Serial Ultrasonographic and Real-Time Elastosonographic Assessment of the Ovine Common Calcaneal Tendon, after an Experimentally Induced Tendinopathy
Abstract
1. Introduction
2. Materials and Methods
- Grade 1: Normal tendon with parallel fibers and homogeneous architecture
- Grade 2: Enlarged tendon with bowed margins and homogeneous architecture
- Grade 3: Hypoechoic area with or without tendon enlargement and bowed margins
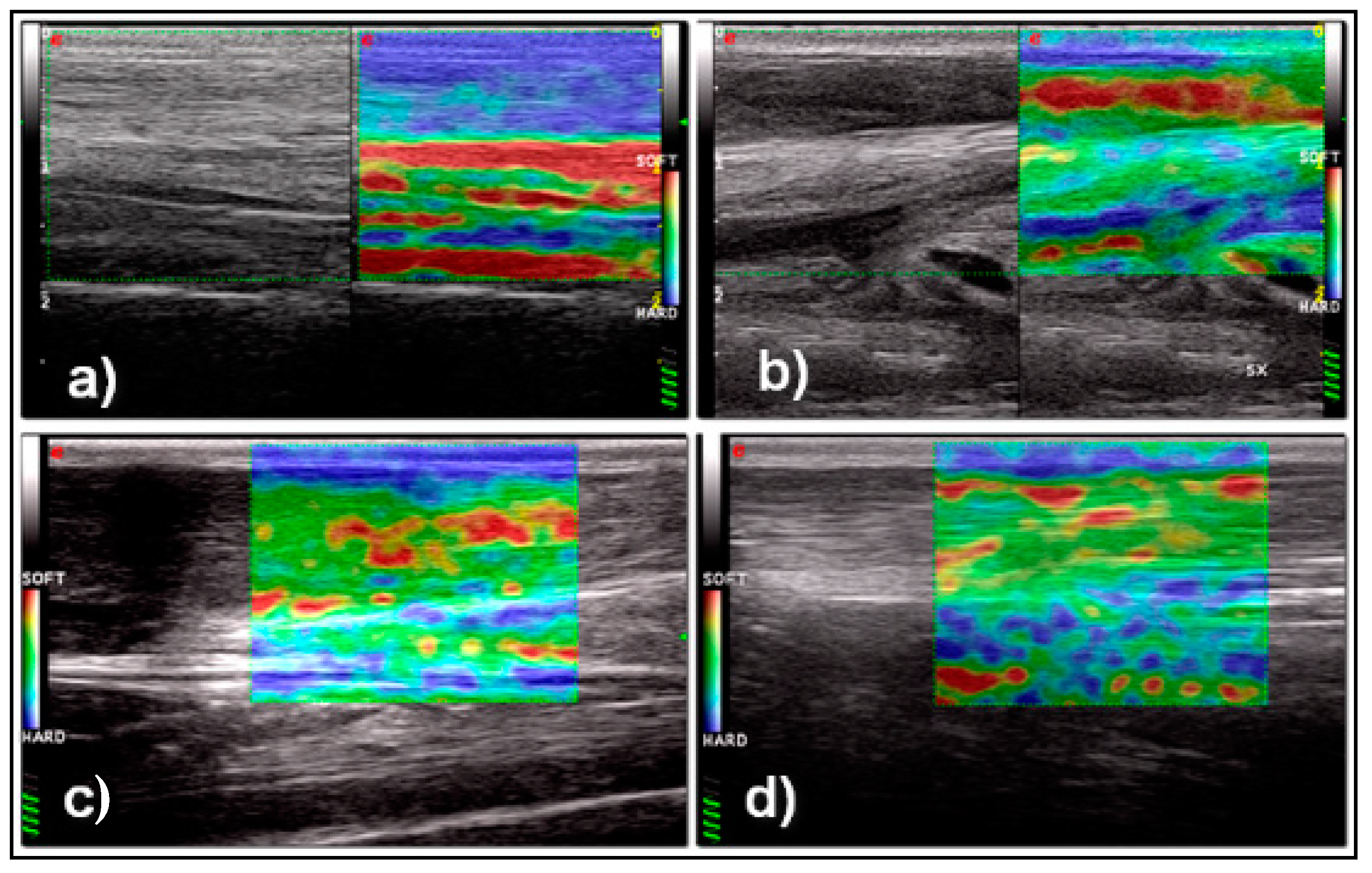
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.Y.; Hua, Y.H. Achilles Tendinopathy: Current Concepts about the Basic Science and Clinical Treatments. BioMed Res. Int. 2016, 2016, 6492597. [Google Scholar] [CrossRef]
- Sadoghi, P.; Rosso, C.; Valderrabano, V.; Leithner, A.; Vavken, P. The role of platelets in the treatment of Achilles tendon injuries. J. Orthop. Res. 2013, 31, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Kjaer, M.; Eliasson, P. Achilles tendon rupture: Treatment and complications—A systematic review. Scand. J. Med. Sci. Sports 2015, 25, e1–e10. [Google Scholar] [CrossRef]
- Evans, E.E.; De Lahunta, A. The muscular system. In Miller’s Anatomy of the Dog, 5th ed.; Saunders and Imprint of Elsevier Inc.: St. Louis, MO, USA, 2019; pp. 272–273. [Google Scholar]
- Gamble, L.-J.; Canapp, D.A.; Canapp, S.O. Evaluation of Achilles Tendon Injuries with Findings from Diagnostic Musculoskeletal Ultrasound in Canines—43 Cases. Vet. Evid. 2017, 2. [Google Scholar] [CrossRef]
