Morphometric Patterns and Blood Biochemistry of Capybaras (Hydrochoerus hydrochaeris) from Human-Modified Landscapes and Natural Landscapes in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Animal Capture and Containment
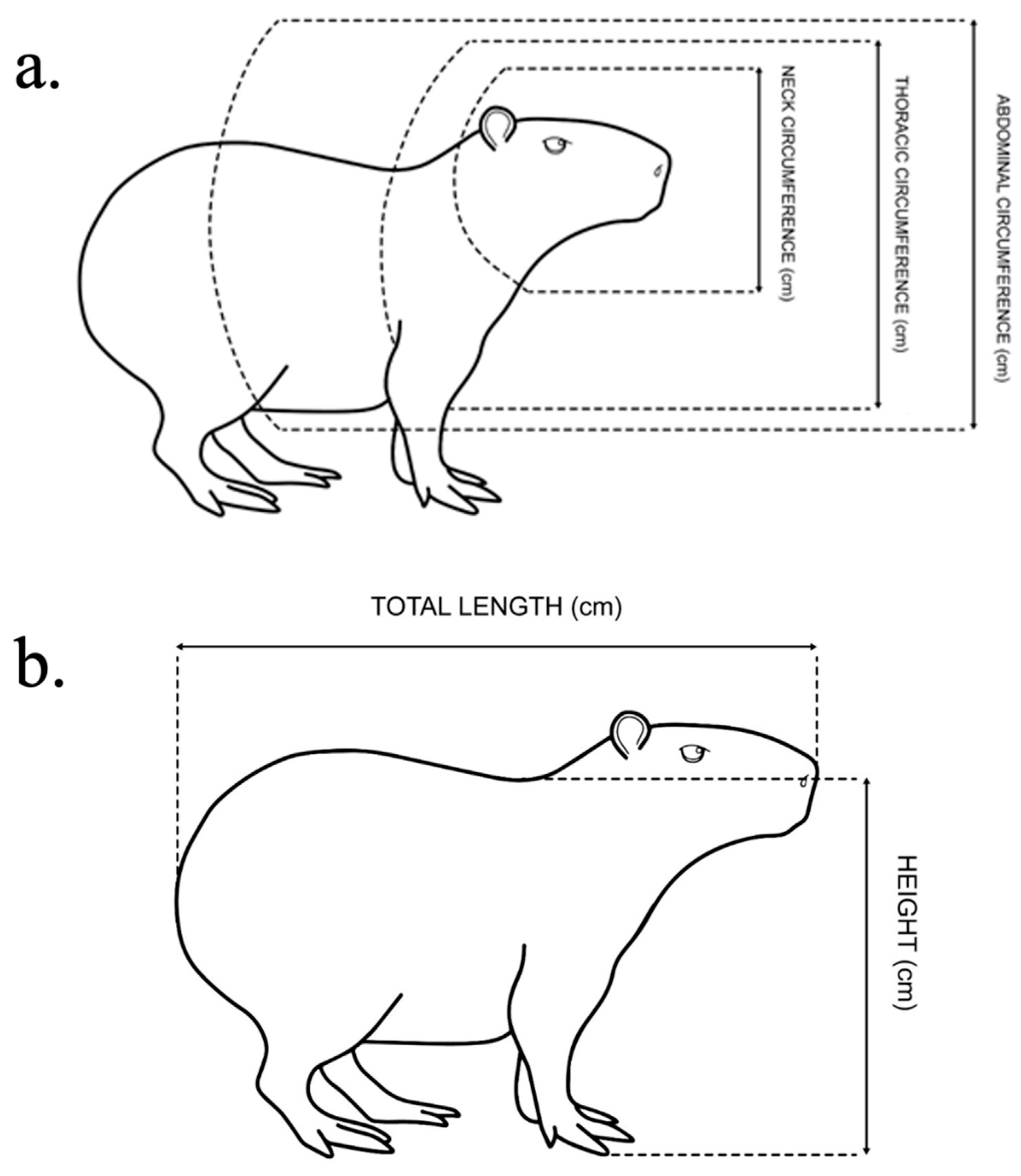
2.3. Biometrical Measures
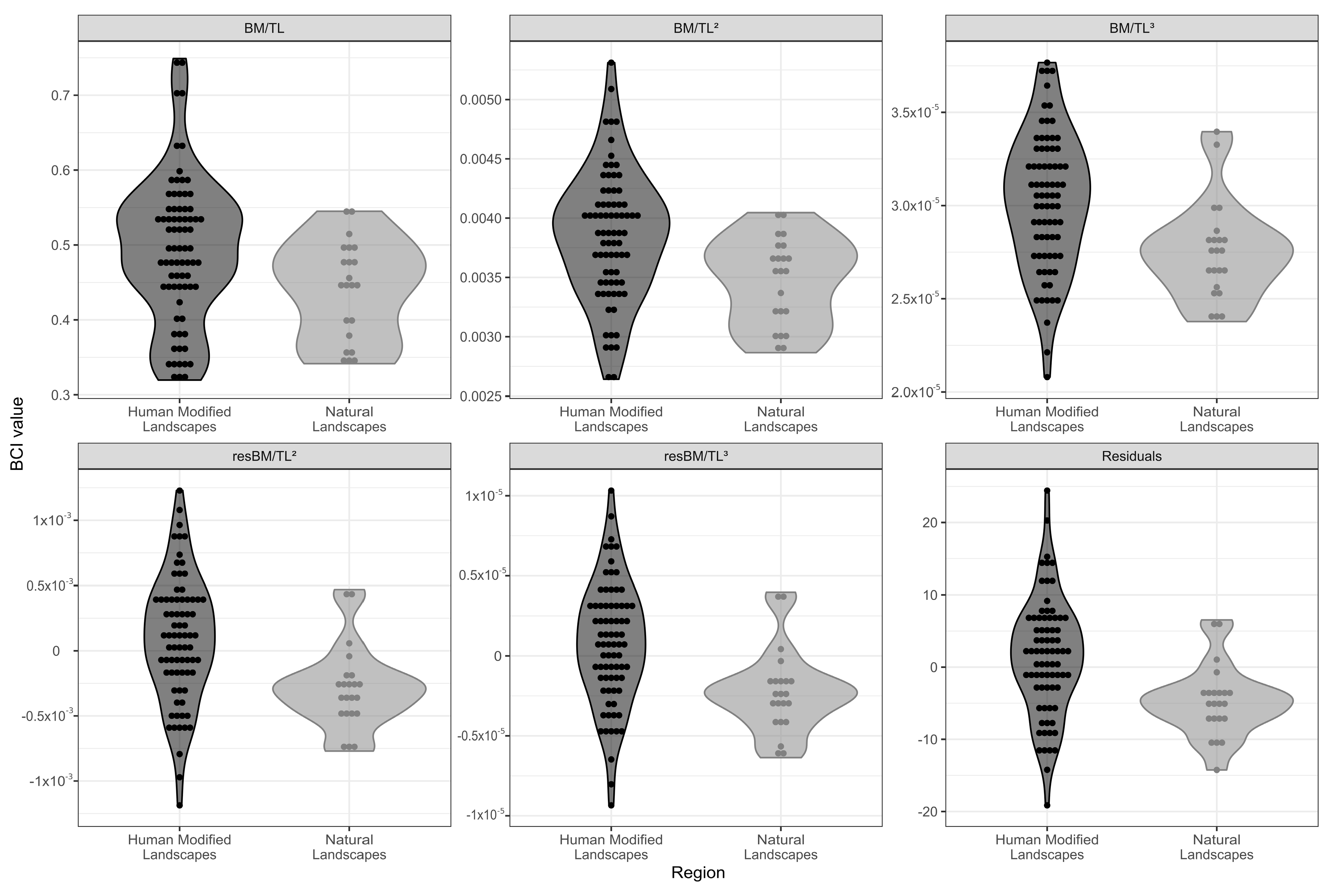
2.4. Definition of Body Condition Index (BCI)
2.5. Fat Amount Estimative
2.6. Blood Collection and Serum Biochemistry
2.7. Statistical Analyses
3. Results
3.1. Capybara Samples
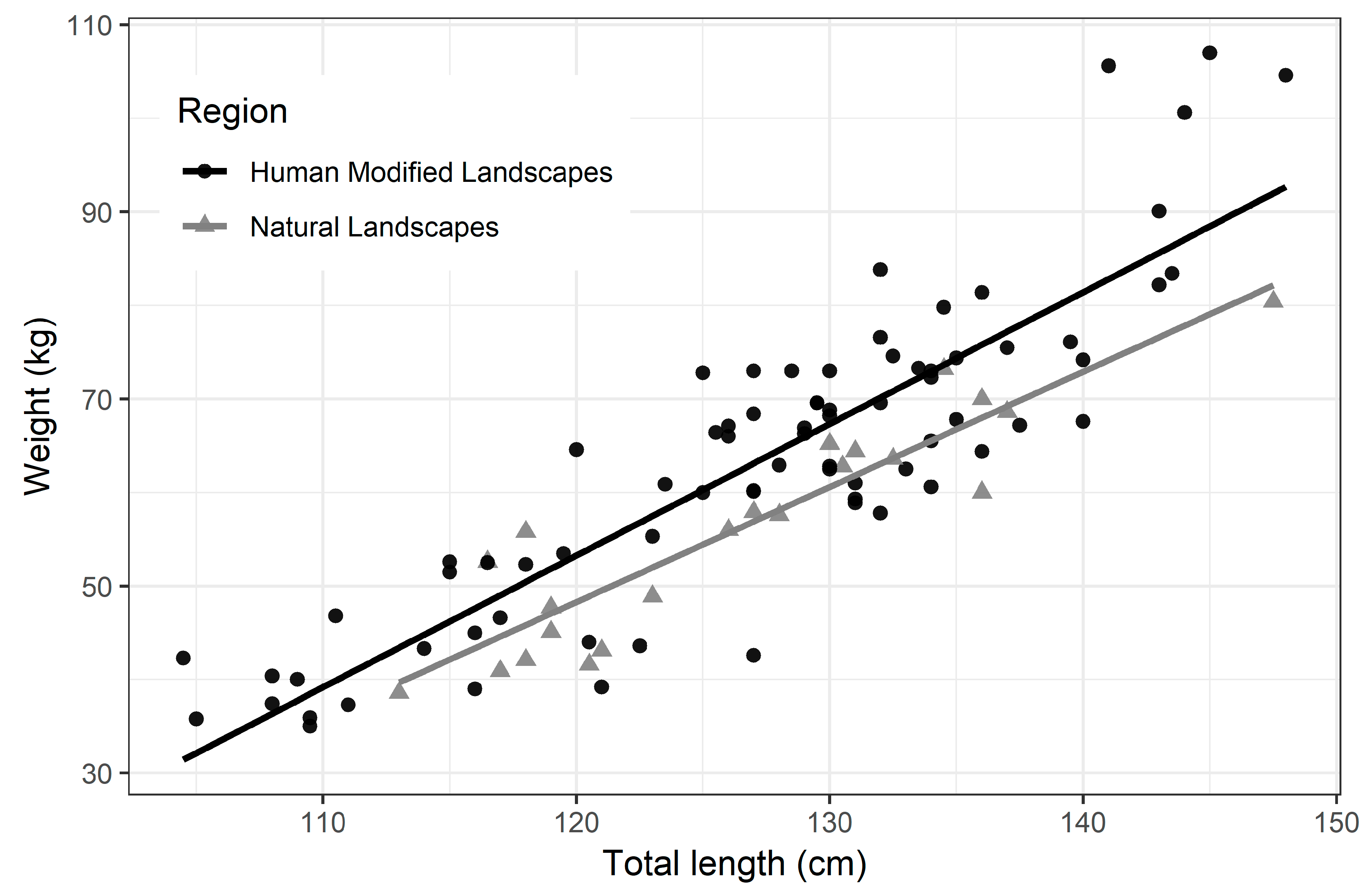
3.2. Biometrics and Body Condition Index (BCI)
3.3. Blood Biochemistry
3.4. Regression Analysis between Capybara Body Mass or BCI and Biochemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alho, C.J.R.; Campos, Z.D.S.; Gonçalves, H.C. Ecologia de capivara (Hydrochaeris hydrochaeris, Rodentia) do Pantanal: I Habitats, densidades e tamanho de grupo. Rev. Bras. Biol. 1987, 47, 87–97. [Google Scholar]
- Jiménez, E.G. El Capibara (Hydrochoerus hydrochaeris). Estado Actual de su Producción; Serie FAO Producción y Sanidad Animal: Roma, Italy, 1995. [Google Scholar]
- Moreira, J.R.; Ferraz, K.M.P.; Herrera, E.A.; Macdonald, D.W. Capybara Demographic Traits. In Capybara: Biology, Use and Conservation of an Exceptional Neotropical Species; Springer: New York, NY, USA, 2013; pp. 147–167. [Google Scholar]
- Herrera, E.A. Capybara social behavior and use of space: Patterns and processes. In Capybara: Biology, Use and Conservation of an Exceptional Neotropical Species; Moreira, J.R., Ferraz, K.M.P.M., Herrera, E.A., Macdonald, D.W., Eds.; Springer: New York, NY, USA, 2013; pp. 195–207. [Google Scholar]
- Macdonald, D.W. Dwindling resources and the social behavior of capybaras, (Hydrochoerus hydrochaeris) (Mammalia). J. Zool. 1981, 194, 371–391. [Google Scholar] [CrossRef]
- Fausto, B.; Fausto, S. História do Brasil; Edusp: São Paulo, Brazil, 1994; Volume 1. [Google Scholar]
- Gheler-Costa, C.; Vettorazzi, C.A.; Pardini, R.; Verdade, L.M. The distribution and abundance of small mammals in agroecosystems of southeastern Brazil. Mammalia 2012, 76, 185–191. [Google Scholar] [CrossRef]
- Verdade, L.M.; Ferraz, K.M.P.M.B. Capybaras in an anthropogenic habitat in southeastern Brazil. Braz. J. Biol. 2006, 66, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, K.M.P.M.B.; Ferraz, S.F.B.; Moreira, J.R.; Couto, H.T.Z.; Verdade, L.M. Capybara (Hydrochoerus hydrochaeris) distribution in agroecosystems: A cross-scale habitat analysis. J. Biogeogr. 2007, 34, 223–230. [Google Scholar] [CrossRef]
- Hume, I.D. Optimal digestive strategies in mammalian herbivores. Physiol. Zool. 1989, 62, 1145–1163. [Google Scholar] [CrossRef]
- Corriale, M.J.; Arias, S.M.; Quintana, R.D. Forage quality of plant species consumed by capybaras (Hydrochoerus hydrochaeris) in the Paraná River Delta, Argentina. Rangel. Ecol. Manag. 2011, 64, 257–263. [Google Scholar] [CrossRef]
