Transcriptomic Profiling of Dromedary Camels Immunised with a MERS Vaccine Candidate
Abstract
:1. Introduction
2. Results
2.1. The Study Animals
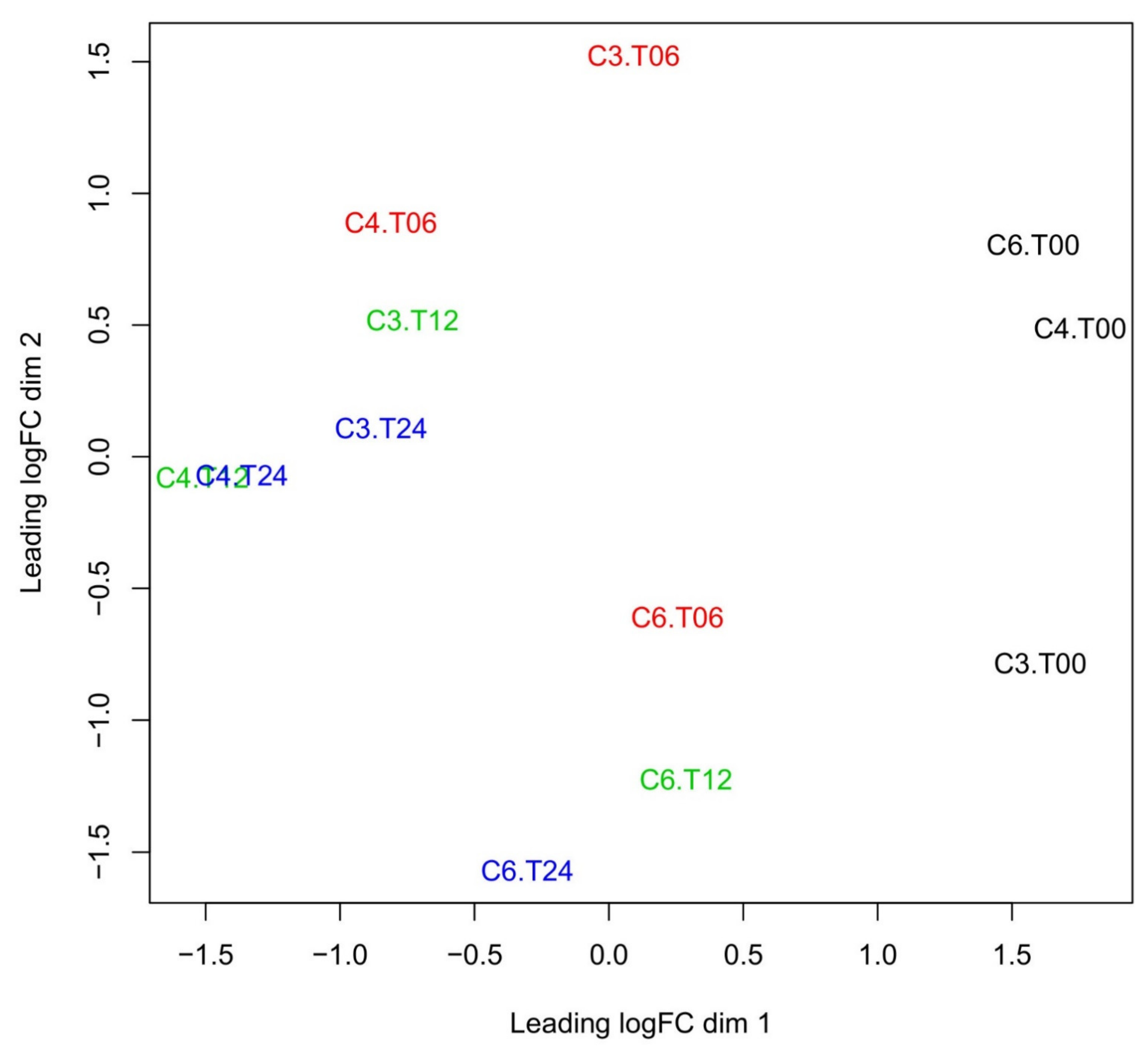
2.2. Changes in Overall Gene Expression
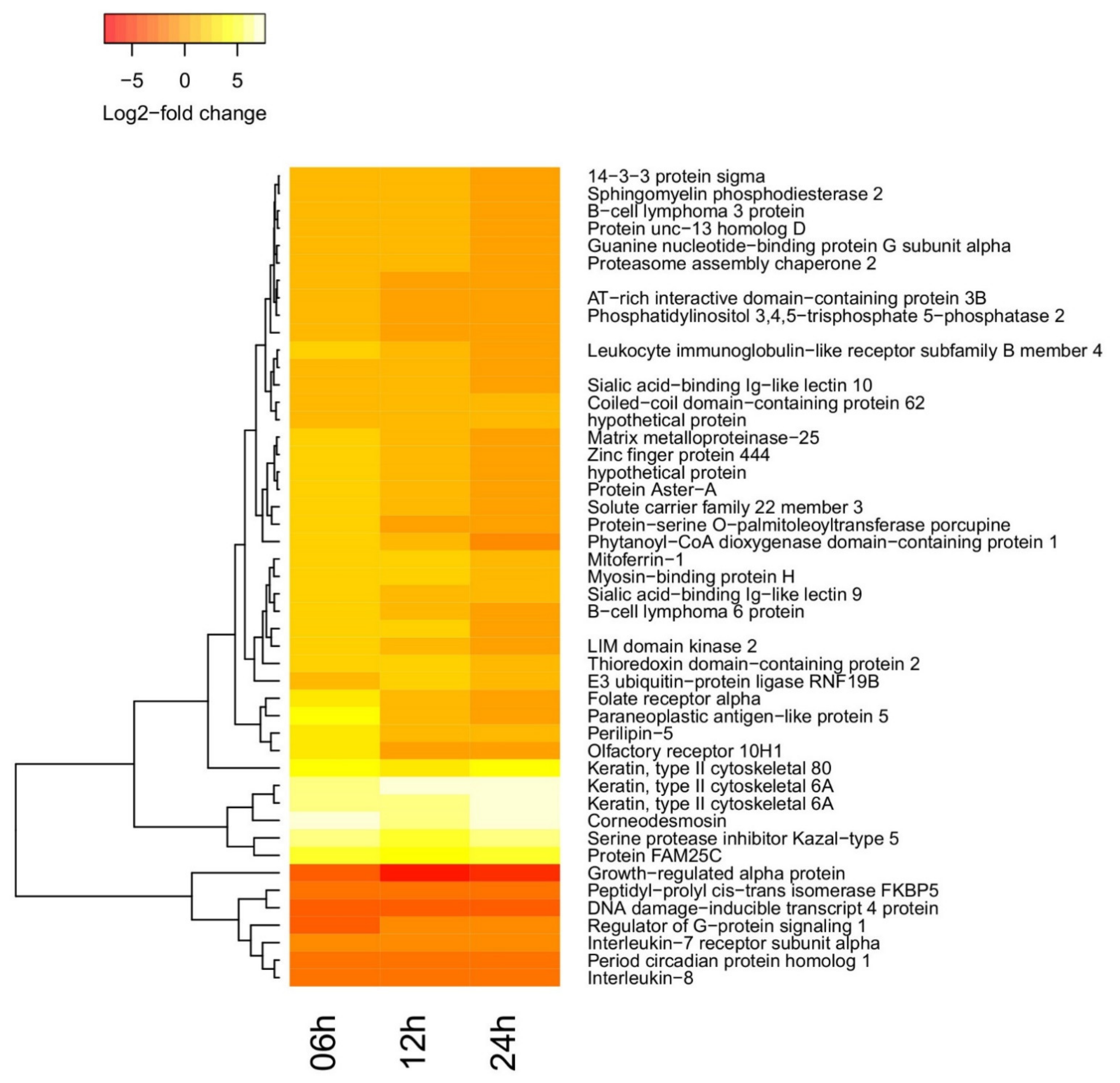
2.3. Time-Dependent Behavior of Gene Regulation
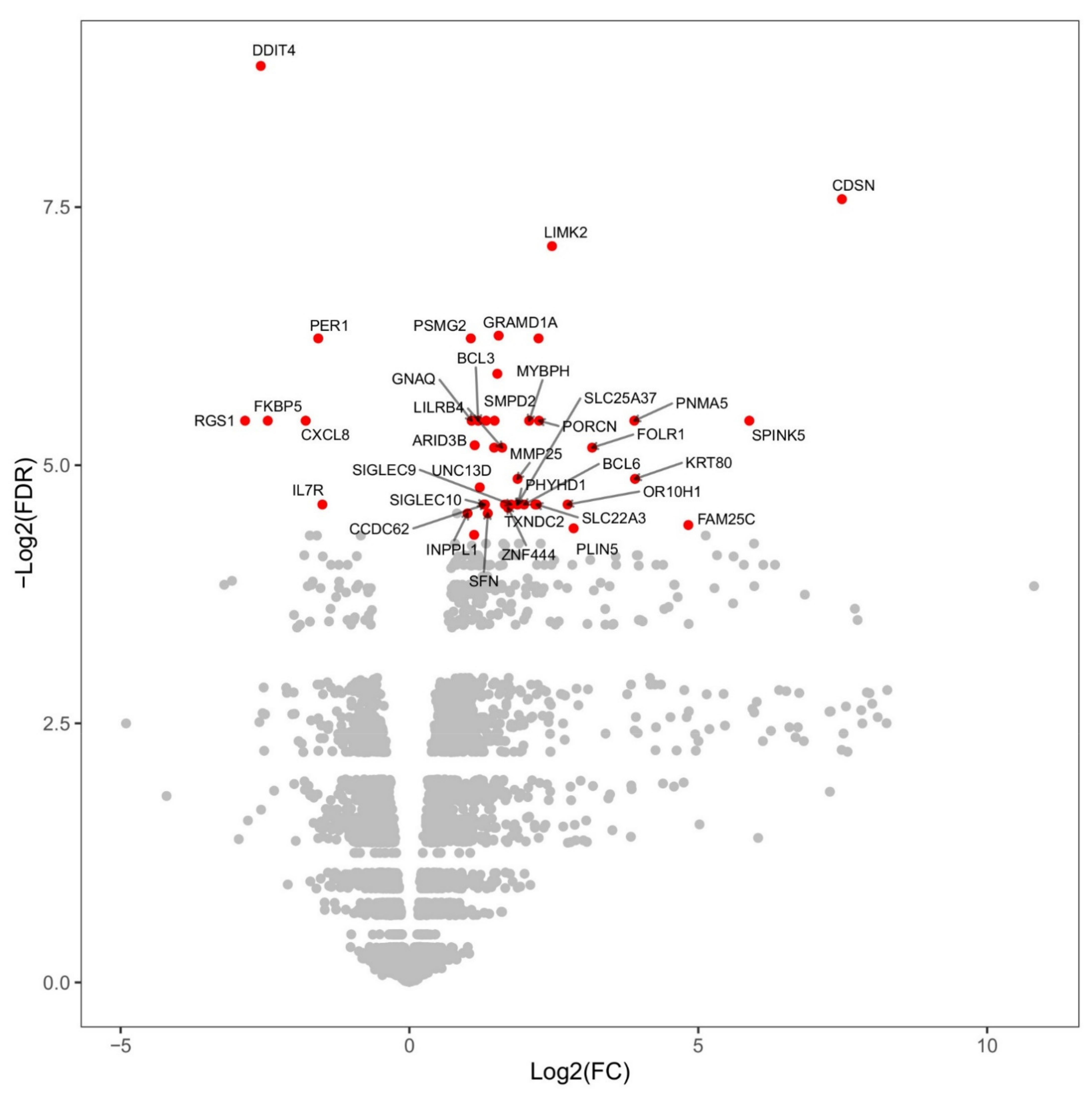
2.4. Functional Enrichment of Differentially Regulated Genes
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Immunizations Vaccination Study
4.2. RNA Sequencing
4.3. Bioinformatics Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- WHO. WHO|Middle East Respiratory Syndrome Coronavirus (MERS-CoV); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Alagaili, A.N.; Briese, T.; Mishra, N.; Kapoor, V.; Sameroff, S.C.; de Wit, E.; Munster, V.J.; Hensley, L.E.; Zalmout, I.S.; Kapoor, A.; et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio 2014, 5, e00884-14. [Google Scholar] [CrossRef] [Green Version]
- Mackay, I.M.; Arden, K.E. Middle East respiratory syndrome: An emerging coronavirus infection tracked by the crowd. Virus Res. 2015, 202, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea, 2015. Osong Public Health Res. Perspect. 2015, 6, 269–278. [CrossRef] [PubMed] [Green Version]
- Alshukairi, A.N.; Zheng, J.; Zhao, J.; Nehdi, A.; Baharoon, S.A.; Layqah, L.; Bokhari, A.; Al Johani, S.M.; Samman, N.; Boudjelal, M.; et al. High Prevalence of MERS-CoV Infection in Camel Workers in Saudi Arabia. mBio 2018, 9, e01985-18. [Google Scholar] [CrossRef] [Green Version]
- Conzade, R.; Grant, R.; Malik, M.R.; Elkholy, A.; Elhakim, M.; Samhouri, D.; Ben Embarek, P.K.; Van Kerkhove, M.D. Reported direct and indirect contact with dromedary camels among laboratory-confirmed MERS-CoV cases. Viruses 2018, 10, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alharbi, N.K.; Ibrahim, O.H.; Alhafufi, A.; Kasem, S.; Aldowerij, A.; Albrahim, R.; Abu Ubaidah, A.; Resolution, A.; Bayoumi, F.A.; Al-Mansour, A.M.; et al. Challenge infection model for MERS-CoV based on naturally infected camels. Virol. J. 2020, 17, 77. [Google Scholar] [CrossRef]