- Harasen, G. Ruptures of the common calcaneal tendon. Can. Vet. J. 2006, 47, 1219–1220. [Google Scholar]
- Cervi, M.; Brebner, N.; Liptak, J. Short- and long-term outcomes of primary Achilles tendon repair in cats: 21 cases. Vet. Comp. Orthop. Traumatol. 2010, 23, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, W.I.; Rist, P. Achilles tendon repair in dogs using the semitendinosus muscle: Surgical technique and short-term outcome in five dogs. Vet. Surg. 2009, 38, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Produman, C.J. Common calcaneal tendinitis in a horse. Equine Vet. Educ. 1992, 4, 277–279. [Google Scholar] [CrossRef]
- Minei, S.; Cinti, F.; Pompei, B.; Abrescia, P. Treatment of Common Calcaneal Tendon Rupture Using a Central Gastrocnemius Turnover Aponeurotic Flap Technique in a Dog. VCOT Open 2020, 3, 84–89. [Google Scholar] [CrossRef]
- Kramer, M.; Gerwing, M.; Michele, U.; Schimke, E.; Kindler, S. Ultrasonographic examination of injuries to the Achilles tendon in dogs and cats. J. Small Anim. Pract. 2001, 42, 531–535. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamaguchi, S.; Sasho, T.; Fukawa, T.; Akatsu, Y.; Akagi, R.; Yamaguchi, T.; Takahashi, K.; Nagashima, K.; Takahashi, K. Quantitative US Elastography Can Be Used to Quantify Mechanical and Histologic Tendon Healing in a Rabbit Model of Achilles Tendon Transection. Radiology 2017, 283, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Garra, B.S. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007, 23, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.S.; Faschingbauer, R.; Jaschke, W.R. Is sonoelastography of value in assessing tendons? Semin. Musculoskelet. Radiol. 2010, 14, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Washburn, N.; Onishi, K.; Wang, J.H. Ultrasound elastography and ultrasound tissue characterisation for tendon evaluation. J. Orthop. Transl. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- Drakonaki, E.E.; Allen, G.M.; Wilson, D.J. Ultrasound elastography for musculoskeletal applications. Br. J. Radiol. 2012, 85, 1435–1445. [Google Scholar] [CrossRef]
- Piccionello Palumbo, A.; Serrani, D.; Busoni, V.; Salvaggio, A.; Bonazzi, M.; Bergamino, C.; Volta, A. Sonoelastographic Features of the Patellar Ligament in Clinically Normal Dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, 279–284. [Google Scholar] [CrossRef] [PubMed]
- McCagherty, J.; Longo, M.; Pennington, C.; Liuti, T.; Morrison, L.R.; Brown, H.; Clements, D.N. Effect of Stifle Flexion Angle on the Repeatability of Real-Time Elastosonography of the Patellar Ligament in Medium- to Large-Breed Dogs. Vet. Comp. Orthop. Traumatol. 2020, 33, 391–397. [Google Scholar] [CrossRef]
- Lustgarten, M.; Redding, W.R.; Labens, R.; Morgan, M.; Davis, W.; Seiler, G.S. Elastographic characteristics of the metacarpal tendons in horses without clinical evidence of tendon injury. Vet. Radiol. Ultrasound 2014, 55, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.; Redding, W.R.; Labens, R.; Davis, W.; Daniel, T.M.; Griffith, E.; Seiler, G.S. Elastographic evaluation of naturally occuring tendon and ligament injuries of the equine distal limb. Vet. Radiol. Ultrasound 2015, 56, 670–679. [Google Scholar] [CrossRef]