- Lora, B.A.; Monteiro, M.B.; Assuncao, V.; Frigerio, R. Levantamento Georreferenciado da Expansão da Cultura de cana-de-Açúcar no Estado de São Paulo; Instituto de Eletrotécnica e Energia da USP: São Paulo, Brazil, 2006. [Google Scholar]
- Ezenwa, V.O.; Godsey, M.S.; King, R.J.; Guptill, S.C. Avian diversity and West Nile virus: Testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B 2006, 273, 109–117. [Google Scholar] [CrossRef]
- Swaddle, J.P.; Calos, S.E. Increased avian diversity is associated with lower incidence of human West Nile infection: Observation of the dilution effect. PLoS ONE 2008, 3, e2488. [Google Scholar] [CrossRef]
- Allan, B.F.; Langerhans, R.B.; Ryberg, W.A.; Landesman, W.J.; Griffin, N.W.; Katz, R.S.; Chase, J.M. Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia 2009, 158, 699–708. [Google Scholar] [CrossRef]
- Dizney, L.J.; Ruedas, L.A. Increased host species diversity and decreased prevalence of Sin Nombre virus. Emerg. Infect. Dis. 2009, 15, 1012–1018. [Google Scholar] [CrossRef]
- Clay, C.A.; Lehmer, E.M.; Jeor, S.S.; Dearing, M.D. Sin Nombre virus and rodent species diversity: A test of the dilution and amplification hypotheses. PLoS ONE 2009, 4, e6467. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.S.; Canham, C.D.; Oggenfuss, K.; Winchcombe, R.J.; Keesing, F. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 2006, 4, e145. [Google Scholar] [CrossRef]
- LoGiudice, K.; Duerr, S.T.; Newhouse, M.J.; Schmidt, K.A.; Killilea, M.E.; Ostfeld, R.S. Impact of host community composition on Lyme disease risk. Ecology 2008, 89, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Brunner, J.; Duerr, S.; Killilea, M.; LoGiudice, K.; Schmidt, K.; Vuing, H.; Ostfeld, R.S. Hosts as ecological traps for the vector of Lyme disease. Proc. Biol. Sci. 2009, 276, 3911–3919. [Google Scholar] [CrossRef] [PubMed]
- Polo, G.; Labruna, M.B.; Ferreira, F. Satellite hyperspectral imagery to support tick-borne infectious diseases surveillance. PLoS ONE 2015, 10, e0143736. [Google Scholar] [CrossRef]
- Luz, H.R.; Costa, F.B.; Benatti, H.R.; Ramos, V.N.; Serpa, M.C.D.A.; Martins, T.F.; Acosta, I.C.L.; Ramirez, D.G.; Muñoz-Leal, S.; Ramirez-Hernandez, A.; et al. Epidemiology of capybara-associated Brazilian spotted fever. PLoS Negl. Trop. Dis. 2019, 13, e0007734. [Google Scholar] [CrossRef]
- Kotiaho, J.S.; Marshall, S.D.; Barrow, J.H.; Jakob, E.M.; Uetz, G.W. Estimating fitness: Comparison of body condition indices revisited. Commentary. Oikos 1999, 87, 399–402. [Google Scholar] [CrossRef]
- Pergams, O.R.; Lawler, J.J. Recent and widespread rapid morphological change in rodents. PLoS ONE 2009, 4, e6452. [Google Scholar] [CrossRef][Green Version]
- Labocha, M.K.; Schutz, H.; Hayes, J.P. Which body condition index is best? Oikos 2014, 123, 111–119. [Google Scholar] [CrossRef]
- Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating fitness: A comparison of body condition indices. Oikos 1996, 77, 61–67. [Google Scholar] [CrossRef]
