Erythrogram Patterns in Dogs with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Hematological Parameters
2.3. Statistical Analysis
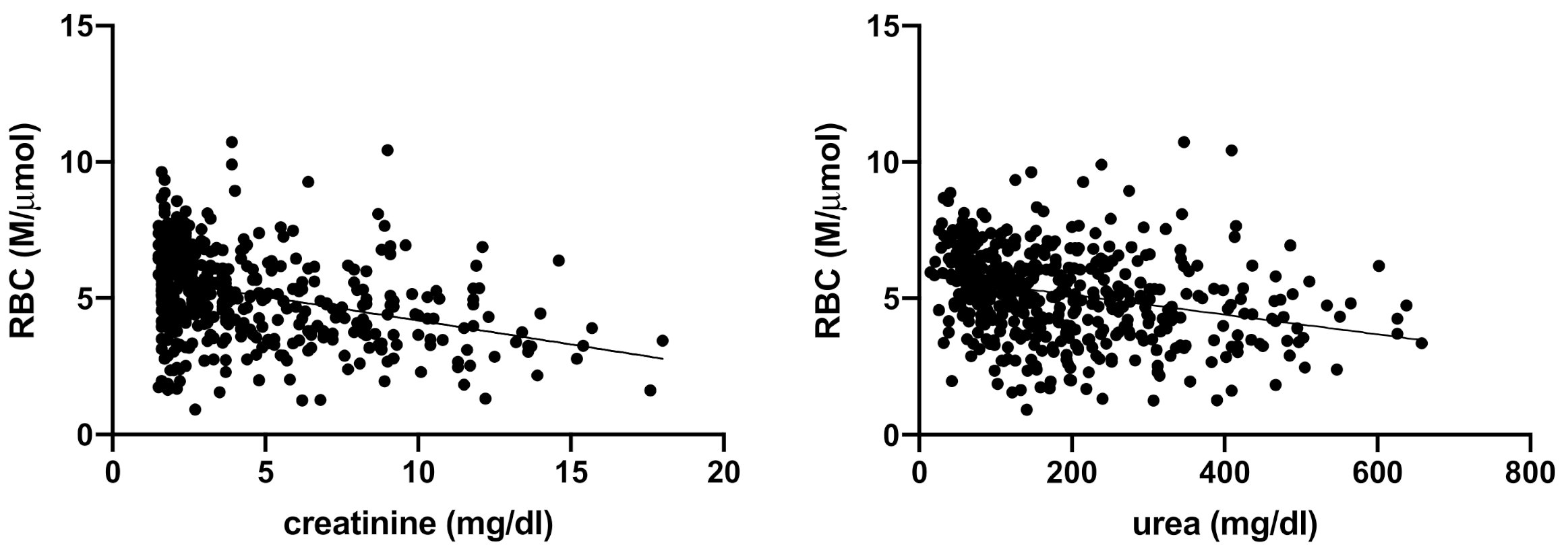
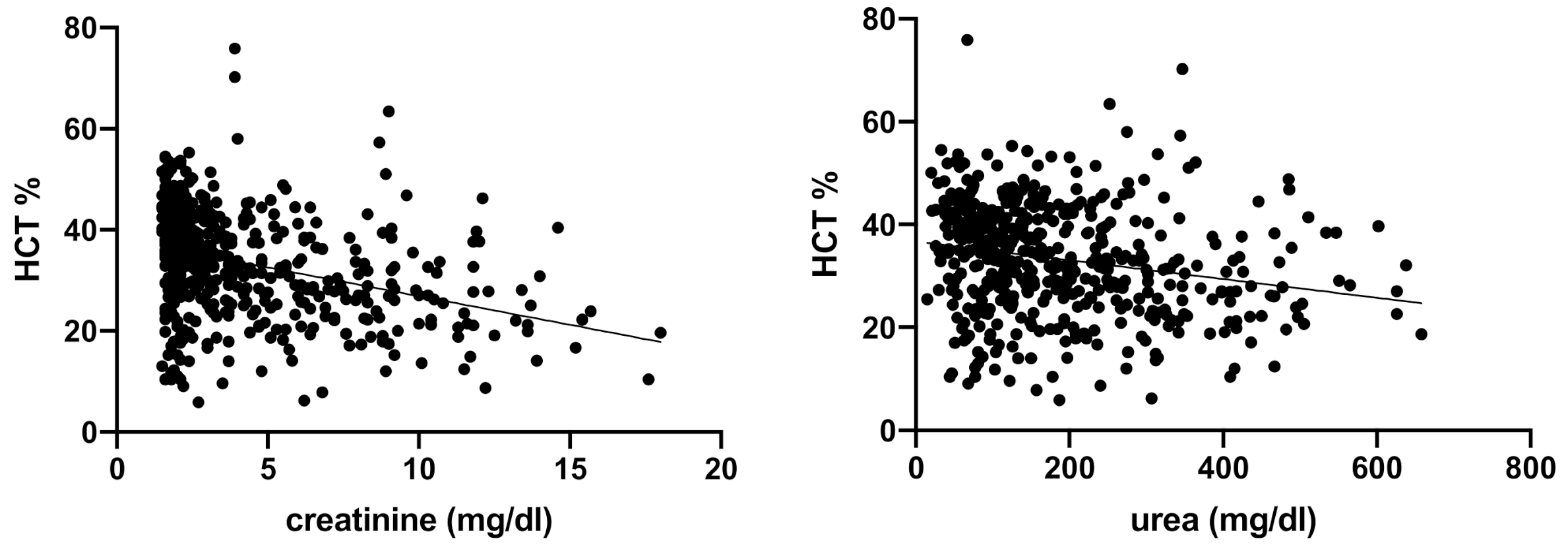
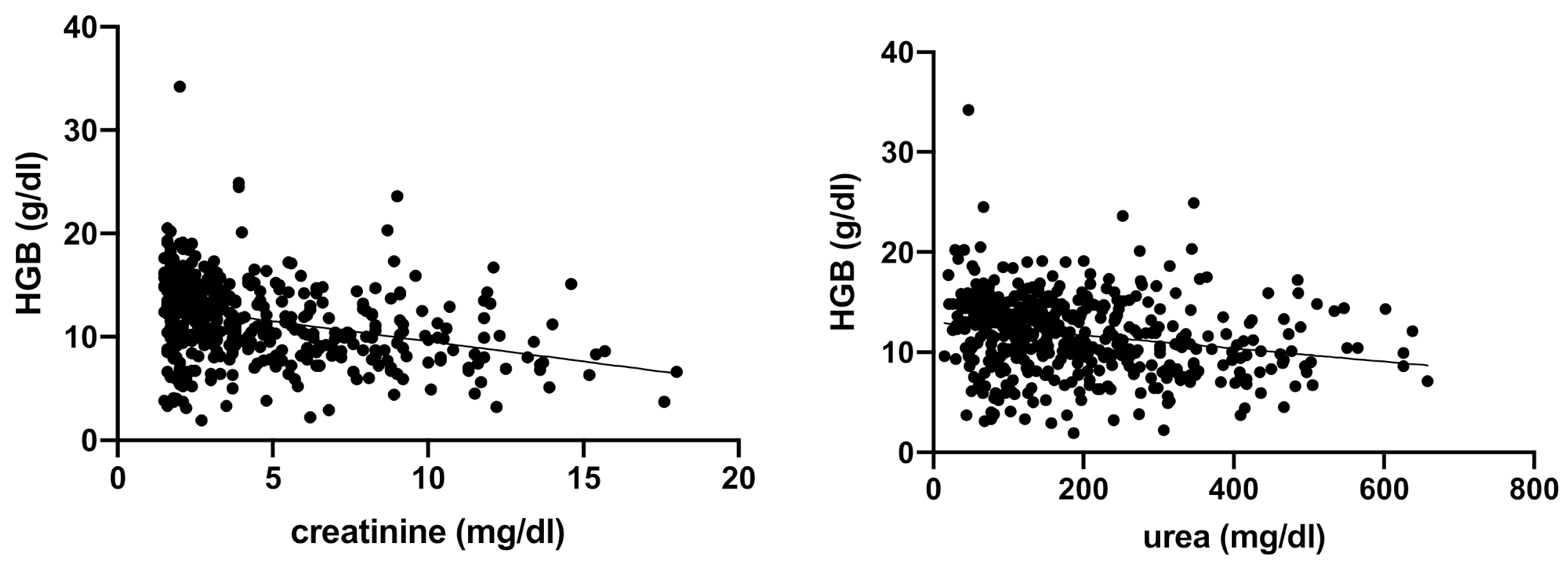
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, L.G.; Giger, U.; Diserens, D.; Nagode, L.A. Anemia of chronic renal failure in dogs. JVIM 1992, 6, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.J. Chronic Kidney Disease. In Nephrology and Urology of Small Animals, 1st ed.; Bartges, J., Polzin, D.J., Eds.; Blackwell Publishing Ltd.: Chicester, UK, 2011; pp. 433–471. [Google Scholar]
- Fry, M.M. Anemia of inflammatory, neoplastic, renal and endocrine diseases. In Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 246–250. [Google Scholar]
- Fiocchi, E.H.; Cowgill, L.D.; Brown, D.C.; Markovich, J.E.; Tucker, S.; Labato, M.A.; Callan, M.B. The use of darbepoetin to stimulate erythropoiesis in the treatment of anemia of chronic kidney disease in dogs. JVIM 2017, 31, 476–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragato, N.; Borges, N.C.; Soares Fioravanti, M.C. B-mode and Doppler ultrasound of chronic kidney disease in dogs and cats. Vet. Res. Commun. 2017, 41, 307–315. [Google Scholar] [CrossRef] [PubMed]
- IRIS Staging of CKD (Modified 2019). Available online: http://www.iris-kidney.com (accessed on 1 January 2021).
- Tvedten, H. Laboratory and clinical diagnosis of anemia. In Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 152–161. [Google Scholar]
- Villiers, E. Disorders of erythrocytes. In BSAVA Manual of Canine and Feline Clinical Pathology, 3rd ed.; Villers, E., Ristic, J., Eds.; BSAVA: Gloucester, UK, 2016; pp. 38–66. [Google Scholar]