- Tamura, N.; Nukada, T.; Kato, T.; Kuroda, T.; Kotoyori, Y.; Fukuda, K.; Kasashima, Y. The use of sonoelastography to assess the recovery of stiffness after equine superficial digital flexor tendon injuries: A preliminary prospective longitudinal study of the healing process. Equine Vet. J. 2017, 49, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.S.; Miyamoto, H.; Bellmann-Weiler, R.; Feuchtner, G.M.; Wick, M.C.; Jaschke, W.R. Sonoelastography: Musculoskeletal applications. Radiology 2014, 272, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Fusini, F.; Langella, F.; Busilacchi, A.; Tudisco, C.; Gigante, A.; Massé, A.; Bisicchia, S. Real-time sonoelastography: Principles and clinical applications in tendon disorders. A systematic review. Muscles Ligaments Tendons J. 2018, 7, 467–477. [Google Scholar] [CrossRef]
- Klauser, A.S.; Miyamoto, H.; Tamegger, M.; Faschingbauer, R.; Moriggl, B.; Klima, G.; Feuchtner, G.M.; Kastlunger, M.; Jaschke, W.R. Achilles tendon assessed with sonoelastography: Histologic agreement. Radiology 2013, 267, 837–842. [Google Scholar] [CrossRef]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef] [PubMed]