- Hepp, G.R.; Blohm, R.J.; Reynolds, R.E.; Hines, J.E.; Nichols, J.D. Physiological condition of autumn-banded mallards and its relationship to hunting vulnerability. J. Wildl. Manag. 1986, 50, 177–183. [Google Scholar] [CrossRef]
- Lunn, N.J.; Boyd, I.L. Effects of maternal age and condition on parturition and the perinatal period of Antarctic fur seals. J. Zool. 1993, 229, 55–67. [Google Scholar] [CrossRef]
- Huot, J.; Poulle, M.L.; Crete, M. Evaluation of several indices for assessment of coyote (Canis latrans) body composition. Canad. J. Zool. 1995, 73, 1620–1624. [Google Scholar] [CrossRef]
- Tierney, M.; Hindell, M.; Lea, M.A.; Tollit, D. A comparison of techniques used to estimate body condition of southern elephant seals (Mirounga leonina). Wildl. Res. 2001, 28, 581–588. [Google Scholar] [CrossRef]
- Bercovitch, F.B.; Widdig, A.; Trefilov, A.; Kessler, M.J.; Berard, J.D.; Schmidtke, J.; Nurnberg, P.; Krawczak, M. A longitudinal study of age-specific reproductive output and body condition among male rhesus macaques, Macaca mulatta. Naturwissenschaften 2003, 90, 309–312. [Google Scholar] [CrossRef]
- Cavallini, P. Comparison of body condition indices in the red fox (Fissipedia, Canidae). Mammalia 1996, 60, 449–462. [Google Scholar] [CrossRef]
- Tella, J.L.; Forero, M.G.; Donázar, J.A.; Negro, J.J.; Hiraldo, F. Non-adaptive adoptions of nestlings in the colonial lesser kestrel: Proximate causes and fitness consequences. Behav. Ecol. Sociobiol. 1997, 40, 253–260. [Google Scholar] [CrossRef]
- McElroy, E.J.; Marien, C.; Meyers, J.J.; Irschick, D.J. Do displays send information about ornament structure and male quality in the ornate tree lizard, Urosaurus ornatus? Ethology 2007, 113, 1113–1122. [Google Scholar] [CrossRef]
- Gould, S.J. Allometry in primates, with emphasis on scaling and the evolution of the brain. Contrib. Primatol. 1975, 5, 244–292. [Google Scholar]
- Thrall, M.A.; Weiser, G.; Allison, R.W.; Campbell, T.W. Veterinary Hematology and Clinical Chemistry; John Wiley & Sons: Oxford, UK, 2012. [Google Scholar]
- Clark, M.; Hoenig, M. Metabolic effects of obesity and its interaction with endocrine diseases. Vet. Clin. N. Am. 2016, 46, 797–815. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Tratado de Fisiologia Médica; Elsevier: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Murphy, D.; Reid, S.W.J.; Graham, P.A.; Love, S. Fructosamine measurement in ponies: Validation and response following experimental cyathostome infection. Res. Vet. Sci. 1997, 63, 113–118. [Google Scholar] [CrossRef]
- Van Liew, J.B.; Davis, P.J.; Davis, F.B.; Bernardis, L.L.; Deziel, M.R.; Marinucci, L.N.; Kumar, D. Effects of aging, diet, and sex on plasma glucose, fructosamine, and lipid concentrations in barrier-raised Fischer 344 rats. J. Gerontol. 1993, 48, B184–B190. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity: Preventing and Managing the Global Epidemic; Report of WHO Consultation on Obesity, Technical Report Series, No. 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Latimer, K.S. Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 5th ed.; John Wiley & Sons: Oxford, UK, 2011. [Google Scholar]
