Variation in the Distribution of Nosema Species in Honeybees (Apis mellifera Linnaeus) between the Neighboring Countries Estonia and Latvia
Abstract
1. Introduction
2. Materials and Methods
2.1. The Geographical Location of Apiaries and Honey Bee Sampling
2.2. DNA Extraction and Analysis
2.3. Flow Cytometry and Spore Counting
2.4. Statistics
3. Results
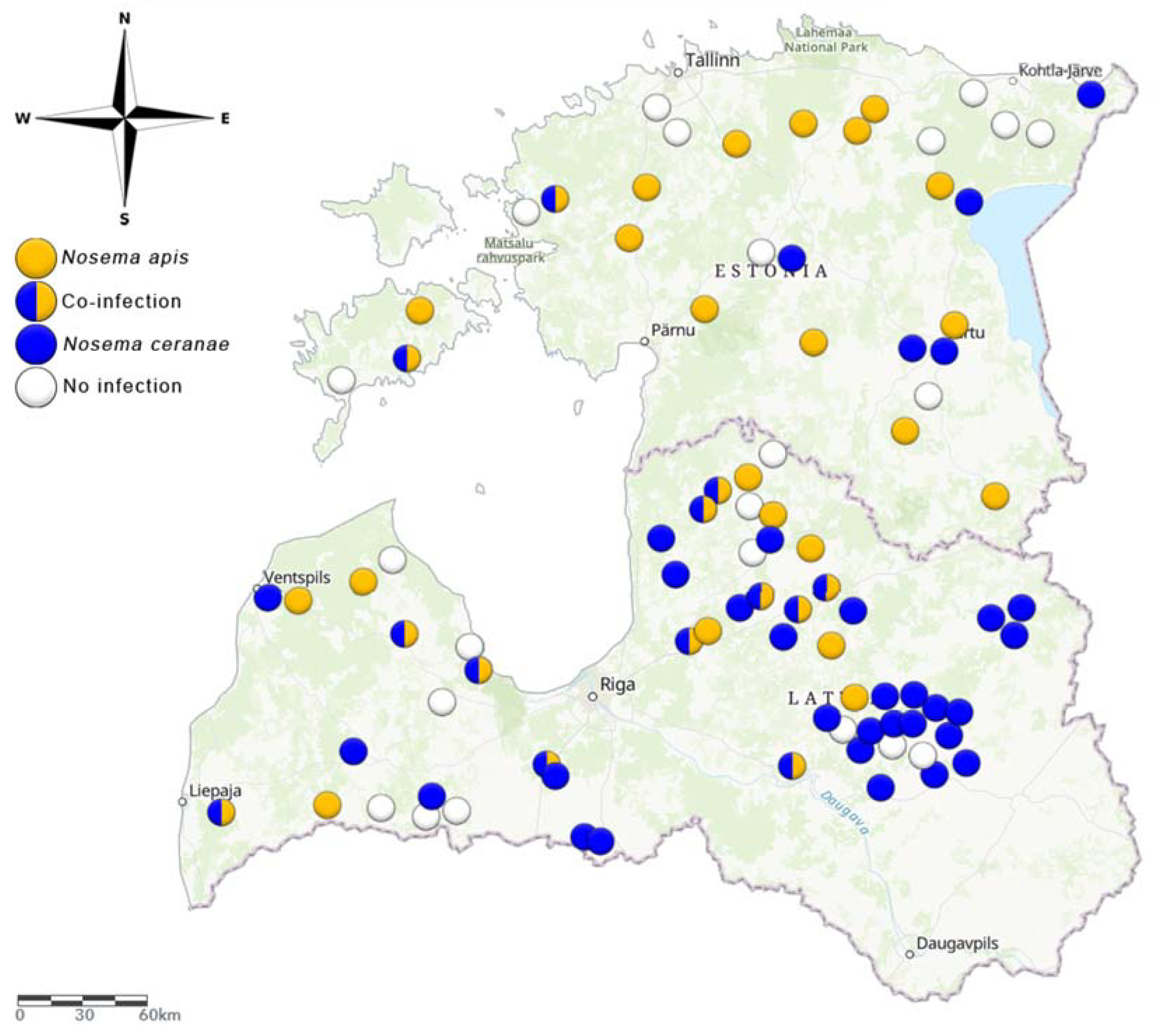
3.1. Prevalence of N. apis and N. ceranae
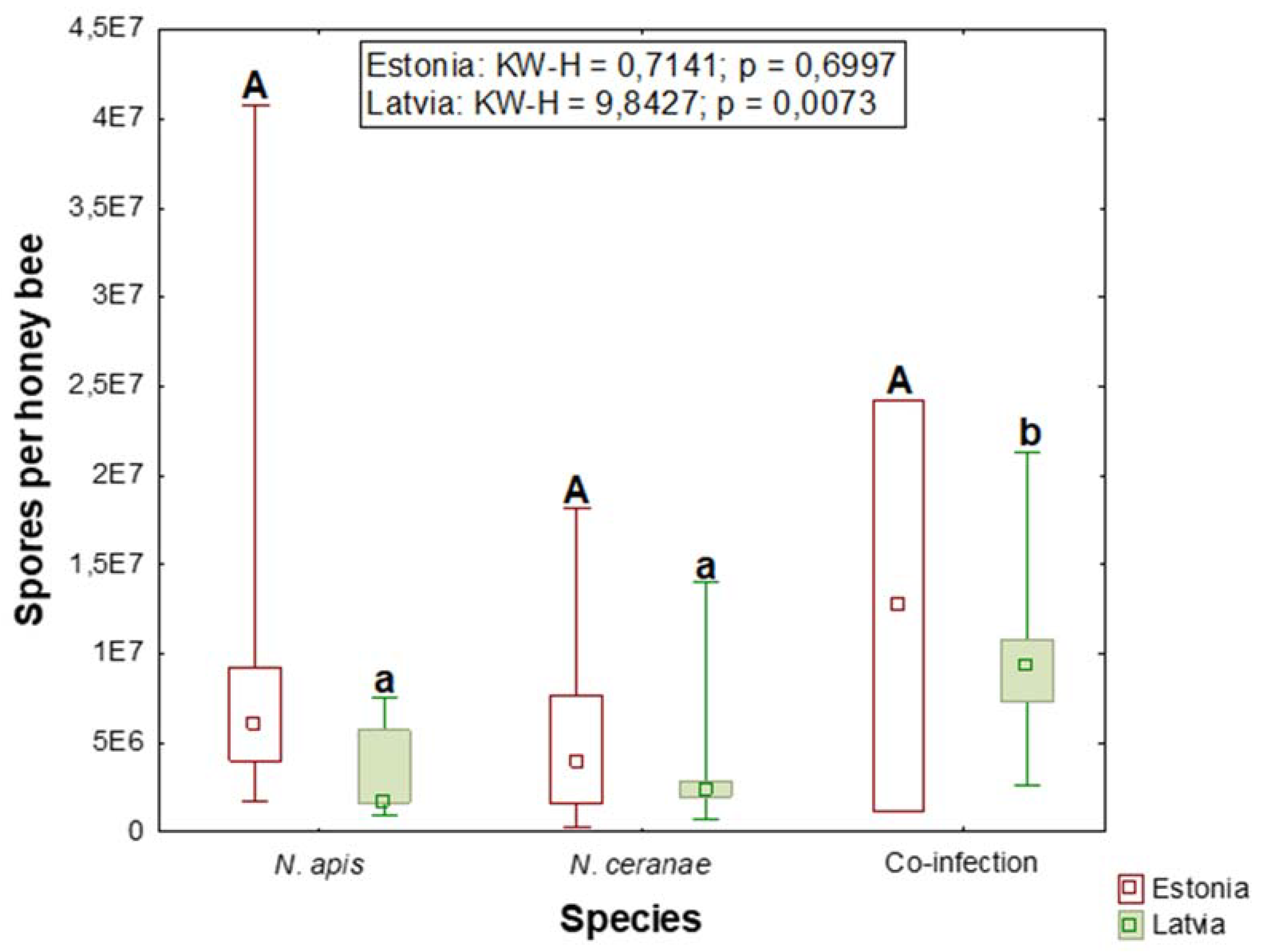
3.2. Spore Quantity
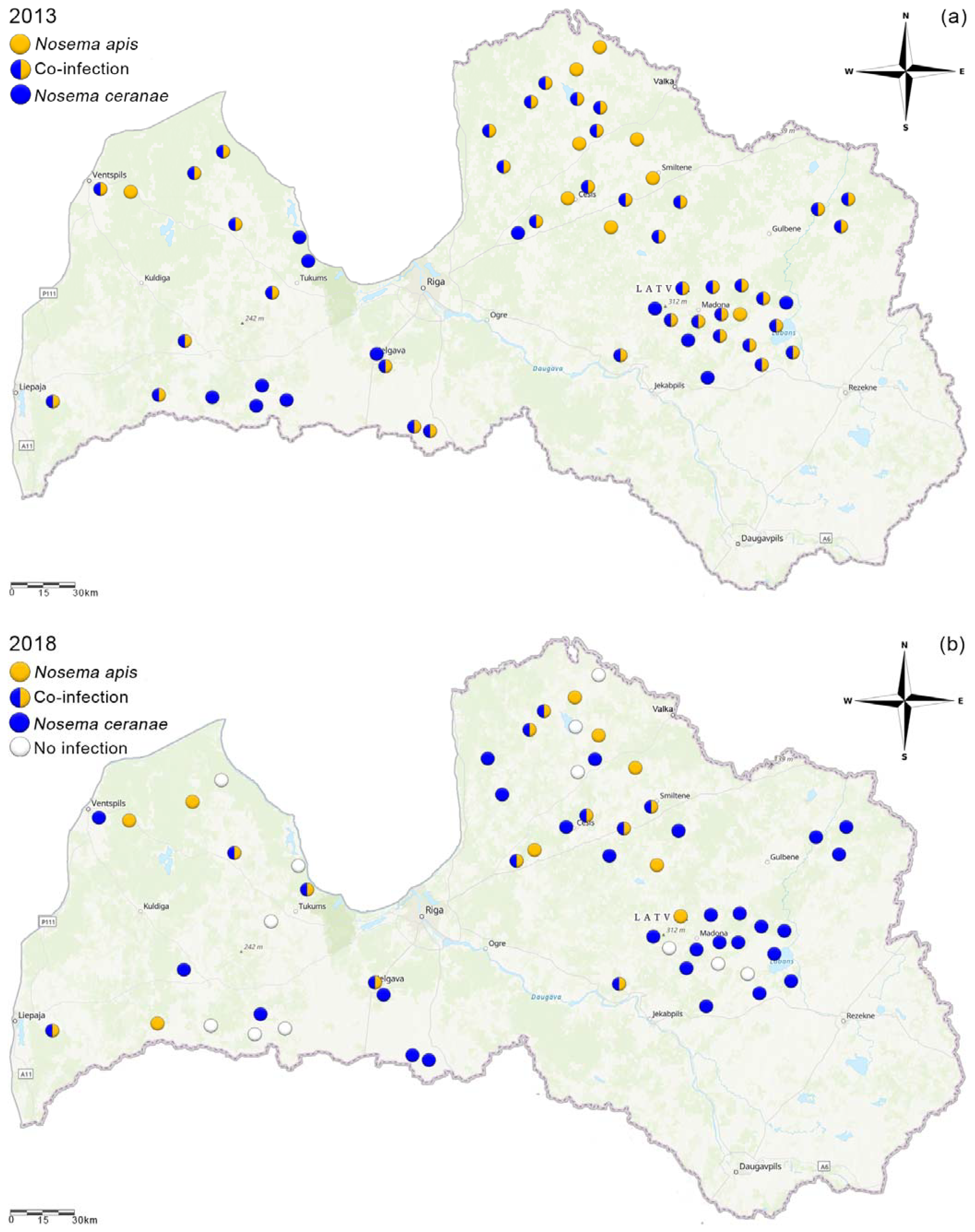
3.3. Species Distribution Changes in Latvia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacques, A.; Laurent, M.; Consortium, E.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.-P. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef] [PubMed]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Wilson, K. Honeybee nutrition is linked to landscape composition. Ecol. Evol. 2014, 4, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Karise, R.; Raimets, R.; Bartkevics, V.; Pugajeva, I.; Pihlik, P.; Keres, I.; Williams, I.H.; Viinalass, H.; Mänd, M. Are