- Archambault, J.M.; Wiley, J.P.; Bray, R.C.; Verhoef, M.; Wiseman, D.A.; Elliott, P.D. Can sonography predict the outcome in patients with achillodynia? J. Clin. Ultrasound 1998, 26, 335–339. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Kramer, M.; Gerwing, M.; Schimke, E. Diagnostik und Therapie ausgewahlter Sehnen und Muskelerkrankungen beim Hund. Teil I: Die Sonographie der Achillessehne beim Hund. Kleintierpraxis 1993, 38, 703–711. [Google Scholar]
- Martinello, T.; Gomiero, C.; Perazzi, A.; Iacopetti, I.; Gemignani, F.; DeBenedictis, G.M.; Ferro, S.; Zuin, M.; Martines, E.; Brun, P.; et al. Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet. Res. 2018, 14, 202. [Google Scholar] [CrossRef]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. C Embryo Today 2013, 99, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Ochi, M.; Ikuta, Y. Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Arch. Orthop. Trauma Surg. 1997, 116, 454–462. [Google Scholar] [CrossRef]
- Maranho, D.A.; Nogueira-Barbosa, M.H.; Simão, M.N.; Volpon, J.B. Ultrasonographic evaluation of Achilles tendon repair after percutaneous sectioning for the correction of congenital clubfoot residual equinus. J. Pediatr. Orthop. 2009, 29, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Mangat, K.S.; Kanwar, R.; Johnson, K.; Korah, G.; Prem, H. Ultrasonographic phases in gap healing following Ponseti-type Achilles tenotomy. J. Bone Jt. Surg. Am. 2010, 92, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.L.; Lavy, C.B. Correlation of clinical and ultrasonographic findings after Achilles tenotomy in idiopathic club foot. J. Bone Jt. Surg. Br. 2006, 88, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Nasr, P.; Berman, L.; Rehm, A. Ultrasonographic findings after Achilles tenotomy during Ponseti treatment for clubfeet: Is ultrasound a reliable tool to assess tendon healing? J. Child. Orthop. 