- Mones, A.; Ojasti, J. Hydrochoerus hydrochaeris. Mamm. Species 1986, 264, 1–7. [Google Scholar] [CrossRef]
- Ferraz, K.M.P.M.B.; Bonach, K.; Verdade, L.M. Relationship between body mass and body length in capybaras (Hydrochoerus hydrochaeris). Biota Neotrop. 2005, 5, 197–200. [Google Scholar] [CrossRef]
- Bressan, M.C.; Miguel, G.Z.; Faria, P.B.; Vieira, J.O.; Oda, S.H.I. Rendimento de carcaça e de cortes comerciais de capivaras (Hydrochaeris hydrochaeris L. 1766). Ciênc. Agrotec. 2002, 26, 1588–1593. [Google Scholar]
- Schulte-Hostedde, A.I.; Millar, J.S.; Hickling, G.J. Evaluating body condition in small mammals. Canad. J. Zool. 2001, 79, 1021–1029. [Google Scholar] [CrossRef]
- Angelieri, C.C.S.; Adams-Hosking, C.; Ferraz, K.M.P.M.B.; de Souza, M.P.; McAlpine, C.A. Using species distribution models to predict potential landscape restoration effects on puma conservation. PLoS ONE 2016, 11, e0145232. [Google Scholar] [CrossRef]
- Trombulak, S.C. Running speed and body mass in Belding’s ground squirrels. J. Mammal. 1989, 70, 194–197. [Google Scholar] [CrossRef]
- Selk, G.E.; Wettemann, R.P.; Lusby, K.S.; Oltjen, J.W.; Mobley, S.L.; Rasby, R.J.; Garmendia, J.C. Relationships among weight change, body condition and reproductive performance of range beef cows. J. Anim. Sci. 1988, 66, 3153–3159. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U. The evolution of body size: What keeps organisms small? Q. Rev. Biol. 2000, 75, 385–407. [Google Scholar] [CrossRef]
- Schluter, D.; Price, T.D.; Rowe, L. Conflicting selection pressures and life history trade-offs. Proc. R. Soc. Lond. B Biol. Sci. 1991, 246, 11–17. [Google Scholar]
- Pryce, J.E.; Coffey, M.P.; Simm, G. The relationship between body condition score and reproductive performance. J. Dairy Sci. 2001, 84, 1508–1515. [Google Scholar] [CrossRef]
- Roche, J.R.; Lee, J.M.; Macdonald, K.A.; Berry, D.P. Relationships among body condition score, body weight, and milk production variables in pasture-based dairy cows. J. Dairy Sci. 2007, 90, 3802–3815. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Day, M.L.; Zalesky, D.D.; Clutter, A.; Kittok, R.J.; Kinder, J.E. Effects of 17 β-estradiol and diets varying in energy on secretion of luteinizing hormone in beef heifers. J. Anim. Sci. 1987, 64, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Méndez, O.; Barragán, K.B. Determinación de Parámetros Fisiológicos, Hematológicos y de Química Sanguínea en Chigüiros silvestres (Hydrochoerus hydrochaeris) en el departamento de Casanare. In El Chigüiro Hydrochoerus hydrochaeris en la Orinoquía Colombiana: Ecología, Manejo Sostenible y Conservación; López-Arévalo, H.F., Sánchez-Palomino, P., Montenegro, O.L., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2014; pp. 185–196. [Google Scholar]