pesticide residues in honey related to oilseed rape treatments? Chemosphere 2017, 188, 389–396. [Google Scholar] [CrossRef]
- Raimets, R.; Bontšutšnaja, A.; Bartkevics, V.; Pugajeva, I.; Kaart, T.; Puusepp, L.; Pihlik, P.; Keres, I.; Viinalass, H.; Mänd, M.; et al. Pesticide residues in beehive matrices are dependent on collection time and matrix type but independent of proportion of foraged oilseed rape and agricultural land in foraging territory. Chemosphere 2020, 238, 124555. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.Y.; Chantawannakul, P.; McAfee, A. Varroa destructor: A Complex Parasite, Crippling Honey Bees Worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef]
- Hiiesaar, K.; Jõgar, K.; Williams, I.H.; Kruus, E.; Metspalu, L.; Luik, A.; Ploomi, A.; Eremeev, V.; Karise, R.; Mänd, M. Factors affecting development and overwintering of second generation Colorado Potato Beetle (Coleoptera: Chrysomelidae) in Estonia in 2010. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013, 63, 506–515. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Fries, I. Nosema Apis—A Parasite in the Honey Bee Colony. Bee World 1993, 74, 5–19. [Google Scholar] [CrossRef]
- Fries, I.; Feng, F.; da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Forsgren, E.; Fries, I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 2010, 170, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-F.; Jiang, J.-H.; Chen, Y.-W.; Wang, C.-H. A Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie 2007, 38. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Meana, A.; Higes, M.; Martin, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef]
- Sinpoo, C.; Paxton, R.J.; Disayathanoowat, T.; Krongdang, S.; Chantawannakul, P. Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response. J. Insect Physiol. 2018, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pacini, A.; Mira, A.; Molineri, A.; Giacobino, A.; Bulacio Cagnolo, N.; Aignasse, A.; Zago, L.; Izaguirre, M.; Merke, J.; Orellano, E.; et al. Distribution and prevalence of Nosema apis and N. ceranae in temperate and subtropical eco-regions of Argentina. J. Invertebr. Pathol. 2016, 141, 34–37. [Google Scholar] [CrossRef]
- Papini, R.; Mancianti, F.; Canovai, R.; Cosci, F.; Rocchigiani, G.; Benelli, G.; Canale, A. Prevalence of the microsporidian Nosema ceranae in honeybee (Apis mellifera) apiaries in Central Italy. Saudi J. Biol. Sci. 2017, 24, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Vugrek, O.; Grilec, D.; Petrinec, Z. Prevalence and distribution of Nosema ceranae in Croatian honeybee colonies. Vet. Med. 2010, 55, 457–462. [Google Scholar] [CrossRef]
- Matović, K.; Vidanović, D.; Manić, M.; Stojiljković, M.; Radojičić, S.; Debeljak, Z.; Šekler, M.; Ćirić, J. Twenty-five-year study of Nosema spp. in honey bees (Apis mellifera) in Serbia. Saudi J. Biol. Sci. 2020, 27, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Odemer, R. Nosema Ceranae—A New Threat to Honey Bees (Apis mellifera)? 2021. Available online: https://osf.io/47df8/ (accessed on 16 March 2021).