2014, 8, 405–411. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Tsujii, A.; Nakamura, N.; Mitsuoka, T. Ultrasonographic Evaluation of the Early Healing Process After Achilles Tendon Repair. Orthop. J. Sports Med. 2018, 6, 2325967118789883. [Google Scholar] [CrossRef]
- Drakonaki, E.E.; Allen, G.M.; Wilson, D.J. Real-time ultrasound elastography of the normal Achilles tendon: Reproducibility and pattern description. Clin. Radiol. 2009, 64, 1196–1202. [Google Scholar] [CrossRef]
- De Zordo, T.; Chhem, R.; Smekal, V.; Feuchtner, G.; Reindl, M.; Fink, C.; Faschingbauer, R.; Jaschke, W.; Klauser, A.S. Real-time sonoelastography: Findings in patients with symptomatic achilles tendons and comparison to healthy volunteers. Ultraschall Med. 2010, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for the Evaluation of Patients with Tendinopathies. Acad. Radiol. 2016, 23, 1204–1213. [Google Scholar] [CrossRef]
- Aubry, S.; Nueffer, J.P.; Tanter, M.; Becce, F.; Vidal, C.; Michel, F. Viscoelasticity in Achilles tendonopathy: Quantitative assessment by using real-time shear-wave elastography. Radiology 2015, 274, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, P.H.; Izvernariu, D.A.; Iancu, C.; Dinu, G.O.; Crişan, D.; Popescu, S.A.; Şirli, R.L.; Nistor, B.M.; RăuŢia, I.C.; Lăzureanu, D.C.; et al. Evaluation of normal and pathological Achilles tendon by real-time shear wave elastography. Rom. J. Morphol. Embryol. 2016, 57 (Suppl. 2), 785–790. [Google Scholar] [PubMed]