- Matus, J.R.C.; Pulido, J.A.R. Estudio del perfil hemático y metabólico de chigüiros (Hydrochaeris hydrochaeris) (Linnaeus, 1766) en confinamiento. Orinoquía 2010, 14, 95–109. [Google Scholar]
- López-Gómez, J.J.; Pérez Castrillón, J.L.; de Luis Román, D.A. Impact of obesity on bone metabolism. Endocrinol. Nutr. 2016, 63, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Cândido, F.; Bressan, J. Vitamin D: Link between Osteoporosis, Obesity, and Diabetes? Int. J. Mol. Sci. 2014, 15, 6569–6591. [Google Scholar] [CrossRef]
- Lopes, B.; McEvoy, J.F.; Morato, R.G.; Luz, H.R.; Costa, F.B.; Benatti, H.R.; Dias, T.C.; Rocha, V.J.; Nascimento, V.R.; Piovezan, U.; et al. Human-modified landscapes alter home range and movement patterns of capybaras. J. Mammal. 2021, 102, 319–332. [Google Scholar] [CrossRef]
- Lord, R.D.A. Descriptive account of capybara behaviour. Stud. Neotrop. Fauna Environ. 1994, 29, 11–22. [Google Scholar] [CrossRef]
- Porfírio, G.E.O. Ecologia Alimentar da Onça-Pintada (Panthera onca) na Sub-Região do Pantanal de Miranda. Master’s Thesis, Universidade Federal do Mato Grosso do Sul, Campo Grande, Brazil, 2009. [Google Scholar]
- Cavalcanti, S.M.; Gese, E.M. Kill rates and predation patterns of jaguars (Panthera onca) in the southern Pantanal, Brazil. J. Mammal. 2010, 91, 722–736. [Google Scholar] [CrossRef]
- Marketon, J.I.W.; Glaser, R. Stress hormones and immune function. Cell. Immunol. 2008, 252, 16–26. [Google Scholar] [CrossRef]
- Clinchy, M.; Sheriff, M.J.; Zanette, L.Y. Predator-induced stress and the ecology of fear. Funct. Ecol. 2013, 27, 56–65. [Google Scholar] [CrossRef]
- Beckerman, A.P.; Wieski, K.; Baird, D.J. Behavioural versus physiological mediation of life history under predation risk. Oecologia 2007, 152, 335–343. [Google Scholar] [CrossRef]
- Hawlena, D.; Schmitz, O.J. Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am. Nat. 2010, 176, 537–556. [Google Scholar] [CrossRef]
- McPeek, M.A. The growth/predation risk trade-off: So what is the mechanism? Am. Nat. 2004, 163, E88–E111. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Garner, W.; Speicher, C.; Penn, G.M.; Holliday, J.; Glaser, R. Psychosocial modifiers of immunocompetence in medical students. Psychosom. Med. 1984, 46, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Mehl, V.S.; Penn, G.; Speicher, C.E.; Kiecolt-Glaser, J.K. Stress-associated changes in plasma immunoglobulin levels. Int. J. Psychosom. 1986, 33, 41–42. [Google Scholar] [PubMed]
- Paterson, J. Capture myopathy. In Zoo Animal and Wildlife Immobilization and Anesthesia; West, G., Heard, D., Caulkett, N., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 115–121. [Google Scholar]
