Signalment and Clinical Data of Cats with Exocrine Pancreatic Insufficiency Diagnosed Using Feline Trypsin-like Immunoreactivity in Routine Diagnostics
Abstract
:1. Introduction
2. Materials and Methods
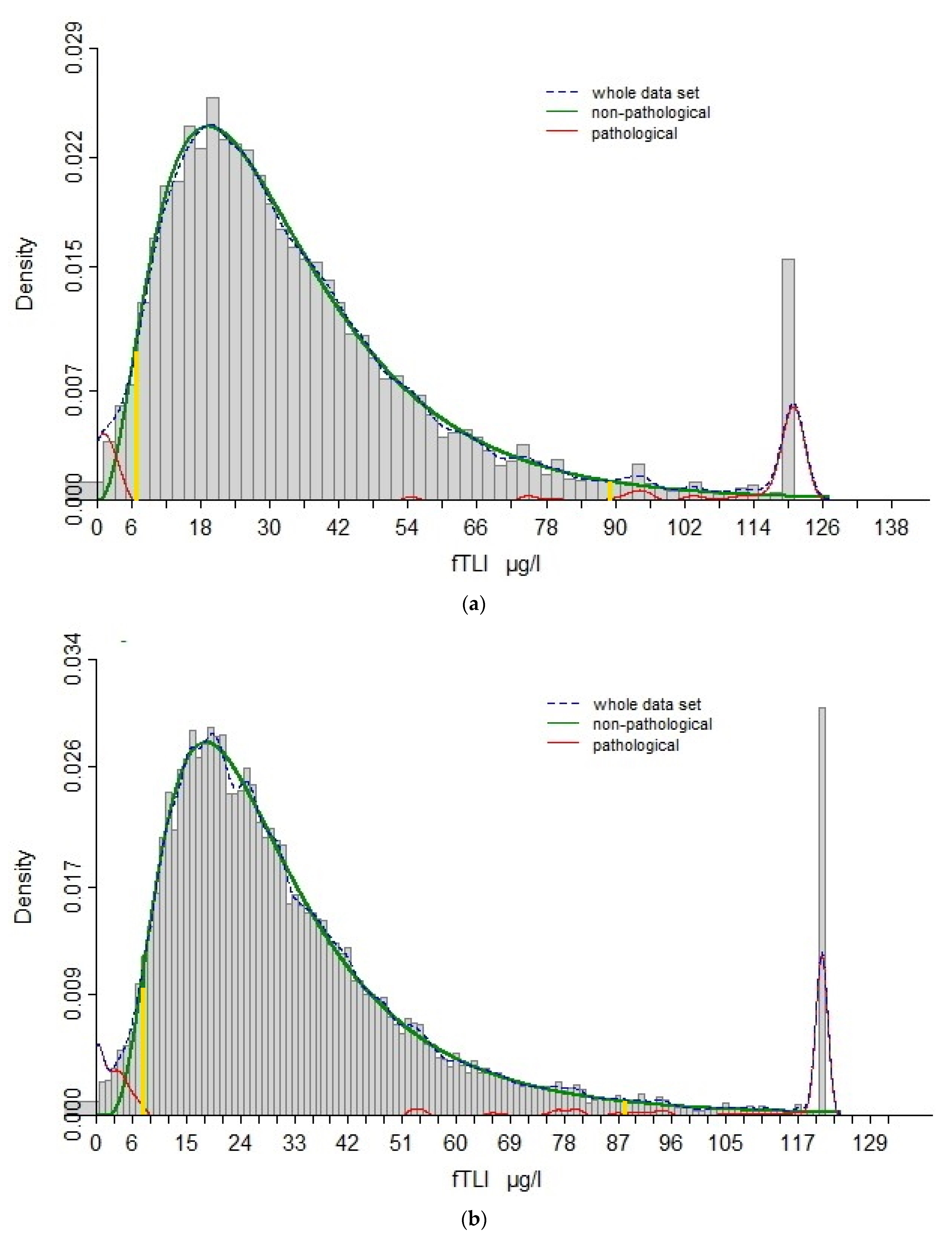
2.1. Serum fTLI Concentration
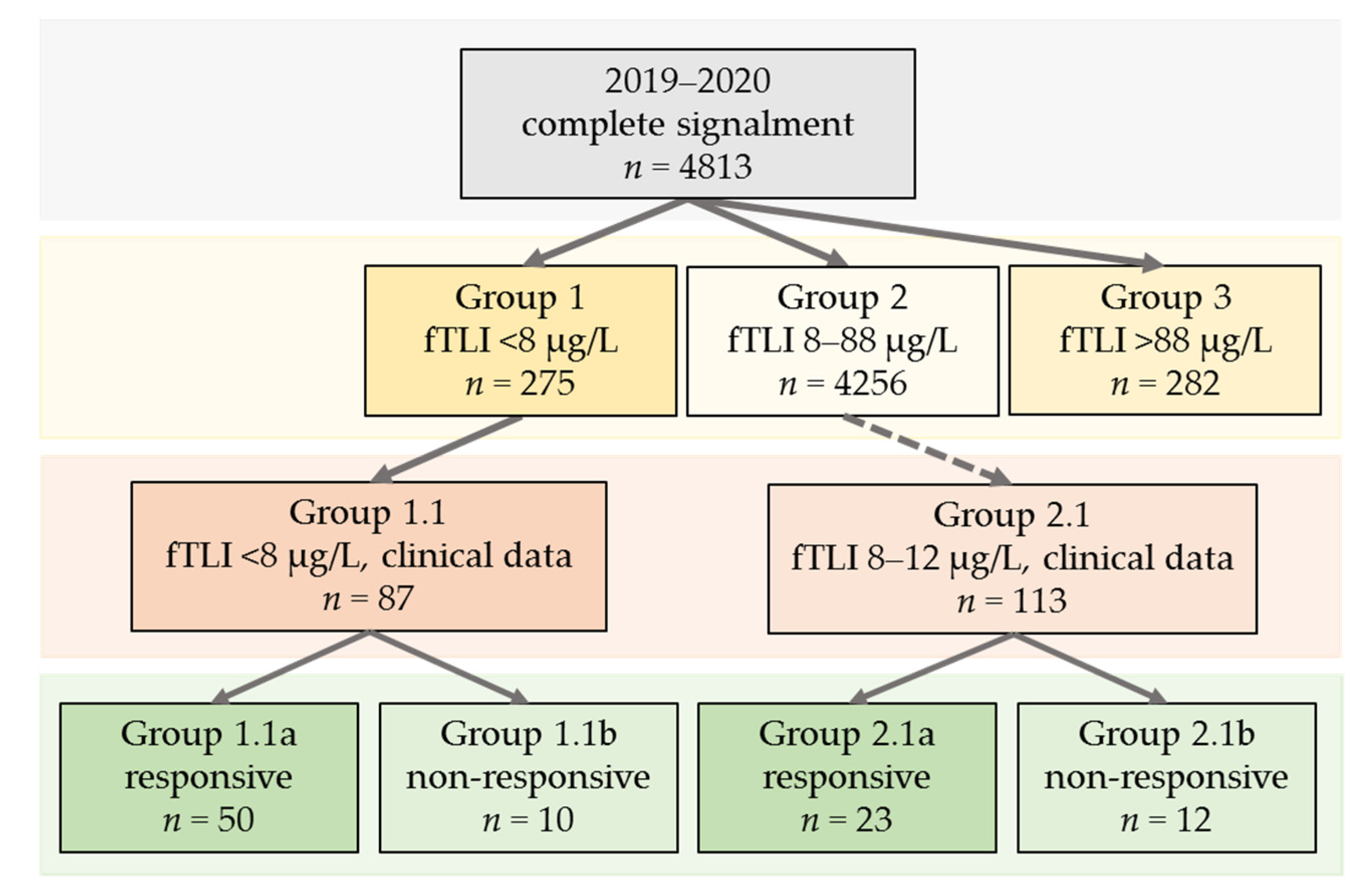
2.2. Signalment and Clinical Data
- Group 1 (serum fTLI < 8 µg/L, decreased).