- Galletti, S.; Oliva, F.; Masiero, S.; Frizziero, A.; Galletti, R.; Schiavone, C.; Salini, V.; Abate, M. Sonoelastography in the diagnosis of tendinopathies: An added value. Muscles Ligaments Tendons J. 2016, 5, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.C.; Schneider, M.E.; Malliaras, P.; Chadwick, M.; Connell, D.A. Diagnostic performance of axial-strain sonoelastography in confirming clinically diagnosed Achilles tendinopathy: Comparison with B-mode ultrasound and color Doppler imaging. Ultrasound Med Biol. 2015, 41, 15–25. [Google Scholar] [CrossRef] [PubMed]
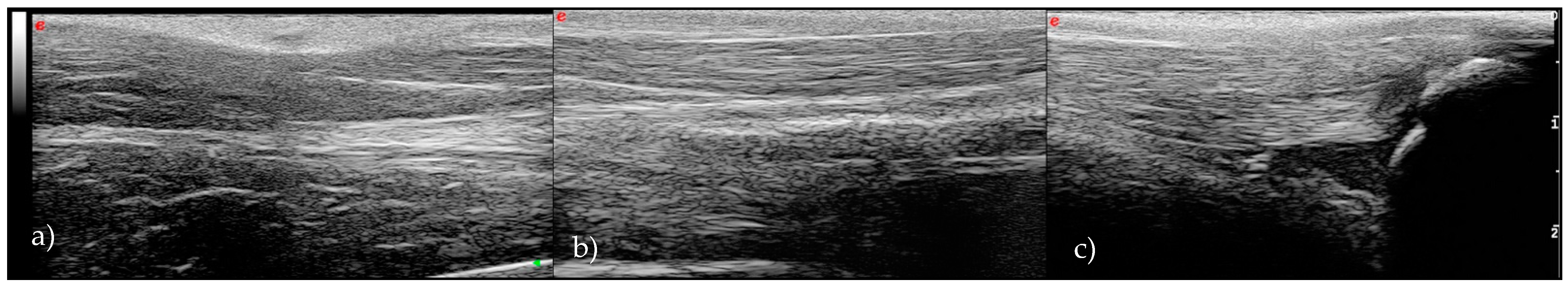
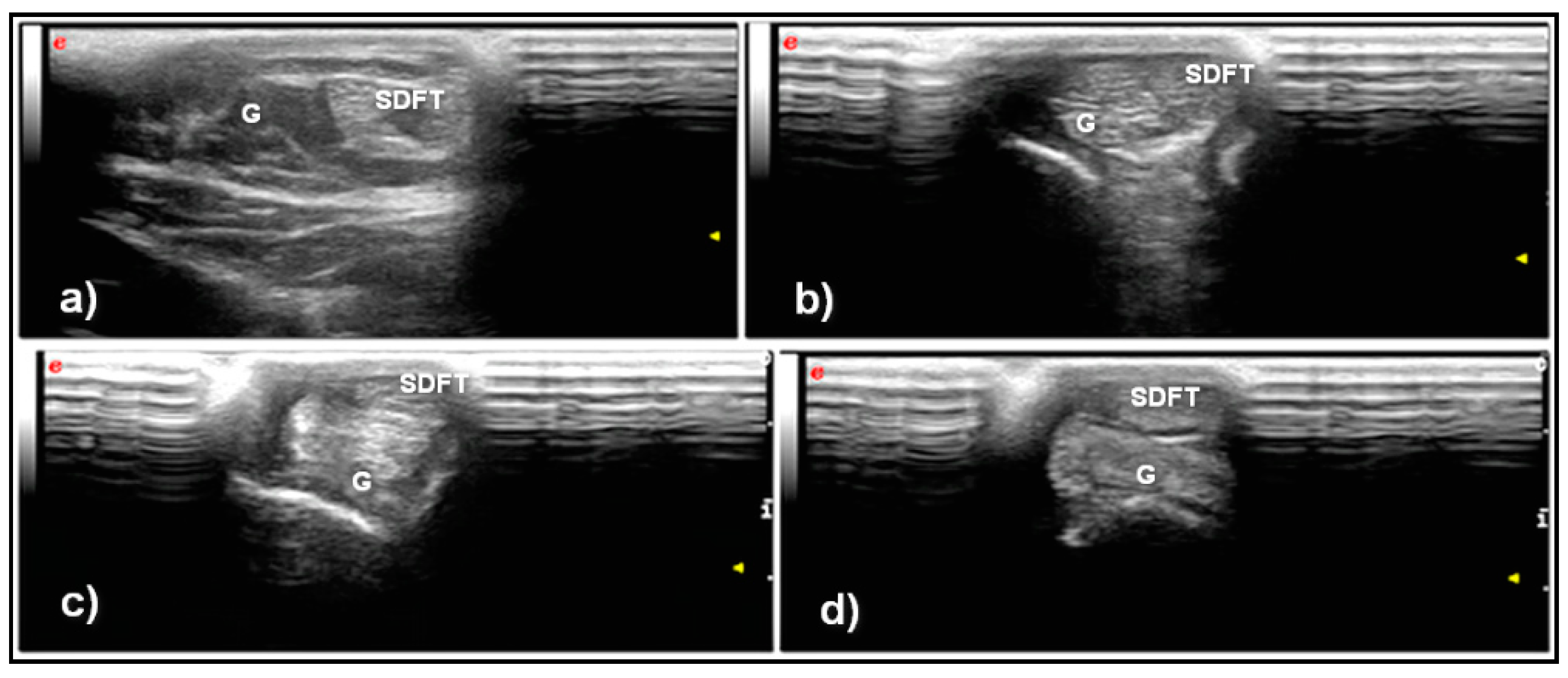
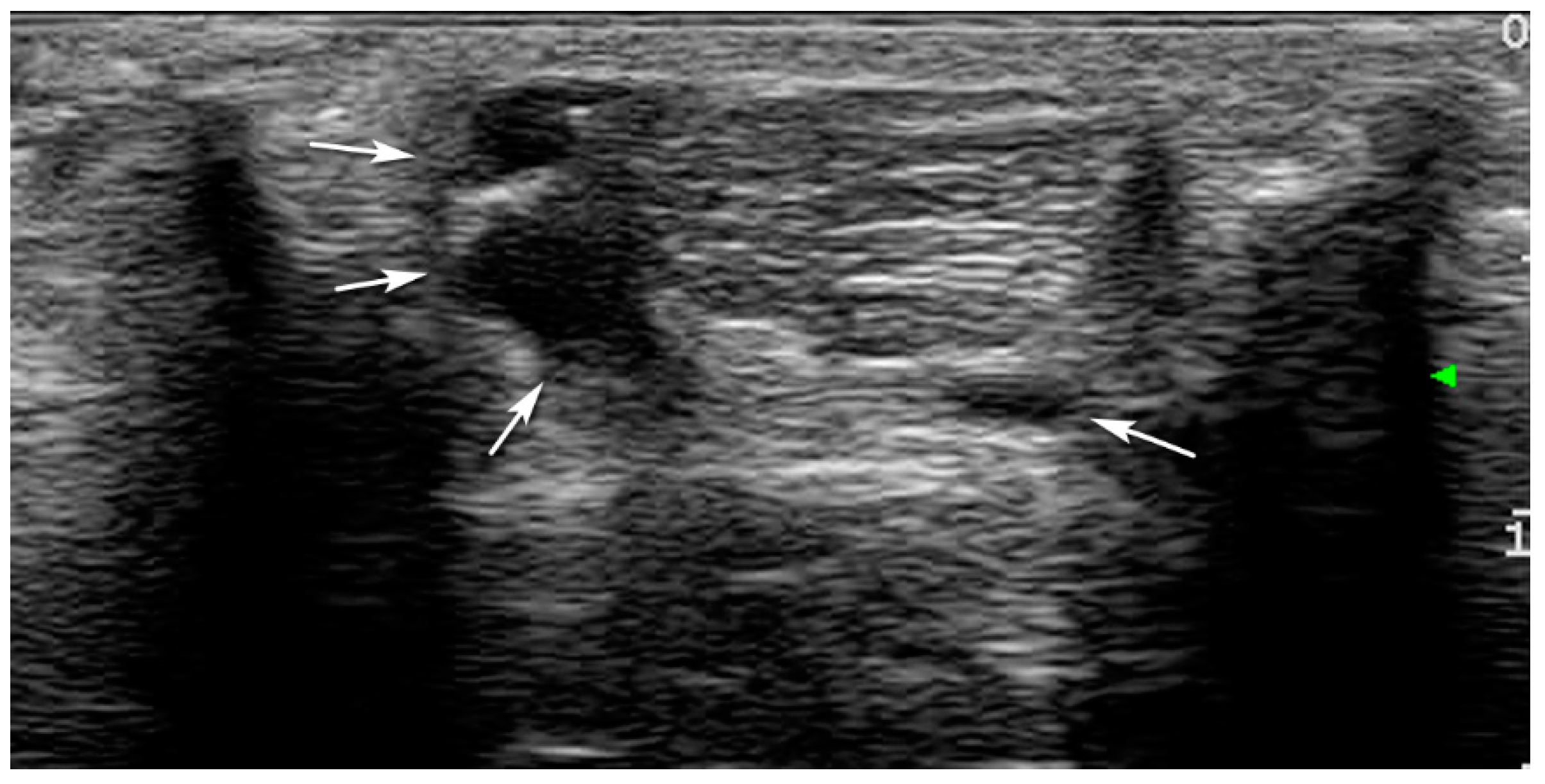
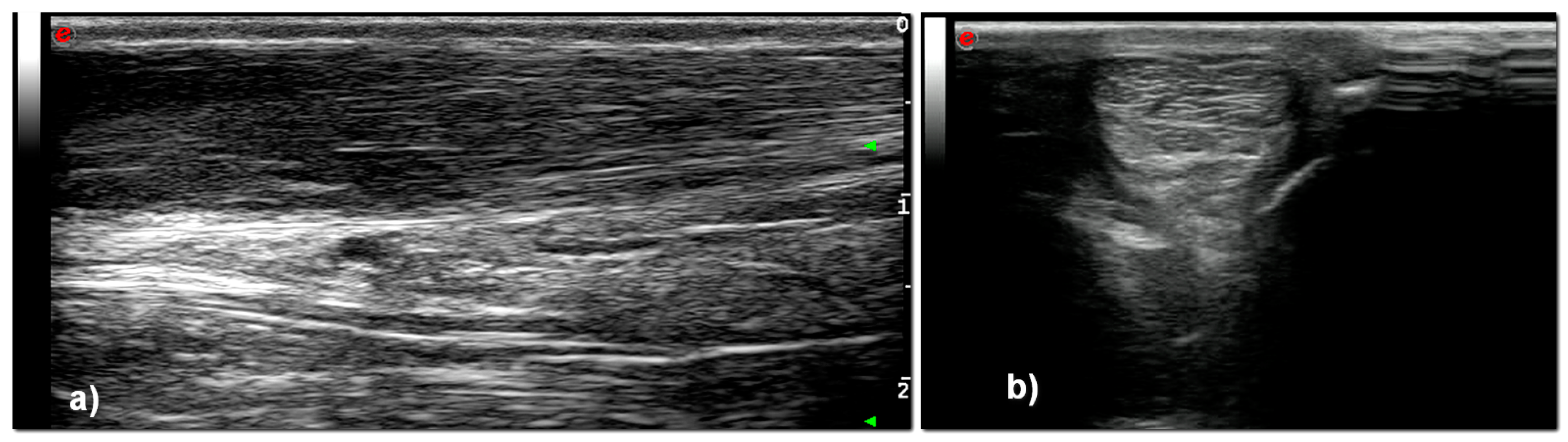

| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
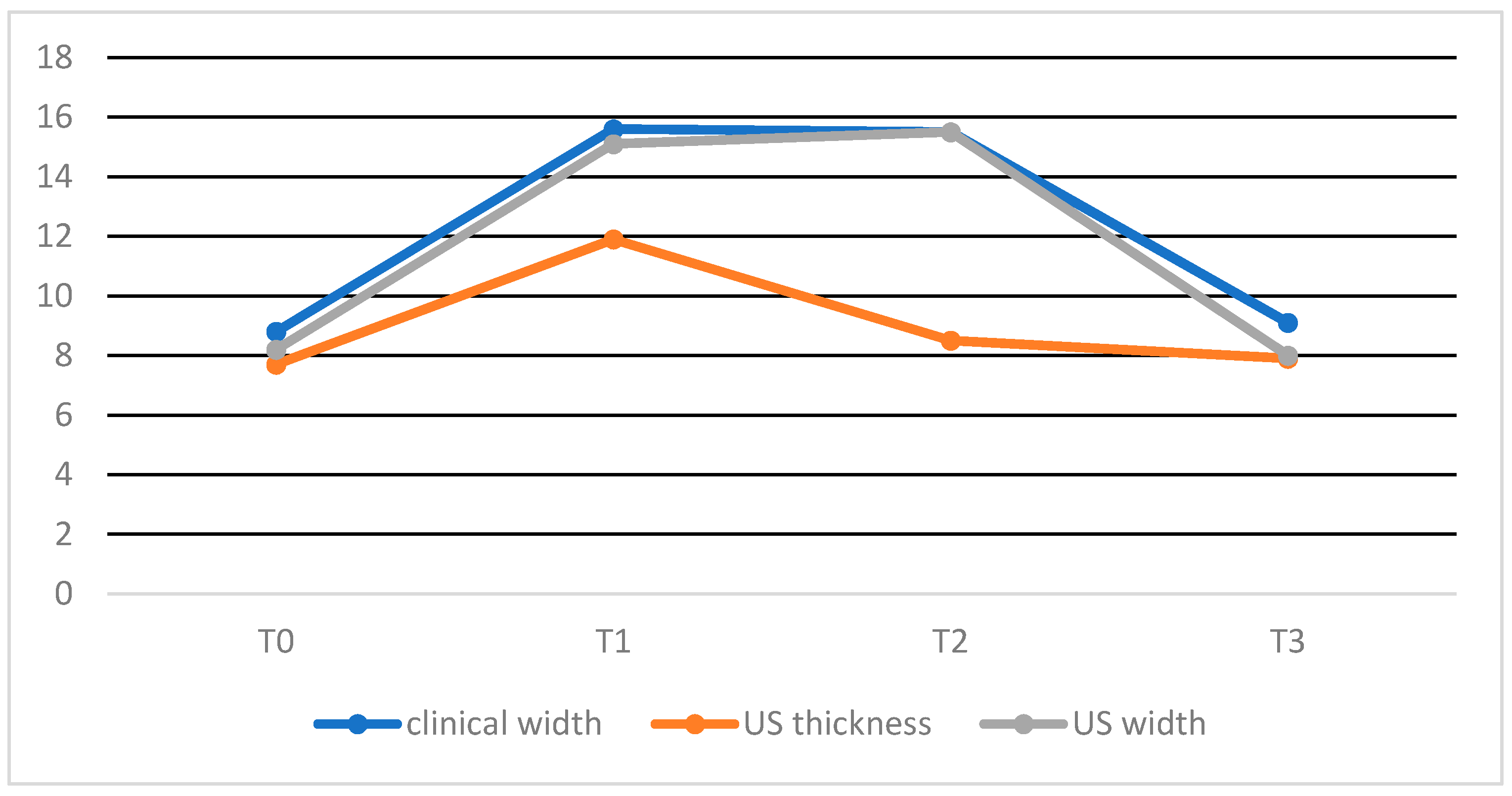
| Mean +/− SD clinical CCT width | 8.8 +/− 1.6 mm | 15.6 +/− 1.6 mm (*) | 15.5 +/− 2.3 mm (†) | 9.1 +/− 1.1 mm (x,‡) |
| Mean +/− SD US CCT thickness | 7.7 +/− 2.0 mm | 11.9 +/− 2.9 mm (*) | 8.5 +/− 1.9 mm (°) | 7.9 +/− 0.8 mm (x) |
| Mean +/− SD US CCT width | 8.2 +/− 1.6 mm | 15.1 +/− 1.6 mm (*) | 15.5 +/− 1.6 mm (†,°) | 8.0 +/− 1.5 mm (*,x) |
| Mean +/− SD Elx-t%hrd | 89.9 +/− 15.3 | 40 +/− 25.1 (*) | 31.8 +/− 19 (†) | 47.2 +/− 29.1 (§) |