- Dias, T.C.; Stabach, J.A.; Huang, Q.; Labruna, M.B.; Leimgruber, P.; Ferraz, K.M.; Lopes, B.; Luz, H.R.; Costa, F.B.; Benatti, H.R.; et al. Habitat selection in natural and human-modified landscapes by capybaras (Hydrochoerus hydrochaeris), an important host for Amblyomma sculptum ticks. PLoS ONE 2020, 15, e0229277. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.L.P.; Benatti, H.R.; Luz, H.R.; Costa, F.B.; Pacheco, R.C.; Labruna, M.B. Endoparasites of capybaras (Hydrochoerus hydrochaeris) from anthropized and natural areas of Brazil. Rev. Bras. Parasitol. Vet. 2021, 30, e027420. [Google Scholar] [CrossRef]
- Bovo, A.A.A.; Ferraz, K.M.P.; Verdade, L.M.; Moreira, J.R. Capybaras (Hydrochoerus hydrochaeris) in anthropogenic environments: Challenges and conflicts. In Biodiversity in Agricultural Landscapes of Southeastern Brazil; Gheler-Costa, C., Lyra-Jorge, M.C., Verdade, L.M., Eds.; De Gruyter: Berlin, Germany, 2016; pp. 178–189. [Google Scholar]
- Rocha, V.J.; Sekiama, M.L.; Gonçalves, D.D.; Sampieri, B.R.; Barbosa, G.P.; Dias, T.C.; Rossi, H.R.; Souza, P.F.P. Capivaras (Hydrochoerus hydrochaeris) e a presença do carrapato (Amblyomma sculptum) no campus da UFSCar-Araras, São Paulo. Ciênc. Anim. Bras. 2017, 18, e-44671. [Google Scholar] [CrossRef]
- Abra, F.D.; Granziera, B.M.; Huijser, M.P.; Ferraz, K.M.P.M.B.; Haddad, C.M.; Paolino, R.M. Pay or prevent? Human safety, costs to society and legal perspectives on animal-vehicle collisions in São Paulo state, Brazil. PLoS ONE 2019, 14, e0215152. [Google Scholar] [CrossRef]
- Passos Nunes, F.B.; da Silva, S.C.; Cieto, A.D.; Labruna, M.B. The dynamics of ticks and capybaras in a residential park area in southeastern Brazil: Implications for the risk of Rickettsia rickettsii infection. Vector-Borne Zoonotic Dis. 2019, 19, 711–716. [Google Scholar] [CrossRef]
- Labruna, M.B. Brazilian spotted fever: The role of capybaras. In Capybara: Biology, Use and Conservation of an Exceptional Neotropical Species; Moreira, J.R., Ferraz, K.M.P.M., Herrera, E.A., Macdonald, D.W., Eds.; Springer: New York, NY, USA, 2013; pp. 371–383. [Google Scholar]
- Polo, G.; Acosta, C.M.; Labruna, M.B.; Ferreira, F. Transmission dynamics and control of Rickettsia rickettsii in populations of Hydrochoerus hydrochaeris and Amblyomma sculptum. PLoS Negl. Trop. Dis. 2017, 11, e0005613. [Google Scholar] [CrossRef]

| Location | Municipality/State | Approximate Coordinates |
|---|---|---|
| Human Modified Landscapes (HMLs) | ||
| University of São Paulo campus of Piracicaba | Piracicaba/SP | 22°43′05.1″ S 47°36′39.6″ W |
| Carioba sewage treatment station | Americana/SP | 22°42′35.7″ S 47°20′24.5″ W |
| Federal University of São Carlos campus of Araras | Araras/SP | 22°18′34.4″ S 47°23′00.2″ W |
| Private Company | Tatuí/SP | 23°22′42.4″ S 47°55′01.0″ W |
| University of São Paulo campus of Pirassununga | Pirassununga/SP | 21°56′52.4″ S 47°27′13.6″ W |
| University of São Paulo campus of Ribeirão Preto | Ribeirão Preto/SP | 21°10′03.9″ S 47°51′30.7″ W |
| Avaré State Park | Avaré/SP | 23°05′59.2″ S 48°54′32.9″ W |
| Natural Landscapes (NLs) | ||
