Sheep as a Potential Model of Intradiscal Infection by the Bacterium Cutibacterium acnes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia
2.3. MRI
2.4. Bacterial Injectate
2.5. Injection Protocol
2.6. Euthanasia and Post Mortem Procedures
2.7. Disc Tissue Sampling
2.8. Statistical Analysis
3. Results
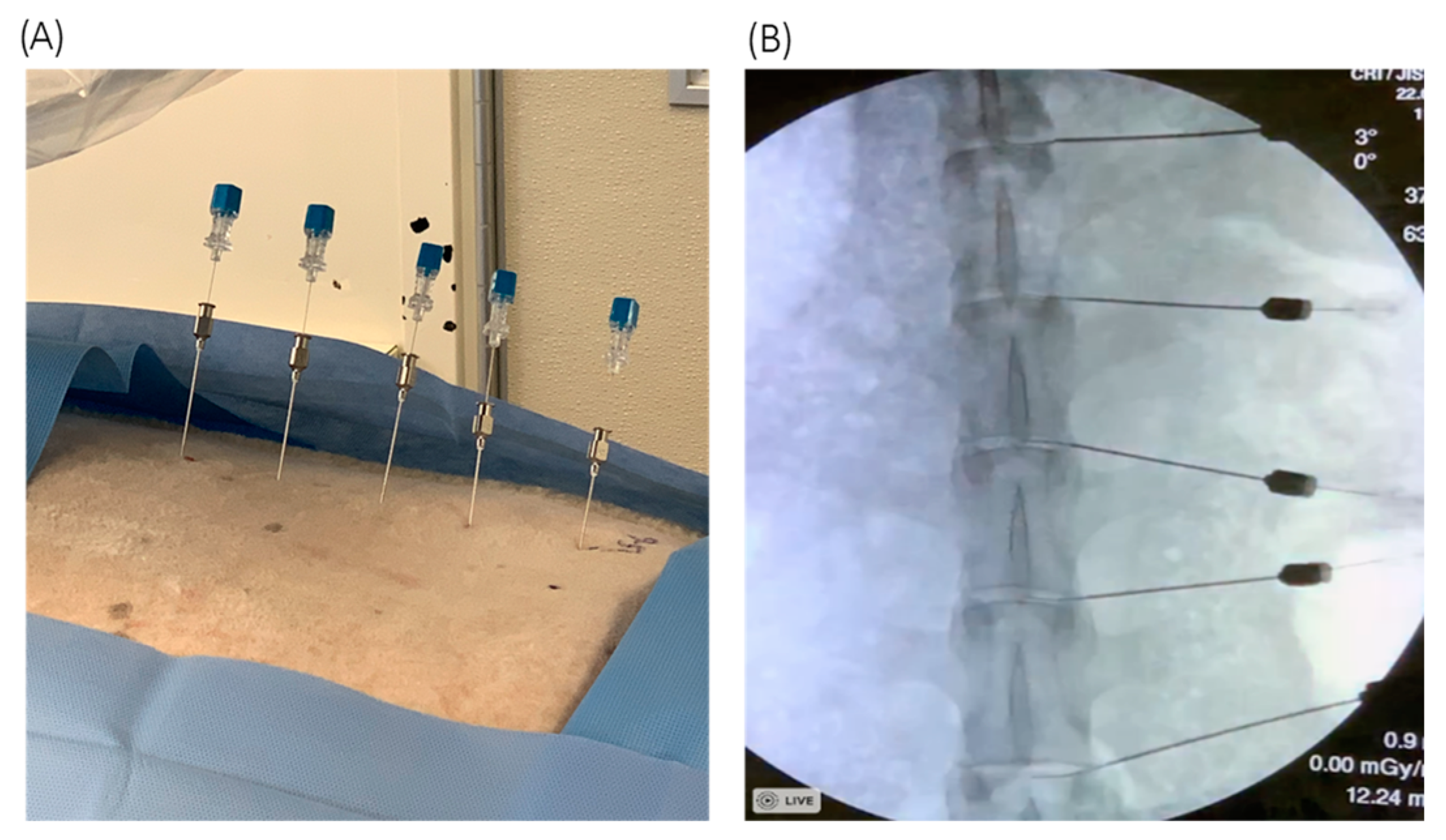
3.1. Percutaneous Injection of C. acnes into Sheep IVDs under Fluoroscopic Guidance
3.2. Animal Welfare
3.3. Evidence of Short-Term C. acnes Colonisation
3.4. MRI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study; IHME: Seattle, WA, USA, 2018. [Google Scholar]
- Urban, J.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luoma, K.; Riihimäki, H.; Luukkonen, R.; Raininko, R.; Viikari-Juntura, E.; Lamminen, A. Low back pain in relation to lumbar disc degeneration. Spine 2000, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Stirling, A.; Worthington, T.; Rafiq, M.; Lambert, P.A.; Elliot, T.S. Association between sciatica and Propionibacterium acnes. Lancet 2001, 357, 2024–2025. [Google Scholar] [CrossRef]
- Coscia, M.; Denys, G.; Wack, M. Propionibacterium acnes, coagulase-negative staphylococcus, and the “biofilm-like” intervertebral disc. Spine 2016, 41, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Aghazadeh, J.; Mirzaei, F.; Ziaeii, E.; Alavi, S.A.N. Propionibacterium acnes infection in disc material and different antibiotic susceptibility in patients with lumbar disc herniation. Int. J. Spine Surg. 2019, 13, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Tangavel, C.; Aiyer, S.N.; Nayagam, S.M.; Raveendran, M.; Demonte, N.L.; Subbaiah, P.; Kanna, R.; Shetty, A.P.; Dharmalingam, K. ISSLS PRIZE IN CLINICAL SCIENCE 2017: Is infection the possible initiator of disc disease? An insight from proteomic analysis. Eur. Spine J. 2017, 26, 1384–1400. [Google Scholar] [PubMed]
- Bivona, L.J.; Camacho, J.E.; Usmani, F.; Nash, A.; Bruckner, J.J.; Hughes, M.; Bhandutia, A.K.; Koh, E.Y.; Banagan, K.E.; Gelb, D.E.; et al. The prevalence of bacterial infection in patients undergoing elective ACDF for degenerative cervical spine conditions: A prospective cohort study with contaminant control. Glob. Spine J. 2021, 11, 13–20. [Google Scholar] [CrossRef]
- Lin, Y.; Jiao, Y.; Yuan, Y.; Zhou, Z.; Zheng, Y.; Xiao, J.; Li, C.; Chen, Z.; Cao, P. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell apoptosis via the TLR2/JNK/mitochondrial-mediated pathway. Emerg. Microbes Infect. 2018, 7, 1. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Y.; Zhou, Z.; Jiao, Y.; Li, C.; Zheng, Y.; Lin, Y.; Xiao, J.; Chen, Z.; Cao, P. Association between chronic inflammation and latent infection of Propionibacterium acnes in non-pyogenic degenerated intervertebral discs: A pilot study. Eur. Spine J. 2018, 27, 2506–2517. [Google Scholar] [CrossRef]