- Group 2 (serum fTLI 8–88 µg/L, reference interval).
- Group 3 (serum fTLI > 88 µg/L, increased).
- Group 1.1 (serum fTLI <8 µg/L and clinical data available).
- Group 2.1 (serum fTLI 8–12 µg/L and clinical data available).
3. Results
3.1. Re-Evaluation of Reference Interval
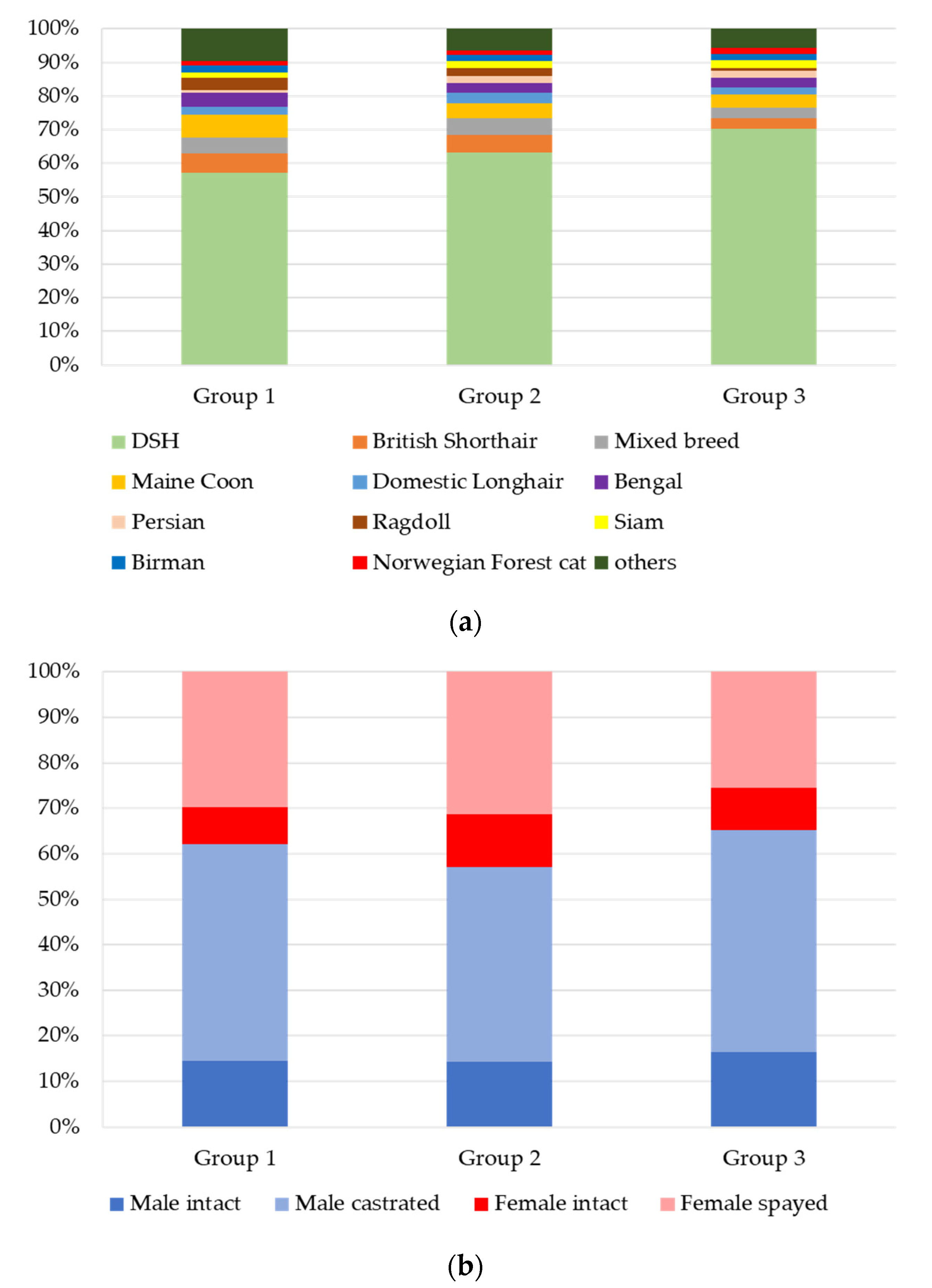
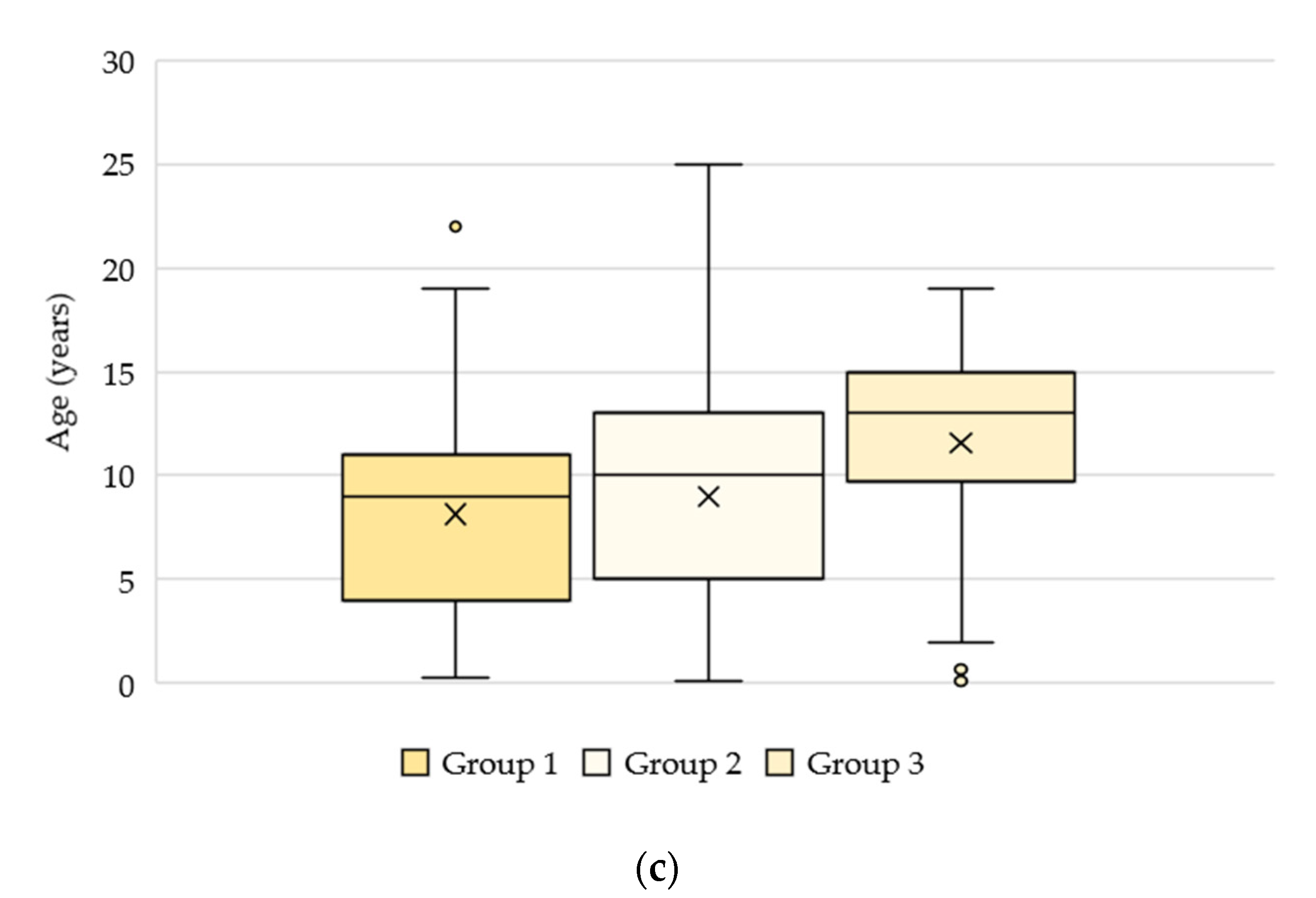
3.2. Signalment and Clinical Data
3.2.1. Groups 1, 2, and 3: n = 4813
3.2.2. Group 1.1 (Serum fTLI <8 µg/L and Clinical Data Available): n = 87
3.2.3. Group 2.1 (Serum fTLI 8–12 µg/L and Clinical Data Available): n = 113
3.2.4. Treatment Response in Groups 1.1 and 2.1 Combined
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steiner, J.M. Exocrine Pancreatic Insufficiency in the Cat. Top. Companion Anim. Med. 2012, 27, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Parnell, N.K.; Hohenhaus, A.E.; Moore, G.E.; Rondeau, M.P. Feline exocrine pancreatic insufficiency: 16 cases (1992–2007). J. Feline Med. Surg. 2009, 11, 935–940. [Google Scholar] [CrossRef] [PubMed]
- German, A.J. Exocrine Pancreatic Insufficiency in the Dog: Breed Associations, Nutritional Considerations, and Long-term Outcome. Top. Companion Anim. Med. 2012, 27, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Westermarck, E.; Wiberg, M. Exocrine Pancreatic Insufficiency in the Dog: Historical Background, Diagnosis, and Treatment. Top. Companion Anim. Med. 2012, 27, 96–103. [Google Scholar] [CrossRef]
- Watson, P.J. Exocrine pancreatic insufficiency as an end stage of pancreatitis in four dogs. J. Small Anim. Pract. 2003, 44, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.M.; Williams, D.A. Serum Feline Trypsin-Like Immunoreactivity in Cats with Exocrine Pancreatic Insufficiency. J. Vet. Intern. Med. 2000, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Xenoulis, P.G.; Zoran, D.L.; Fosgate, G.T.; Suchodolski, J.S.; Steiner, J.M. Feline Exocrine Pancreatic Insufficiency: A Retrospective Study of 150 Cases. J. Vet. Intern. Med. 2016, 30, 1790–1797. [Google Scholar] [CrossRef] [Green Version]