- Weiss, D.J. Uniform evaluation and semiquantitative reporting of hematologic data in veterinary laboratories. Vet. Clin. Pathol. 1984, 13, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Brzozka, A.; Franczyk, B.; Olszewski, R.; Rysz, J. The influence of inflammation on anemia in CKD patients. Int. J. Mol. Sci. 2020, 21, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cases, A.; Egocheaga, M.I.; Tranche, S.; Pallares, V.; Ojeda, R.; Gorriz, J.L.; Portoles, J.M. Anemia of chronic kidney disease: Protocol of study, management and referral to nephrology. Nefrologia 2018, 38, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bowry, S.K.; Gatti, E. Impact of hemodialysis therapy on anemia of chronic kidney disease: The potential mechanisms. Blood Purif. 2011, 32, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.L.; Elwood, C.; Murphy, K.F.; Gajanayake, I.; Syme, H.M. Juvenile nephropathy in 37 boxer dogs. J. Small Anim. Pract. 2007, 48, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Bartges, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Lothrop, C.D. Serum erythropoietin concentrations measured by radiommunoassay in normal, polycythemic, and anemic dogs and cats. JVIM 1994, 8, 18–25. [Google Scholar]
- Christian, J.A. Erythrokinetics and erythrocyte destruction. In Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 136–143. [Google Scholar]
- Dorgalaleh, A.; Mahmudi, M.; Tabibian, S.; Kashani Khatib, Z.; Hossein Tamaddon, G.; Sanei Moghaddam, E.; Bamedi, T.; Alizadeh, S.; Moradi, E. Anemia and thrombocytopenia in acute and chronic renal failure. Int. J. Hematol. Oncol. Stem Cell Res. 2013, 7, 34–39. [Google Scholar] [PubMed]
- Paltrinieri, S.; Bertazzolo, W.; Giordano, A. Ematologia ed Emostasi. In Patologia Clinica del Cane e del Gatto, Approccio Pratico alla Diagnostica di Laboratorio, 1st ed.; Paltrinieri, S., Bertazzolo, W., Giordano, A., Eds.; Elsevier: Milano, Italy, 2017; pp. 22–64. [Google