- Tang, G.; Wang, Z.; Chen, J.; Zhang, Z.; Qian, H.; Chen, Y. Latent infection of low-virulence anaerobic bacteria in degenerated lumbar intervertebral discs. BMC Musculoskelet. Disord. 2018, 19, 445. [Google Scholar] [CrossRef]
- Rollason, J.; McDowell, A.; Albert, H.B.; Barnard, E.; Worthington, T.; Hilton, A.C.; Vernallis, A.; Patrick, S.; Elliott, T.; Lambert, P. Genotypic and antimicrobial characterization of Propionibacterium acnes isolates from surgically excised lumbar disc herniations. BioMed Res. Int. 2013, 2013, 530382. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Chen, Y.; Chen, J.; Wang, Z.; Jiang, W. Higher proportion of low-virulence anaerobic bacterial infection in young patients with intervertebral disc herniation. Exp. Ther. Med. 2019, 18, 3085–3089. [Google Scholar] [CrossRef] [PubMed]
- Dudli, S.; Liebenberg, E.; Magnitsky, S.; Miller, S.; Demir-Deviren, S.; Lotz, J.C. Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with modic changes. J. Orthop. Res. 2016, 34, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and acne vulgaris: New insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.A.; Banerjee, A.; Dipreta, J.A. Propionibacterium acnes osteomyelitis after intraosseous cannulation in a child. Case Rep. Orthop. 2019, 7170154. [Google Scholar] [CrossRef]
- Ely, A.L.; Neely, K. Propionibacterium acnes endophthalmitis following Baerveldt glaucoma device implantation. J. Glaucoma 2020, 29, e71–e73. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.A.; Clarke, D.; Do, T.; Gilbert, S.C.; Mannocci, F.; Beighton, D. Propionibacterium acnes and Staphylococcus epidermidis isolated from refractory endodontic lesions are opportunistic pathogens. J. Clin. Microbiol. 2010, 48, 3859–3869. [Google Scholar] [CrossRef]
- Piper, K.E.; Jacobson, M.J.; Cofield, R.H.; Sperling, J.W.; Sanchez-Sotelo, J.; Osmon, D.R.; McDowell, A.; Patrick, S.; Steckelberg, J.M.; Mandrekar, J.N.; et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 2009, 47, 1878–1884. [Google Scholar] [CrossRef]
- Beaver, M.; Lagundzin, D.; Thapa, I.; Lee, J.; Ali, H.; Kielian, T.; Skar, G.L. C. acnes central nervous system catheter infection induces long-term changes in the CSF proteome. Infect. Immun. 2020, IAI.00531-20. [Google Scholar] [CrossRef]
- Cohen, R.J.; Shannon, B.A.; McNeal, J.E.; Shannon, T.; Garrett, K.L. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: A possible link to cancer evolution? J. Urol. 2005, 173, 1969–1974. [Google Scholar] [CrossRef]
- Eishi, Y. Etiologic link between sarcoidosis and Propionibacterium acnes. Respir. Investig. 2013, 51, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Capoor, M.N.; Ruzicka, F.; Schmitz, J.E.; James, G.A.; Machackova, T.; Jancalek, R.; Smrcka, M.; Lipina, R.; Ahmed, F.S.; Alamin, T.F.; et al. Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS ONE 2017, 12, e0174518. [Google Scholar]