- Steiner, J.M.; Medinger, T.L.; Williams, D.A. Development and validation of a radioimmunoassay for feline trypsin-like immunoreactivity. Am. J. Vet. Res. 1996, 57, 1417–1420. [Google Scholar]
- Steiner, V.J.M.; Williams, D.A.; Moeller, E.M.; Melgarejo, T. Development and validation of an enzyme-linked immunosorbent assay for feline trypsin-like immunoreactivity. Am. J. Vet. Res. 2000, 61, 620–623. [Google Scholar] [CrossRef]
- A Williams, D.; Batt, R.M. Sensitivity and specificity of radioimmunoassay of serum trypsin-like immunoreactivity for the diagnosis of canine exocrine pancreatic insufficiency. J. Am. Vet. Med Assoc. 1988, 192, 195–201. [Google Scholar]
- Bridges, C.S.; Miller, P.S.; Lidbury, J.A.; Suchodolski, J.S.; Yi, Y.; Engelhardt, J.; Steiner, J.M. Validation of a radioimmunoassay of serum trypsin-like immunoreactivity in ferrets. J. Vet. Diagn. Investig. 2018, 30, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Daste, T.; Dossin, O.; Reynolds, B.S.; Aumann, M. Manual ventilation therapy and aggressive potassium supplementation in the management of respiratory failure secondary to severe hypokalaemia in a cat with exocrine pancreatic insufficiency. J. Feline Med. Surg. 2014, 16, 373–377. [Google Scholar] [CrossRef]
- Perry, L.A.; Williams, D.A.; Pidgeon, G.L.; Boosinger, T.R. Exocrine pancreatic insufficiency with associated coagulopathy in a cat. J. Am. Anim. Hosp. Assoc. 1991, 27, 109–114. [Google Scholar]
- Hanisch, F.; Toresson, L.; Spillmann, T. Cobalaminmangel bei Hund und Katze. Tierarztl. Prax. Ausg. K Kleintiere. Heimtiere. 2018, 46, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Davison, L.J. Diabetes mellitus and pancreatitis-cause or effect? J. Small Anim. Pract. 2015, 56, 50–59. [Google Scholar] [CrossRef]