Scholar]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Duchnowski, P.; Hryniewiecki, T.; Kusmierczyk, M.; Szymanski, P. Anisocytosis predicts postoperative renal replacement therapy in patients undergoing heart valve surgery. Cardiol. J. 2020, 27, 362–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babitt, J.L.; Lin, H.Y. Mechanisma of anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopher, M.M.; Hawkins, M.G.; Burton, A.G. Poikilocytosis in rabbits: Prevalence, type, and association with disease. PLoS ONE 2014, 9, e112455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, A.B.; Voloshyna, I.; DeLeon, J.; Miyawaki, N.; Mattana, J. Cholesterol metabolism in CKD. Am. J. Kidney Dis. 2015, 66, 1071–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warry, E.; Bohn, A.; Emanuelli, M.; Thamm, D.; Lana, S. Disease distribution in canine patients with acanthocytosis: 123 cases. Vet. Clin. Pathol. 2013, 42, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.W. Evaluation of erythrocytes; Abnormal erythtocyte morphology. In Veterinary Hematology, a Diagnostic Guide and Color Atlas, 2nd ed.; Harvey, J.W., Ed.; Elsevier: St. Louis, MO, USA, 2012; pp. 59–70. [Google Scholar]
| IRIS 2 (n = 108) | IRIS 3 (n = 77) | IRIS 4 (n = 117) | p-Value | |
|---|---|---|---|---|
| Frequency of different types of anemia | 0.203 | |||
| Microcytic–Hypochromic | 1/108 (1%) | 2/77 (3%) | 0/117 (0%) | |
| Microcytic–Normochromic | 26/108 (24%) | 11/77 (14%) | 22/117 (19%) | |
| Microcytic–Hyperchromic | 1/108 (1%) | 3/77 (4%) | 6/117 (5%) | |
| Normocytic–Normochromic | 74/108 (69%) | 57/77 (74%) | 78/117 (67%) | |
| Normocytic–Hyperchromic | 2/108 (2%) | 0/77 (0%) | 2/117 (2%) | |
| Macrocytic–Hypochromic | 3/108 (3%) | 0/77 (0%) | 3/117 (3%) | |
| Macrocytic–Normochromic | 1/108 (1%) | 4/77 (5%) | 6/117 (5%) | |
| IRIS 2 | IRIS 3 | IRIS 4 | p-Value | |
|---|---|---|---|---|