- Ohrt-Nissen, S.; Fritz, B.G.; Walbom, J.; Kragh, K.N.; Bjarnsholt, T.; Dahl, B.; Manniche, C. Bacterial biofilms: A possible mechanism for chronic infection in patients with lumbar disc herniation—A prospective proof-of-concept study using fluorescence in situ hybridization. APMIS 2018, 126, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Georgy, M.M.; Vaida, F.; Stern, M.; Murphy, K. Association between type I modic changes and Propionibacterium acnes infection in the cervical spine: An observational study. AJNR 2018, 39, 1764–1767. [Google Scholar] [CrossRef]
- Albert, H.B.; Lambert, P.; Rollason, J.; Sorensen, J.S.; Worthington, T.; Pedersen, M.B.; Nørgaard, H.S.; Vernallis, A.; Busch, F.; Manniche, C.; et al. Does nuclear tissue infected with bacteria following disc herniations lead to modic changes in the adjacent vertebrae? Eur. Spine J. 2013, 22, 690–696. [Google Scholar] [CrossRef]
- Aghazadeh, J.; Salehpour, F.; Ziaeii, E.; Javanshir, N.; Samadi, A.; Sadeghi, J.; Mirzaei, F.; Naseri Alavi, S.A. Modic changes in the adjacent vertebrae due to disc material infection with Propionibacterium acnes in patients with lumbar disc herniation. Eur. Spine J. 2017, 26, 3129–3134. [Google Scholar] [CrossRef]
- Shan, Z.; Zhang, X.; Li, S.; Yu, T.; Liu, J.; Zhao, F. Propionibacterium acnes incubation in the discs can result in time-dependent modic changes: A long-term rabbit model. Spine (Phila Pa 1976) 2017, 42, 1595–1603. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Y.; Yuan, Y.; Jiao, Y.; Xiao, J.; Zhou, Z.; Cao, P. Modic changes and disc degeneration caused by inoculation of Propionibacterium acnes inside intervertebral discs of rabbits: A pilot study. Biomed. Res. Int. 2016, 2016, 9612437. [Google Scholar]
- Daly, C.; Ghosh, P.; Jenkin, G.; Oehme, D.; Goldschlager, T. A review of animal models of intervertebral disc degeneration: Pathophysiology, regeneration, and translation to the clinic. Biomed. Res. Int. 2016, 2016, 5952165. [Google Scholar] [CrossRef]
- O’Connell, G.D.; Vresilovic, E.J.; Elliott, D.M. Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976) 2007, 32, 328–333. [Google Scholar] [CrossRef]
- Cappello, R.; Bird, J.L.; Pfeiffer, D.; Bayliss, M.T.; Dudhia, J. Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine (Phila Pa 1976) 2006, 31, 873–882; discussion 883. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Balian, G.; Li, X.J. Animal models for disc degeneration-an update. Histol. Histopathol. 2018, 33, 543–554. [Google Scholar] [PubMed]
- Wilke, H.J.; Kettler, A.; Wenger, K.H.; Claes, L.E. Anatomy of the sheep spine and its comparison to the human spine. Anat. Rec. 1997, 247, 542–555. [Google Scholar] [CrossRef]
- Lipp, C.; Kirker, K.; Agostinho, A.; James, G.; Stewart, P. Testing wound dressings using an in vitro wound model. J. Wound Care 2010, 19, 220–226. [Google Scholar] [CrossRef] [PubMed]
- AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Available online: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (accessed on 1 December 2020).