- Arzideh, F.; Wosniok, W.; Gurr, E.; Hinsch, W.; Schumann, G.; Weinstock, N.; Haeckel, R. A plea for intra-laboratory reference limits. Part 2. A bimodal retrospective concept for determining reference limits from intra-laboratory databases demonstrated by catalytic activity concentrations of enzymes. Clin. Chem. Lab. Med. 2007, 45, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, T.; Wittker, A.; Teigelkamp, S.; Eim, C.; Burkhardt, E.; Eigenbrodt, E.; Sziegoleit, A. An Immunoassay for Canine Pancreatic Elastase 1 as an Indicator for Exocrine Pancreatic Insufficiency in Dogs. J. Vet. Diagn. Investig. 2001, 13, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Spillmann, T.; Eigenbrodt, E.; Sziegoleit, A. Die Bestimmung und klinische Relevanz der fäkalen pankreatischen Elastase beim Hund. Tierarztl. Prax. Ausg. K Kleintiere. Heimtiere 1998, 26, 364–368. [Google Scholar]
- Steiner, J.; Rehfeld, J.; Pantchev, N. Evaluation of Fecal Elastase and Serum Cholecystokinin in Dogs with a False Positive Fecal Elastase Test. J. Vet. Intern. Med. 2010, 24, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Carro, T.; A Williams, D. Relationship between dietary protein concentration and serum trypsin-like immunoreactivity in dogs. Am. J. Vet. Res. 1989, 50, 2105–2107. [Google Scholar]
- Batchelor, D.J.; Noble, P.-J.M.; Cripps, P.J.; Taylor, R.H.; McLean, L.; Leibl, M.A.; German, A.J. Breed Associations for Canine Exocrine Pancreatic Insufficiency. J. Vet. Intern. Med. 2007, 21, 207. [Google Scholar] [CrossRef]
- Kook, P.H.; Zerbe, P.; Reusch, C.E. Exokrine Pankreasinsuffizienz bei der Katze. Schweiz Arch Tierheilkd 2011, 153, 19–25. [Google Scholar] [CrossRef]