| Frequency of anemia | 0.0001 | |||
| Anemia | (n = 108) 108/231 (47%) | (n = 77) 77/109 (71%) | (n = 117) 117/142 (82%) | |
| Degree of anemia | 0.0001 | |||
| Mild (HCT 37–30%) | (n = 104) 49/104 (47%) | (n = 76) 34/76 (45%) | (n = 115) 27/115 (23%) | |
| Moderate (HCT 29–20%) | 34/104 (33%) | 34/76 (45%) | 62/115 (54%) | |
| Severe (HCT < 19%) | 21/104 (20%) | 8/76 (10%) | 26/115 (23%) | |
| Degree of regeneration | 0.0001 | |||
| None (RET < 60,000/μL) | (n = 104) 78/104 (75%) | (n = 76) 61/76 (80%) | (n = 115) 109/115 (95%) | |
| Mild (RET 60,000–150,000/μL) | 20/104 (19%) | 12/76 (16%) | 5/115 (4%) | |
| Moderate (RET > 150,000/μL) | 6/104 (6%) | 3/7 (4%) | 1/115 (0.8%) | |
| Parameter | Reference Range | CKD (n = 482) | IRIS 2 (n = 231) | IRIS 3 (n = 109) | IRIS 4 (n = 142) | p-Value |
|---|---|---|---|---|---|---|
| RBC (M/µL) | 5.6–8.8 | 5.17 ± 1.64 | 5.86 (4.76–6.7) | 5.0 (4.0–6.0) | 4.2 (3.3–5.2) | <0.0001 |
| HCT (%) | 37.3–61.7 | 33.2 ± 10.7 | 37.6 (30.4–43.9) | 32.1 (26.0–39.2) | 27.3 (21.2–335.0) | <0.0001 |
| HGB (g/dL) | 13.1–20.5 | 11.9 (9.2–14.4) | 13.3 (10.8–15.2) | 11.2 (13.8–9.5) | 9.8 (12.1–7.7) | <0.0001 |
| MCV (fL) | 61.6–73.5 | 64.6 (61.5–67.5) | 64.8 (61.6–67.2) | 64.8 (61.8–68.0) | 64 (60.8–67.7) | 0.53 |
| MCH (pg) | 21.2–25.9 | 22.8 (21.8–23.7) | 22.6 (21.6–23.6) | 23 (22.0–23.7) | 23 (21.9–24) | 0.11 |
| MCHC (g/dL) | 32.0–37.9 | 35.3 (34.1–36.3) | 35.1 (34.0–35.9) | 35.3 (33.9–36.4) | 35.7 (34.5–36.7) | 0.0002 |
| RDW (%) | 13.6–21.7 | 16.2 (14.9–18.2) | 16.5 (15.0–18.8) | 16 (14.7–18.3) | 15.8 (14.7–17.7) | 0.024 |
| RET (K/µL) | 10–110 | 24 (1–166) | 34 (3–166) | 23 (12–56) | 16 (1–78) | 0.003 |
| Degree | IRIS 2 | IRIS 3 | IRIS 4 | p-Value | |
|---|---|---|---|---|---|
| Anisocytosis (291/482; 60%) | weak | 90 | 45 | 56 | 0.77 |
| moderate | 40 | 15 | 25 | ||
| marked | 7 | 5 | 8 | ||