- Elliott, D.M.; Yerramalli, C.S.; Beckstein, J.C.; Boxberger, J.I.; Johannessen, W.; Vresilovic, E.J. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine (Phila Pa 1976) 2008, 33, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Zamora, T.; Palma, J.; Andia, M.; Garcia, P.; Wozniak, A.; Solar, A.; Campos, M. Effect of Propionibacterium acnes (PA) injection on intervertebral disc degeneration in a rat model: Does it mimic modic changes? Orthop. Traumatol. Surg. Res. 2017, 103, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, D.B.; Vaghasia, A.M.; Yu, S.H.; Mak, T.N.; Brüggemann, H.; Nelson, W.G.; De Marzo, A.M.; Yegnasubramanian, S.; Sfanos, K.S. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate 2013, 73, 1007–1105. [Google Scholar]
- Olsson, J.; Drott, J.B.; Laurantzon, L.; Laurantzon, O.; Bergh, A.; Elgh, F. Chronic prostatic infection and inflammation by Propionibacterium acnes in a rat prostate infection model. PLoS ONE 2012, 7, e51434, Erratum in: PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coscia, E.C.; Abutaleb, N.S.; Hostetter, B.; Seleem, M.N.; Breur, G.J.; McCain, R.R.; Crain, C.J.; Slaby, O.; Capoor, M.N.; McDowell, A.; et al. Sheep as a Potential Model of Intradiscal Infection by the Bacterium Cutibacterium acnes. Vet. Sci. 2021, 8, 48. https://doi.org/10.3390/vetsci8030048
Coscia EC, Abutaleb NS, Hostetter B, Seleem MN, Breur GJ, McCain RR, Crain CJ, Slaby O, Capoor MN, McDowell A, et al. Sheep as a Potential Model of Intradiscal Infection by the Bacterium Cutibacterium acnes. Veterinary Sciences. 2021; 8(3):48. https://doi.org/10.3390/vetsci8030048
Chicago/Turabian StyleCoscia, Erin C., Nader S. Abutaleb, Bradley Hostetter, Mohamed N. Seleem, Gert J. Breur, Robyn R. McCain, Christa J. Crain, Ondrej Slaby, Manu N. Capoor, Andrew McDowell, and et al. 2021. "Sheep as a Potential Model of Intradiscal Infection by the Bacterium Cutibacterium acnes" Veterinary Sciences 8, no. 3: 48. https://doi.org/10.3390/vetsci8030048
APA StyleCoscia, E. C., Abutaleb, N. S., Hostetter, B., Seleem, M. N., Breur, G. J., McCain, R. R., Crain, C. J., Slaby, O., Capoor, M. N., McDowell, A., Ahmed, F. S., Vijayanpillai, V., Narayanan, S. K., & Coscia, M. F. (2021). Sheep as a Potential Model of Intradiscal Infection by the Bacterium Cutibacterium acnes. Veterinary Sciences, 8(3), 48. https://doi.org/10.3390/vetsci8030048