- Watanabe, T.; Hoshi, K.; Zhang, C.; Ishida, Y.; Sakata, I. Hyperammonaemia due to cobalamin malabsorption in a cat with exocrine pancreatic insufficiency. J. Feline Med. Surg. 2012, 14, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, M.E.; Nurmi, A.-K.; Westermarck, E. Serum Trypsinlike Immunoreactivity Measurement for the Diagnosis of Subclinical Exocrine Pancreatic Insufficiency. J. Vet. Intern. Med. 1999, 13, 426–432. [Google Scholar] [CrossRef]
- Wiberg, M.E.; Westermarck, E. Subclinical exocrine pancreatic insufficiency in dogs. J. Am. Vet. Med Assoc. 2002, 220, 1183–1187. [Google Scholar] [CrossRef]
- Hulsebosch, S.E.; Palm, C.A.; Segev, G.; Cowgill, L.D.; Kass, P.H.; Marks, S.L. Evaluation of Canine Pancreas-Specific Lipase Activity, Lipase Activity, and Trypsin-Like Immunoreactivity in an Experimental Model of Acute Kidney Injury in Dogs. J. Vet. Intern. Med. 2016, 30, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A.; Majumdar, A.P.; Dubick, M.A.; Vesenka, G.D.; Mar, G.; Geokas, M.C. Biochemical changes in the pancreas of rats with chronic renal failure. Am. J. Physiol. 1985, 249, F518–F523. [Google Scholar] [CrossRef]
- Mansfield, C.S.; Jones, B.R. Plasma and urinary trypsinogen activation peptide in healthy dogs, dogs with pancreatitis and dogs with other systemic diseases. Aust. Vet. J. 2000, 78, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef]
- Steiner, J.M.; Williams, D.A. Influence of feeding on serum feline trypsin-like immunoreactivity. Am. J. Vet. Res. 1999, 60, 895–897. [Google Scholar]
- Saver, A.T.; Steiner, J.M.; Hetzel, S.J.; Lidbury, J.A.; Suchodolski, J.S.; Pritchard, J.C. Effect of withholding food on serum concentrations of cobalamin, folate, trypsin-like immunoreactivity, and pancreatic lipase immunoreactivity in healthy dogs. Am. J. Vet. Res. 2021, 82, 367–373. [Google Scholar] [CrossRef] [PubMed]

| Variable | Categories | Responder | Non-Responder | Total Number (n) | p Value |
|---|---|---|---|---|---|