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Blazyte-Cereskiene, L.; Skrodenyte-Arbaciauskiene, V.; Radžiutė, S.; Nedveckytė, I.; Buda, V. Honey Bee Infection Caused by Nosema spp. in Lithuania. J. Apic. Sci. 2016, 60. [Google Scholar] [CrossRef]
- Holt, H.L.; Aronstein, K.A.; Grozinger, C.M. Chronic parasitization by Nosema microsporidia causes global expression changes in core nutritional, metabolic and behavioral pathways in honey bee workers (Apis mellifera). BMC Genom. 2013, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Evans, J.D.; Murphy, C.; Gutell, R.; Zuker, M.; Gundensen-Rindal, D.; Pettis, J.S. Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera. J. Eukaryot. Microbiol. 2009, 56, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; García-Palencia, P.; Urbieta, A.; Nanetti, A.; Martín-Hernández, R. Nosema apis and Nosema ceranae Tissue Tropism in Worker Honey Bees (Apis mellifera). Vet. Pathol. 2020, 57, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Emsen, B.; De la Mora, A.; Lacey, B.; Eccles, L.; Kelly, P.G.; Medina-Flores, C.A.; Petukhova, T.; Morfin, N.; Guzman-Novoa, E. Seasonality of Nosema ceranae Infections and Their Relationship with Honey Bee Populations, Food Stores, and Survivorship in a North American Region. Vet. Sci. 2020, 7, 131. [Google Scholar] [CrossRef]
- van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An Overview of Recent Scientific Advances and Their Significance for Apiculture. J. Agric. Food Chem. 2014, 62, 2728–2737. [Google Scholar] [CrossRef]
- Costa, C.; Lodesani, M.; Maistrello, L. Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 2009, 41, 141–150. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Control of the microsporidian parasite Nosema ceranae in honey bees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef]
- Botías, C.; Anderson, D.L.; Meana, A.; Garrido-Bailón, E.; Martín-Hernández, R.; Higes, M. Further evidence of an oriental origin for Nosema ceranae (Microsporidia: Nosematidae). J. Invertebr. Pathol. 2012, 110, 108–113. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Meana, A. Nosema ceranae in Europe: An emergent type C nosemosis. Apidologie 2010, 41, 375–392. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Bartolomé, C.; Chejanovsky, N.; Conte, Y.L.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef]
- Natsopoulou, M.E.; Doublet, V.; Paxton, R.J. European isolates of the Microsporidia Nosema apis and Nosema ceranae have similar virulence in laboratory tests on European worker honey bees. Apidologie 2016, 47, 57–65. [Google Scholar] [CrossRef][Green Version]
- Gisder, S.; Schüler, V.; Horchler, L.L.; Groth, D.; Genersch, E. Long-Term Temporal Trends of Nosema spp. Infection Prevalence in Northeast Germany: Continuous Spread of Nosema ceranae, an Emerging Pathogen of Honey Bees (Apis mellifera), but No General Replacement of Nosema apis. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Odemer, R.; Nilles, L.; Linder, N.; Rosenkranz, P. Sublethal effects of clothianidin and Nosema spp. on the longevity and foraging activity of free flying honey bees. Ecotoxicol. Lond. Engl. 2018, 27, 527–538. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Nozal, M.J.; del Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.-L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Ostroverkhova, N.V.; Konusova, O.L.; Kucher, A.N.; Kireeva, T.N.; Rosseykina, S.A. Prevalence of the Microsporidian Nosema spp. in Honey Bee Populations (Apis mellifera) in Some Ecological Regions of North Asia. Vet. Sci. 2020, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Al-Ghamdi, A.; Nuru, A.; Khan, K.A.; Alattal, Y. Geographical distribution and molecular detection of Nosema ceranae from indigenous honey bees of Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 983–991. [Google Scholar] [CrossRef] [PubMed]