- Kasem, S.; Qasim, I.; Al-Hufofi, A.; Hashim, O.; Alkarar, A.; Abu-Obeida, A.; Gaffer, A.; Al-Fadil, A.; Zaki, A.; Al-Rumaihi, A.; et al. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J. Infect. Public Health 2018, 11, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K. Vaccines against Middle East respiratory syndrome coronavirus for humans and camels. Rev. Med. Virol. 2016, 27, e1971. [Google Scholar] [CrossRef]
- Yong, C.Y.; Ong, H.K.; Yeap, S.K.; Ho, K.L.; Tan, W.S. Recent Advances in the Vaccine Development against Middle East Respiratory Syndrome-Coronavirus. Front. Microbiol. 2019, 10, 1781. [Google Scholar] [CrossRef] [Green Version]
- Margalida, A.; Bogliani, G.; Bowden, C.G.R.; Donázar, J.A.; Genero, F.; Gilbert, M.; Karesh, W.B.; Kock, R.A.; Lubroth, J.; Manteca, X.; et al. Science and regulation. One Health approach to use of veterinary pharmaceuticals. Science 2014, 346, 1296–1298. [Google Scholar] [PubMed] [Green Version]
- Zhou, Y.; Jiang, S.; Du, L. Prospects for a MERS-CoV spike vaccine. Expert Rev. Vaccines 2018, 17, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alharbi, N.K.; Qasim, I.; Almasoud, A.; Aljami, H.A.; Alenazi, M.W.; Alhafufi, A.; Aldibasi, O.S.; Hashem, A.M.; Kasem, S.; Albrahim, R.; et al. Humoral Immunogenicity and Efficacy of a Single Dose of ChAdOx1 MERS Vaccine Candidate in Dromedary Camels. Sci. Rep. 2019, 9, 16292. [Google Scholar] [CrossRef]
- Haagmans, B.L.; van den Brand, J.M.A.; Victor, S.R.; Volz, A.; Wohlsein, P.; Smits, S.L.; Schipper, D.; Bestebroer, T.M.; Okba, N.M.A.; Fux, R.; et al. An orthopoxvirus-based vaccine reducesvirus excretion after MERS-CoV infectionin dromedary camels. Science 2016, 351, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Muthumani, K.; Falzarano, D.; Reuschel, E.L.; Tingey, C.; Flingai, S.; Villarreal, D.O.; Wise, M.C.; Patel, A.; Izmirly, A.; Aljuaid, A.; et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci. Transl. Med. 2015, 7, 301ra132. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, N.K.; Padron-Regalado, E.; Thompson, C.P.; Kupke, A.; Wells, D.; Sloan, M.A.; Grehan, K.; Temperton, N.; Lambe, T.; Warimwe, G.; et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine 2017, 35, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Munster, V.J.; Wells, D.; Lambe, T.; Wright, D.; Fischer, R.J.; Bushmaker, T.; Saturday, G.; van Doremalen, N.; Gilbert, S.C.; de Wit, E.; et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines 2017, 2, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folegatti, P.M.; Bittaye, M.; Flaxman, A.; Lopez, F.R.; Bellamy, D.; Kupke, A.; Mair, C.; Makinson, R.; Sheridan, J.; Rohde, C.; et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: A dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 816–826. [Google Scholar] [CrossRef]
- Knabl, L.; Lee, H.K.; Wieser, M.; Mur, A.; Zabernigg, A.; Knabl, L.; Rauch, S.; Bock, M.; Schumacher, J.; Kaiser, N.; et al. Impact of BNT162b first vaccination on the immune transcriptome of elderly patients infected with the B.1.351 SARS-CoV-2 variant. medRxiv 2021. [Google Scholar] [CrossRef]
- Maruyama, S.R.; Carvalho, B.; González-Porta, M.; Rung, J.; Brazma, A.; Gustavo Gardinassi, L.; Ferreira, B.R.; Banin, T.M.; Veríssimo, C.J.; Katiki, L.; et al. Blood transcriptome profile induced by an efficacious vaccine formulated with salivary antigens from cattle ticks. NPJ Vaccines 2019, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Christian, L.M.; Porter, K.; Karlsson, E.; Schultz-Cherry, S.; Iams, J.D. Serum proinflammatory cytokine responses to influenza virus vaccine among women during pregnancy versus non-pregnancy. Am. J. Reprod. Immunol. 2013, 70, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Leonard, E.J.; Yoshimura, T. Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]). Am. J. Respir. Cell Mol. Biol. 1990, 2, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Talaat, K.R.; Halsey, N.A.; Cox, A.B.; Coles, C.L.; Durbin, A.P.; Ramakrishnan, A.; Bream, J.H. Rapid changes in serum cytokines and chemokines in response to inactivated influenza vaccination. Influenza Other Respir. Viruses 2018, 12, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Damme, J.; Rampart, M.; Conings, R.; Decock, B.; Van Osselaer, N.; Willems, J.; Billiau, A. The neutrophil-activating proteins interleukin 8 and beta-thromboglobulin: In vitro and in vivo comparison of NH2-terminally processed forms. Eur. J. Immunol. 1990, 20, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Schorr, W. Role of interleukin-8 in neutrophil signaling. Curr. Opin. Hematol. 2000, 7, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Hatzi, K.; Nance, J.P.; Kroenke, M.A.; Bothwell, M.; Haddad, E.K.; Melnick, A.; Crotty, S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med. 2015, 212, 539–553. [Google Scholar] [CrossRef] [Green Version]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.-H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef] [Green Version]
- Bentebibel, S.-E.; Schmitt, N.; Banchereau, J.; Ueno, H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc. Natl. Acad. Sci. USA 2011, 108, E488–E497. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, S.; Katikaneni, D.S.; Gogoi, H.; Jin, L. Monocyte-Derived Dendritic Cells (moDCs) Differentiate into Bcl6+ Mature moDCs to Promote Cyclic di-GMP Vaccine Adjuvant-Induced Memory TH Cells in the Lung. J. Immunol. 2021, 206, 2233–2245. [Google Scholar] [CrossRef]
- Bunting, K.L.; Melnick, A.M. New effector functions and regulatory mechanisms of BCL6 in normal and malignant lymphocytes. Curr. Opin. Immunol. 2013, 25, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Arima, M.; Fukuda, T.; Tokuhisa, T. Role of the Transcriptional Repressor BCL6 in Allergic Response and Inflammation. World Allergy Organ. J. 2008, 1, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-kappaB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef] [Green Version]