| Pantanal of Poconé | Poconé/MT | 16°29′35.6″ S 56°26′20.8″ W |
| Pantanal of Nhecolândia | Corumbá/MS | 19°14′53.5″ S 57°01′34.0″ W |
| Groups | Municipality | Number of Adult Capybaras | ||||
|---|---|---|---|---|---|---|
| Total Sampled | Recaptures | Included in the Study | ||||
| Males | Females | Total | ||||
| HMLs | Piracicaba | 38 | 13 | 3 | 22 | 25 |
| Americana | 12 | 2 | 5 | 5 | 10 | |
| Araras | 17 | 2 | 1 | 14 | 15 | |
| Tatuí | 14 | 0 | 3 | 11 | 14 | |
| Pirassununga | 66 | 13 | 14 | 39 | 53 | |
| Ribeirão Preto | 22 | 10 | 4 | 8 | 12 | |
| Avaré | 5 | 0 | 1 | 4 | 5 | |
| TOTAL | 174 | 40 | 31 | 103 | 134 | |
| NLs | Poconé | 20 | 10 | 4 | 6 | 10 |
| Corumbá | 24 | 2 | 4 | 18 | 22 | |
| TOTAL | 44 | 12 | 8 | 24 | 32 | |
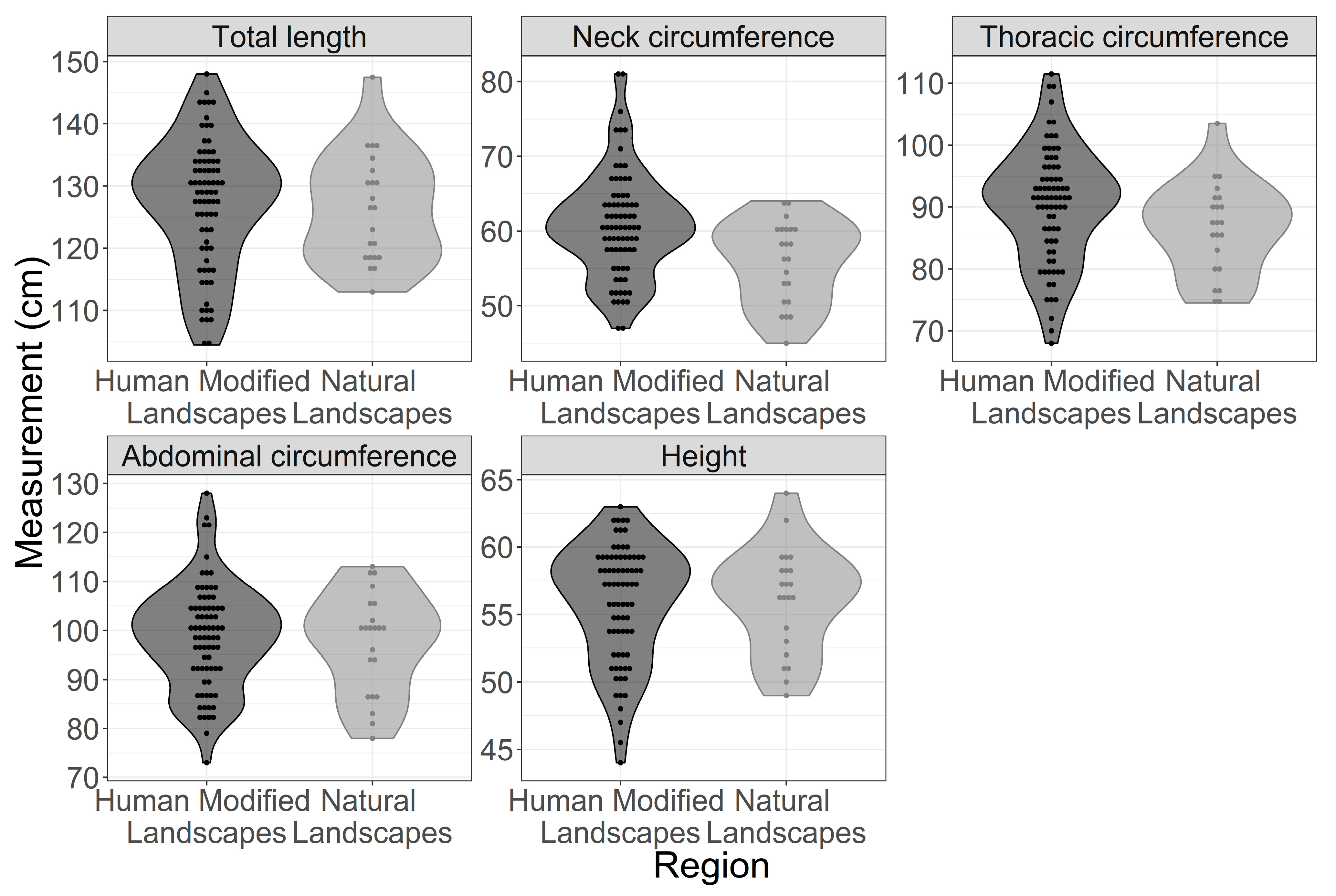
| Measurement | Region | Mean (SD) | Median (IQR) | p-Value |
|---|---|---|---|---|
| Height | HMLs | 55.9 (4.3) | 57 (5.9) | 0.8 |
| NLs | 56.1 (3.9) | 56.8 (5.1) | ||
| Total length | HMLs | 127.3 (10.3) | 129.2 (13.6) | 0.69 |
| NLs | 126.4 (8.7) | 126.5 (13.1) | ||
| Abdominal circumference | HMLs | 98.7 (10.7) | 99.5 (11.8) | 0.6578 |
| NLs | 97.6 (10.3) | 100.2 (15.5) | ||
| Neck circumference | HMLs | 60.9 (7.1) | 60.8 (6.8) | 0.000966 |
| NLs | 55.9 (5.5) | 57.2 (8.5) | ||
| Thoracic circumference | HMLs | 90.4 (9.3) | 91.5 (11.6) | 0.049 |
| NLs | 86.5 (7.4) | 87.5 (10.1) | ||
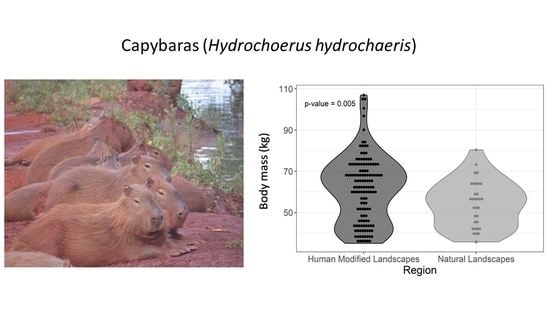
| Body mass | HMLs | 61.2 (16.1) | 62.7 (25.8) | 0.005 |
| NLs | 54.2 (11.2) | 55.7 (18.4) |
| Formula | Region | Mean (SD) | p-Value |
|---|---|---|---|
| BM/TL | HMLs | 0.4928 (0.0965) | 0.005 |
| NLs | 0.4410 (0.0656) | ||
| BM/TL2 | HMLs | 0.0038 (0.0005) | <0.001 |
| NLs | 0.0035 (0.0004) | ||
| BM/TL3 | HMLs | 3.03 × 105 (3.64 × 103) | <0.001 |
| NLs | 2.75 × 105 (2.62 × 103) | ||
| Residue | HMLs | 1.37 (8.01) | <0.001 |
| NLs | −4.74 (4.89) | ||
| resBM/TL2 | HMLs | 8.75 × 105 (0.00048) | <0.001 |
| NLs | −2.9 × 1011 (0.00031) | ||
| resBM/TL3 | HMLs | 71.8 (3.79 × 103) | <0.001 |
| NLs | −2.28 × 103 (2.57 × 103) |
| Serum Biochemical Parameter | Region | Mean (SD) | Median (IQR) | p-Value |
|---|---|---|---|---|
| Albumin (g/dL) | HMLs | 2.8 (0.4) | 2.9 (0.6) | 6.00 × 10−4 |
| NLs | 2.5 (0.4) | 2.5 (0.7) | ||
| Alkaline Phosphatase (U/L) | HMLs | 151.1 (104.7) | 115.3 (148.2) | 0.7756 |
| NLs | 171.5 (163.9) | 92.8 (244.9) | ||
| Aspartate Aminotransferase (U/L) | HMLs | 21.7 (25) | 14 (8.4) | 0.0597 |
| NLs | 13.6 (6.9) | 11.8 (10.4) | ||
| Calcium (mg/dL) | HMLs | 10.3 (1.2) | 10.5 (1.3) | 0.0523 |
| NLs | 10.9 (0.9) | 10.8 (1) | ||
| Creatine Kinase (U/L) | HMLs | 261 (733.2) | 87.3 (100.3) | <0.0001 |
| NLs | 61.4 (77) | 35 (48.2) | ||
| Cholesterol (mg/dL) | HMLs | 50.7 (16.7) | 47.1 (14.5) | <0.0001 |
| NLs | 37.6 (15.4) | 34.1 (16.2) | ||
| Fructosamine (mmol/L) | HMLs | 200.9 (53.5) | 190 (68) | 0.0332 |
| NLs | 175.6 (33.9) | 175 (42) | ||
| Phosphorous (mg/dL) | HMLs | 5.5 (2) | 5 (2.5) | 0.9901 |
