuzumab | HER2 ECD IV | Prevents HER2 homodimerization; Block receptor internalization and degradation; Prevents HER2 shedding; Induces ADCC and apoptosis | HER2-overexpressing invasive, metastatic and early-stage tumors | Klapper et al., 2000 [95]; Cho et al., 2003 [18]; J. Piccart-Gebhart, 2005 [96]; Nahta et al., 2007 [97]; D. Slamon, 2011 [98]; Menyhart et al., 2015 [99]; Richard et al., 2016 [22]; and Kast et al., 2017 [100] | CAT-M | 2+ | 10,000 (EC50 = 3047.89 µg/mL ± 1.43) | 92.6 |
| FMCp | 0 | 10,000 (EC50 = 3243.40 µg/mL ± 2.29) | 60.1 | |||||
| FMCm | 1+ | 10,000 (EC50 = 528.45 µg/mL ± 1.14) | 82.7 | |||||
| T-DM1 | HER2 ECD II; CKAP5 | Prevents HER2 homodimerization; Inhibits microtubule assembly; Induces cell apoptosis | HER2-positive, advanced, early stage and metastatic tumors | Phillips et al., 2008 [19]; Lambert and Chari, 2014 [101]; Von Minckwitz et al., 2019 [24]; Lacasse et al., 2020 [102]; and Liu et al., 2020 [103] | CAT-M | 2+ | 1000 (EC50 = 19.63 µg/mL ± 1.22) | 94.0 |
| FMCp | 0 | 1000 (EC50 = 88.72 µg/mL ± 1.29) | 74.2 | |||||
| FMCm | 1+ | 1000 (EC50 = 52.84 µg/mL ± 1.50) | 53.8 | |||||
| TKi | Target | Mechanism of Action | Breast Cancer Clinical Application | References | FMC In Vitro System | |||
|---|---|---|---|---|---|---|---|---|
| Cell Line | HER2 Status | Concentration (nM) | Cytotoxicity (%) | |||||
| Lapatinib | HER1 and HER2 | Reversible; Prevents EGFR family members phosphorylation | Solid, advanced and metastatic HER2-positive tumors; Valuable in combined protocols | Frenel et al., 2009 [115]; Opdam et al., 2012 [116]; Shi et al., 2016 [117]; and Stanley et al., 2017 [118] | CAT-M | 2+ | 50,000 (IC50 = 3930 nM ± 49) | 100 |
| FMCp | 0 | 50,000 (IC50 = 4870 nM ± 100) | 100 | |||||
| FMCm | 1+ | 100 × 103 (IC50 = 17,470 nM ± 100) | 100 | |||||
| Neratinib | HER1; HER2 and HER4 | Irreversible; Prevents EGFR family members phosphorylation; Surpass lapatinib resistance | Adjuvant treatment of HER2-positive early-stage and metastatic breast cancer | Tiwari et al., 2015 [119]; Sun et al., 2015 [40]; Cocco et al., 2018 [23]; and Food and Drug Administration (FDA) | CAT-M | 2+ | 25 | 33.5 |
| FMCp | 0 | 250 | 79.4 | |||||
| FMCm | 1+ | 1000 | 31.4 | |||||
| Combined Protocol | Blocked Pathways | FMC In Vitro Assay | |||
|---|---|---|---|---|---|
| Cell Line | HER2 Status | Increase in Cell Cytotoxicity (%) | p-Value | ||
| mAbs combination | |||||
| Pertuzumab plus Trastuzumab | HER2 ECD II and HER2 ECD IV | CAT-M | 2+ | 26.4 | 0.0018 |
| FMCp | 0 | 11.7 | 0.0184 | ||
| FMCm | 1+ | 29.5 | <0.001 | ||
| mAb plus TKi | |||||
| Pertuzumab plus Lapatinib | HER2 ECD II; HER1 and HER2 TK domain | CAT-M | 2+ | 69.4 | <0.001 |
| FMCp | 0 | 47.5 | <0.001 | ||
| FMCm | 1+ | 41.5 | <0.001 | ||