| Sex | Male/female | 39/34 | 14/8 | 95 | 0.400 |
| Male intact/male castrated/female intact/female spayed | 8/31/10/24 | 1/13/2/6 | 95 | 0.708 | |
| Age (median) | Numbers in years | 0.3–16 (7) | 1–14 (9) | 95 | 0.269 |
| <1 year/1–10 years/>10 years | 5/45/22 | 0/14/8 | 95 | 0.221 | |
| Breed | DSH/others | 34/39 | 15/7 | 95 | 0.077 |
| Weight loss | Yes/no | 43/21 | 14/6 | 84 | 0.815 |
| Diarrhoea | Yes/no | 49/20 | 12/8 | 89 | 0.353 |
| Vomiting | Yes/no | 33/35 | 8/14 | 90 | 0.322 |
| Polyphagia | Yes/no | 18/48 | 4/17 | 87 | 0.453 |
| Anorexia | Yes/no | 24/43 | 7/14 | 88 | 0.836 |
| Poor hair coat | Yes/no | 21/33 | 8/10 | 72 | 0.679 |
| Lethargy | Yes/no | 22/42 | 6/13 | 83 | 0.822 |
| fTLI concentration (median) | Numbers in µg/L | 0.09–11.8 (6.0) | 0.09–11.6 (8.5) | 95 | 0.022 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törner, K.; Grassinger, J.M.; Weber, C.N.; Aupperle-Lellbach, H.; Cerezo-Echevarria, A.; Müller, E. Signalment and Clinical Data of Cats with Exocrine Pancreatic Insufficiency Diagnosed Using Feline Trypsin-like Immunoreactivity in Routine Diagnostics. Vet. Sci. 2021, 8, 155. https://doi.org/10.3390/vetsci8080155
Törner K, Grassinger JM, Weber CN, Aupperle-Lellbach H, Cerezo-Echevarria A, Müller E. Signalment and Clinical Data of Cats with Exocrine Pancreatic Insufficiency Diagnosed Using Feline Trypsin-like Immunoreactivity in Routine Diagnostics. Veterinary Sciences. 2021; 8(8):155. https://doi.org/10.3390/vetsci8080155
Chicago/Turabian StyleTörner, Katrin, Julia Maria Grassinger, Corinna N. Weber, Heike Aupperle-Lellbach, Argine Cerezo-Echevarria, and Elisabeth Müller. 2021. "Signalment and Clinical Data of Cats with Exocrine Pancreatic Insufficiency Diagnosed Using Feline Trypsin-like Immunoreactivity in Routine Diagnostics" Veterinary Sciences 8, no. 8: 155. https://doi.org/10.3390/vetsci8080155