| NLs | 5.5 (2) | 5.4 (2.3) | ||
| Total Protein (g/dL) | HMLs | 6 (0.6) | 6 (0.7) | 0.0137 |
| NLs | 6.3 (0.6) | 6.5 (1) | ||
| Triglycerides (mg/dL) | HMLs | 83.6 (100.4) | 50.9 (57.4) | 0.9345 |
| NLs | 76 (72.7) | 55.9 (61) | ||
| Urea (mg/dL) | HMLs | 26.1 (16.7) | 24.2 (19.4) | 0.2498 |
| NLs | 28.6 (10.4) | 25.4 (10.5) |
| Parameters | Regression Equation | R2 (%) | p Value |
|---|---|---|---|
| Fructosamine (Fru) | Fru (mmol/L) = 190.1 + 0.1701 body mass | 0.23 | 0.601 |
| Total protein (TP) | TP (g/dL) = 4769 − 3.29 body mass (kg) | 0.03 | 0.829 |
| Aspartate aminotransferase (AST) | AST (U/L) = 33.55 − 0.2299 body mass (kg) | 2.10 | 0.083 |
| Alkaline phosphatase (ALP) | ALP (U/L) = 265.1 − 1.863 body mass (kg) | 6.07 | 0.007 |
| Creatine kinase (CK) | CK (U/L) = 2085 − 25.12 body mass (kg) | 0.89 | 0.306 |
| Urea (Ur) | Ur (mg/dL) = 32.38 − 0.07457 body mass (kg) | 0.54 | 0.381 |
| Calcium (Ca) | Ca (mg/dL) = 10.86 − 0.006089 body mass (kg) | 0.61 | 0.351 |
| Phosphorous (P) | P (mg/dL) = 5.871 − 0.01028 body mass (kg) | 0.65 | 0.337 |
| Cholesterol (Cho) | Cho (mg/dL) = 25.64 + 0.3397 body mass (kg) | 13.73 | <0.001 |
| Triglycerides (Tri) | Tri (mg/dL) = −47.97 + 2.046 body mass (kg) | 12.2 | <0.001 |
| Albumin (Alb) | Alb (g/dL) = 3.111 − 0.005519 body mass (kg) | 4.20 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benatti, H.R.; Luz, H.R.; Lima, D.M.; Gonçalves, V.D.; Costa, F.B.; Ramos, V.N.; Aguiar, D.M.; Pacheco, R.C.; Piovezan, U.; Szabó, M.P.J.; et al. Morphometric Patterns and Blood Biochemistry of Capybaras (Hydrochoerus hydrochaeris) from Human-Modified Landscapes and Natural Landscapes in Brazil. Vet. Sci. 2021, 8, 165. https://doi.org/10.3390/vetsci8080165
Benatti HR, Luz HR, Lima DM, Gonçalves VD, Costa FB, Ramos VN, Aguiar DM, Pacheco RC, Piovezan U, Szabó MPJ, et al. Morphometric Patterns and Blood Biochemistry of Capybaras (Hydrochoerus hydrochaeris) from Human-Modified Landscapes and Natural Landscapes in Brazil. Veterinary Sciences. 2021; 8(8):165. https://doi.org/10.3390/vetsci8080165
Chicago/Turabian StyleBenatti, Hector R., Hermes R. Luz, Daniel M. Lima, Vinicius D. Gonçalves, Francisco B. Costa, Vanessa N. Ramos, Daniel M. Aguiar, Richard C. Pacheco, Ubiratan Piovezan, Matias P. J. Szabó, and et al. 2021. "Morphometric Patterns and Blood Biochemistry of Capybaras (Hydrochoerus hydrochaeris) from Human-Modified Landscapes and Natural Landscapes in Brazil" Veterinary Sciences 8, no. 8: 165. https://doi.org/10.3390/vetsci8080165
APA StyleBenatti, H. R., Luz, H. R., Lima, D. M., Gonçalves, V. D., Costa, F. B., Ramos, V. N., Aguiar, D. M., Pacheco, R. C., Piovezan, U., Szabó, M. P. J., Ferraz, K. M. P. M. B., & Labruna, M. B. (2021). Morphometric Patterns and Blood Biochemistry of Capybaras (Hydrochoerus hydrochaeris) from Human-Modified Landscapes and Natural Landscapes in Brazil. Veterinary Sciences, 8(8), 165. https://doi.org/10.3390/vetsci8080165