- González, S.A.C.; Valencia, G.L.; Cabrera, C.O.; Gómez, S.D.G.; Torres, K.M.; Blandón, K.O.E.; Velázquez, J.G.G.; Paz, L.E.S.; Muñoz, E.T.; Navarro, F.J.M. Prevalence and geographical distribution of Nosema apis and Nosema ceranae in apiaries of Northwest Mexico using a duplex real-time PCR with melting-curve analysis. J. Apic. Res. 2020, 59, 195–203. [Google Scholar] [CrossRef]
- Cilia, G.; Sagona, S.; Giusti, M.; Jarmela dos Santos, P.E.; Nanetti, A.; Felicioli, A. Nosema ceranae infection in honeybee samples from Tuscanian Archipelago (Central Italy) investigated by two qPCR methods. Saudi J. Biol. Sci. 2019, 26, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Mederle, N.; Lobo, M.; Morariu, S.; Morariu, F.; Darabus, G.; Mederle, O.; Matos, O. Microscopic and molecular detection of nosema ceranae in honeybee apis mellifera L. from Romania. Rev. Chim. 2018, 69, 3761–3772. [Google Scholar] [CrossRef]
- Ozgor, E.; Keskin, N. Determination of spore longevity and viability of Nosema apis and Nosema ceranae according to storage conditions. EuroBiotech J. 2017, 1, 217–221. [Google Scholar] [CrossRef]
- Fenoy, S.; Rueda, C.; Higes, M.; Martín-Hernández, R.; del Aguila, C. High-Level Resistance of Nosema ceranae, a Parasite of the Honeybee, to Temperature and Desiccation. Appl. Environ. Microbiol. 2009, 75, 6886–6889. [Google Scholar] [CrossRef]
- Graystock, P.; Goulson, D.; Hughes, W.O.H. Parasites in bloom: Flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151371. [Google Scholar] [CrossRef]
- Meana, A.; Martín-Hernández, R.; Higes, M. The reliability of spore counts to diagnose Nosema ceranae infections in honey bees. J. Apic. Res. 2010, 49, 212–214. [Google Scholar] [CrossRef]
| Name | Primer Sequence | Fragment Size (bp) | Specificity |
|---|---|---|---|
| MnCeranae-F | 5′−CGTTAAAGTGTAGATAAGATGTT−3′ | 143 | N. ceranae |
| MnApis-F | 5′−GCATGTCTTTGACGTACTATG−3′ | 224 | N. apis |
| Muniv-R | 5′−GACTTAGTAGCCGTCTCTC−3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naudi, S.; Šteiselis, J.; Jürison, M.; Raimets, R.; Tummeleht, L.; Praakle, K.; Raie, A.; Karise, R. Variation in the Distribution of Nosema Species in Honeybees (Apis mellifera Linnaeus) between the Neighboring Countries Estonia and Latvia. Vet. Sci. 2021, 8, 58. https://doi.org/10.3390/vetsci8040058
Naudi S, Šteiselis J, Jürison M, Raimets R, Tummeleht L, Praakle K, Raie A, Karise R. Variation in the Distribution of Nosema Species in Honeybees (Apis mellifera Linnaeus) between the Neighboring Countries Estonia and Latvia. Veterinary Sciences. 2021; 8(4):58. https://doi.org/10.3390/vetsci8040058
Chicago/Turabian StyleNaudi, Sigmar, Juris Šteiselis, Margret Jürison, Risto Raimets, Lea Tummeleht, Kristi Praakle, Arvi Raie, and Reet Karise. 2021. "Variation in the Distribution of Nosema Species in Honeybees (Apis mellifera Linnaeus) between the Neighboring Countries Estonia and Latvia" Veterinary Sciences 8, no. 4: 58. https://doi.org/10.3390/vetsci8040058
APA StyleNaudi, S., Šteiselis, J., Jürison, M., Raimets, R., Tummeleht, L., Praakle, K., Raie, A., & Karise, R. (2021). Variation in the Distribution of Nosema Species in Honeybees (Apis mellifera Linnaeus) between the Neighboring Countries Estonia and Latvia. Veterinary Sciences, 8(4), 58. https://doi.org/10.3390/vetsci8040058