- De Goeje, P.L.; Bezemer, K.; Heuvers, M.E.; Dingemans, A.-M.C.; Groen, H.J.; Smit, E.F.; Hoogsteden, H.C.; Hendriks, R.W.; Aerts, J.G.; Hegmans, J.P. Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. Oncoimmunology 2015, 4, e1014242. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wu, Q.; Shi, J.; Guo, W.; Jiang, X.; Zhou, B.; Ren, C. LILRB4, from the immune system to the disease target. Am. J. Transl. Res. 2020, 12, 3149–3166. [Google Scholar] [PubMed]
- Li, S.; Rouphael, N.; Duraisingham, S.; Romero-Steiner, S.; Presnell, S.; Davis, C.; Schmidt, D.S.; Johnson, S.E.; Milton, A.; Rajam, G.; et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. 2014, 15, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartnell, F.; Brown, A.; Capone, S.; Kopycinski, J.; Bliss, C.; Makvandi-Nejad, S.; Swadling, L.; Ghaffari, E.; Cicconi, P.; Del Sorbo, M.; et al. A Novel Vaccine Strategy Employing Serologically Different Chimpanzee Adenoviral Vectors for the Prevention of HIV-1 and HCV Coinfection. Front. Immunol. 2018, 9, 3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco-Sola, S.; Sammeth, M.; Guigó, R.; Ribeca, P. The GEM mapper: Fast, accurate and versatile alignment by filtration. Nat. Methods 2012, 9, 1185–1188. [Google Scholar] [CrossRef]
- Lappalainen, T.; Sammeth, M.; Friedländer, M.R.; Ac’t Hoen, P.; Monlong, J.; Rivas, M.A.; Gonzàlez-Porta, M.; Kurbatova, N.; Griebel, T.; Ferreira, P.G.; et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 2013, 501, 506–511. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
| Gene Set | Timepoint | Enriched Ontologies |
|---|---|---|
| BCL3, BCL6, UNC13D | 6 h | Germinal center formation |
| CXCL8, CXCL1 | 12 h | Chemokine activity, Chemokine receptor binding, Antimicrobial humoral immune response, Response to chemokine, Neutrophil migration, Granulocyte migration, Myeloid leukocyte migration, Cytokine activity, Leukocyte chemotaxis, Cellular response to biotic stimulus, G-protein coupled receptor binding, Cytokine receptor binding, Cell chemotaxis, Response to a molecule of bacterial origin |