| Mean +/− SD Elx-t%sft | 9.9 +/− 15 | 67.4 +/− 26.4 (*) | 52.8 +/− 29.5 (†) | 69.6 +/− 19.2 (§) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrani, D.; Volta, A.; Cingolani, F.; Pennasilico, L.; Di Bella, C.; Bonazzi, M.; Salvaggio, A.; Palumbo Piccionello, A. Serial Ultrasonographic and Real-Time Elastosonographic Assessment of the Ovine Common Calcaneal Tendon, after an Experimentally Induced Tendinopathy. Vet. Sci. 2021, 8, 54. https://doi.org/10.3390/vetsci8040054
Serrani D, Volta A, Cingolani F, Pennasilico L, Di Bella C, Bonazzi M, Salvaggio A, Palumbo Piccionello A. Serial Ultrasonographic and Real-Time Elastosonographic Assessment of the Ovine Common Calcaneal Tendon, after an Experimentally Induced Tendinopathy. Veterinary Sciences. 2021; 8(4):54. https://doi.org/10.3390/vetsci8040054
Chicago/Turabian StyleSerrani, Daniele, Antonella Volta, Franco Cingolani, Luca Pennasilico, Caterina Di Bella, Mattia Bonazzi, Alberto Salvaggio, and Angela Palumbo Piccionello. 2021. "Serial Ultrasonographic and Real-Time Elastosonographic Assessment of the Ovine Common Calcaneal Tendon, after an Experimentally Induced Tendinopathy" Veterinary Sciences 8, no. 4: 54. https://doi.org/10.3390/vetsci8040054
APA StyleSerrani, D., Volta, A., Cingolani, F., Pennasilico, L., Di Bella, C., Bonazzi, M., Salvaggio, A., & Palumbo Piccionello, A. (2021). Serial Ultrasonographic and Real-Time Elastosonographic Assessment of the Ovine Common Calcaneal Tendon, after an Experimentally Induced Tendinopathy. Veterinary Sciences, 8(4), 54. https://doi.org/10.3390/vetsci8040054