| Trastuzumab plus Lapatinib | HER2 ECD IV; HER1 and HER2 TK domain | CAT-M | 2+ | 71.9 | <0.001 |
| FMCp | 0 | 62.0 | <0.001 | ||
| FMCm | 1+ | 27.2 | 0.0017 | ||
| TKi plus mTORi | |||||
| Lapatinib plus Rapamycin | HER1 and HER2 TK domain and mTOR complex | CAT-M | 2+ | 51.9 | 0.0360 |
| FMCp | 0 | 47.5 | <0.001 | ||
| FMCm | 1+ | 85.6 | <0.001 | ||
| Neratinib plus Rapamycin | HER1, HER2 and HER4 TK domain and mTOR complex | CAT-M | 2+ | 47.4 | 0.0044 |
| FMCp | 0 | 44.1 | 0.0034 | ||
| FMCm | 1+ | 66.7 | <0.001 | ||
| Class of the Compound | Mechanism of Action | References | Agent | FDA Approval | FMC In Vitro Assays | |
|---|---|---|---|---|---|---|
| Cell Line | IC50 Value | |||||
| HDACi (µM) | Inhibits histone deacetylases leading to chromatin relaxation and uncontrolled gene expression; Induces cell cytotoxicity and death by apoptosis | Xu et al., 2007 [148]; Chun, 2015 [149]; and FDA | CI-994 | Experimental | CAT-M | 16.470 ± 1.904 |
| FMCp | 9.616 ± 2.150 | |||||
| Panobinostat | Yes; 2015 | CAT-M | 0.042 ± 0.067 | |||
| FMCp | ND # | |||||
| SAHA | Yes; 2006 | CAT-M | 4.416 ± 0.453 | |||
| FMCp | 2.571 ± 0.578 | |||||
| SBHA | Experimental | CAT-M | 45.230 ± 4.692 | |||
| FMCp | 33.830 ± 6.454 | |||||
| Scriptaid | ND | CAT-M | 3.392 ± 0.403 | |||
| FMCp | 3.090 ± 0.691 | |||||
| Trichostatin A | Experimental | CAT-M | 0.263 ± 0.062 | |||
| FMCp | ND # | |||||
| MTi (nM) | Inhibits microtubule polymerization, leading to cytoskeleton disruption; Induces cell cycle arrest and apoptosis | Risinger et al., 2015 [27]; Zang et al., 2018 [29]; Steinmetz and Prota, 2018 [28]; and FDA | Colchicine (Destabilizing agent) | Yes; 2009 | CAT-M | 1.472 ± 0.484 |
| FMCp | 5.876 ± 0.968 | |||||
| Nocodazol (Destabilizing agent) | Experimental | CAT-M | 12.270 ± 3.455 | |||
| FMCp | 30.840 ± 8.499 | |||||
| Vinblastine (Destabilizing agent) | Yes; 2011 | CAT-M | 0.570 ± 1.080 | |||
| FMCp | 6.563 ± 1.514 | |||||
| Paclitaxel (Stabilizing agent) | Yes; 2002 | CAT-M | 1.939 ± 1.134 | |||
| FMCp | 8.646 ± 2.337 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gameiro, A.; Urbano, A.C.; Ferreira, F. Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma. Vet. Sci. 2021, 8, 164. https://doi.org/10.3390/vetsci8080164
Gameiro A, Urbano AC, Ferreira F. Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma. Veterinary Sciences. 2021; 8(8):164. https://doi.org/10.3390/vetsci8080164
Chicago/Turabian StyleGameiro, Andreia, Ana Catarina Urbano, and Fernando Ferreira. 2021. "Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma" Veterinary Sciences 8, no. 8: 164. https://doi.org/10.3390/vetsci8080164
APA StyleGameiro, A., Urbano, A. C., & Ferreira, F. (2021). Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma. Veterinary Sciences, 8(8), 164. https://doi.org/10.3390/vetsci8080164