| DDIT4, PER1 | 12 h, 24 h | Response to a steroid hormone, Negative regulation of phosphorylation |
| CXCL8, KRT64 | 24 h | Entry into the host, Interaction with host, Antimicrobial humoral immune response, Regulation of symbiosis |
| CDSN, KRT64 | 24 h | Keratinization, Cornification, Keratinocyte differentiation, Epidermal cell differentiation, Skin development, Epidermis development |
| Camel | 0 h | 6 h | 12 h | 24 h | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Mapping | Total | Mapping | Total | Mapping | Total | Mapping | |
| 3 | 23.5 M | 73.4% | 23.0 M | 96.4% | 23.0 M | 94.4% | 18.7 M | 96.2% |
| 4 | 18.1 M | 93.3% | 14.8 M | 96.2% | 27.1 M | 94.2% | 27.3 M | 96.0% |
| 6 | 25.5 M | 94.5% | 21.5 M | 96.0% | 31.8 M | 95.3% | 19.2 M | 96.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hala, S.; Ribeca, P.; Aljami, H.A.; Alsagaby, S.A.; Qasim, I.; Gilbert, S.C.; Alharbi, N.K. Transcriptomic Profiling of Dromedary Camels Immunised with a MERS Vaccine Candidate. Vet. Sci. 2021, 8, 156. https://doi.org/10.3390/vetsci8080156
Hala S, Ribeca P, Aljami HA, Alsagaby SA, Qasim I, Gilbert SC, Alharbi NK. Transcriptomic Profiling of Dromedary Camels Immunised with a MERS Vaccine Candidate. Veterinary Sciences. 2021; 8(8):156. https://doi.org/10.3390/vetsci8080156
Chicago/Turabian StyleHala, Sharif, Paolo Ribeca, Haya A. Aljami, Suliman A. Alsagaby, Ibrahim Qasim, Sarah C. Gilbert, and Naif Khalaf Alharbi. 2021. "Transcriptomic Profiling of Dromedary Camels Immunised with a MERS Vaccine Candidate" Veterinary Sciences 8, no. 8: 156. https://doi.org/10.3390/vetsci8080156
APA StyleHala, S., Ribeca, P., Aljami, H. A., Alsagaby, S. A., Qasim, I., Gilbert, S. C., & Alharbi, N. K. (2021). Transcriptomic Profiling of Dromedary Camels Immunised with a MERS Vaccine Candidate. Veterinary Sciences, 8(8), 156. https://doi.org/10.3390/vetsci8080156