| Polychromasia (113/482; 23%) | weak | 59 | 1 | 18 | 0.001 |
| moderate | 9 | 1 | 2 | ||
| marked | 1 | 1 | 1 | ||
| Howell–Jolly bodies (87/482; 18%) | weak | 30 | 11 | 31 | 0.186 |
| moderate | 7 | 0 | 5 | ||
| marked | 3 | 0 | 0 | ||
| Poikilocytosis (135/482; 28%) | weak | 33 | 23 | 29 | 0.315 |
| moderate | 15 | 14 | 7 | ||
| marked | 3 | 4 | 7 | ||
| Echinocyte (73/135; 54%) | weak | 16 | 11 | 17 | 0.464 |
| moderate | 7 | 9 | 12 | ||
| marked | 0 | 1 | 0 | ||
| Fragmented cells (54/135; 40%) | weak | 21 | 7 | 20 | 0.008 |
| moderate | 2 | 4 | 0 | ||
| marked | 0 | 0 | 0 | ||
| Acantocytes (45/135; 33%) | weak | 15 | 5 | 15 | 0.05 |
| moderate | 3 | 5 | 2 | ||
| marked | 0 | 0 | 0 | ||
| Dacriocytes (13/135, 10%) | weak | 4 | 8 | 0 | 0.488 |
| moderate | 0 | 1 | 0 | ||
| marked | 0 | 0 | 0 | ||
| Eccentrocytes (5/135; 4%) | weak | 1 | 1 | 0 | 0.441 |
| moderate | 0 | 0 | 1 | ||
| marked | 1 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, I.; Perondi, F.; Lubas, G.; Gori, E.; Pierini, A.; D’Addetta, A.; Marchetti, V. Erythrogram Patterns in Dogs with Chronic Kidney Disease. Vet. Sci. 2021, 8, 123. https://doi.org/10.3390/vetsci8070123
Lippi I, Perondi F, Lubas G, Gori E, Pierini A, D’Addetta A, Marchetti V. Erythrogram Patterns in Dogs with Chronic Kidney Disease. Veterinary Sciences. 2021; 8(7):123. https://doi.org/10.3390/vetsci8070123
Chicago/Turabian StyleLippi, Ilaria, Francesca Perondi, George Lubas, Eleonora Gori, Alessio Pierini, Alessandra D’Addetta, and Veronica Marchetti. 2021. "Erythrogram Patterns in Dogs with Chronic Kidney Disease" Veterinary Sciences 8, no. 7: 123. https://doi.org/10.3390/vetsci8070123
APA StyleLippi, I., Perondi, F., Lubas, G., Gori, E., Pierini, A., D’Addetta, A., & Marchetti, V. (2021). Erythrogram Patterns in Dogs with Chronic Kidney Disease. Veterinary Sciences, 8(7), 123. https://doi.org/10.3390/vetsci8070123









