Investigation on the Prevalence of Canine Microfilaremia in Thailand Using a Novel Microfluidic Device in Combination with Real-Time PCR
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
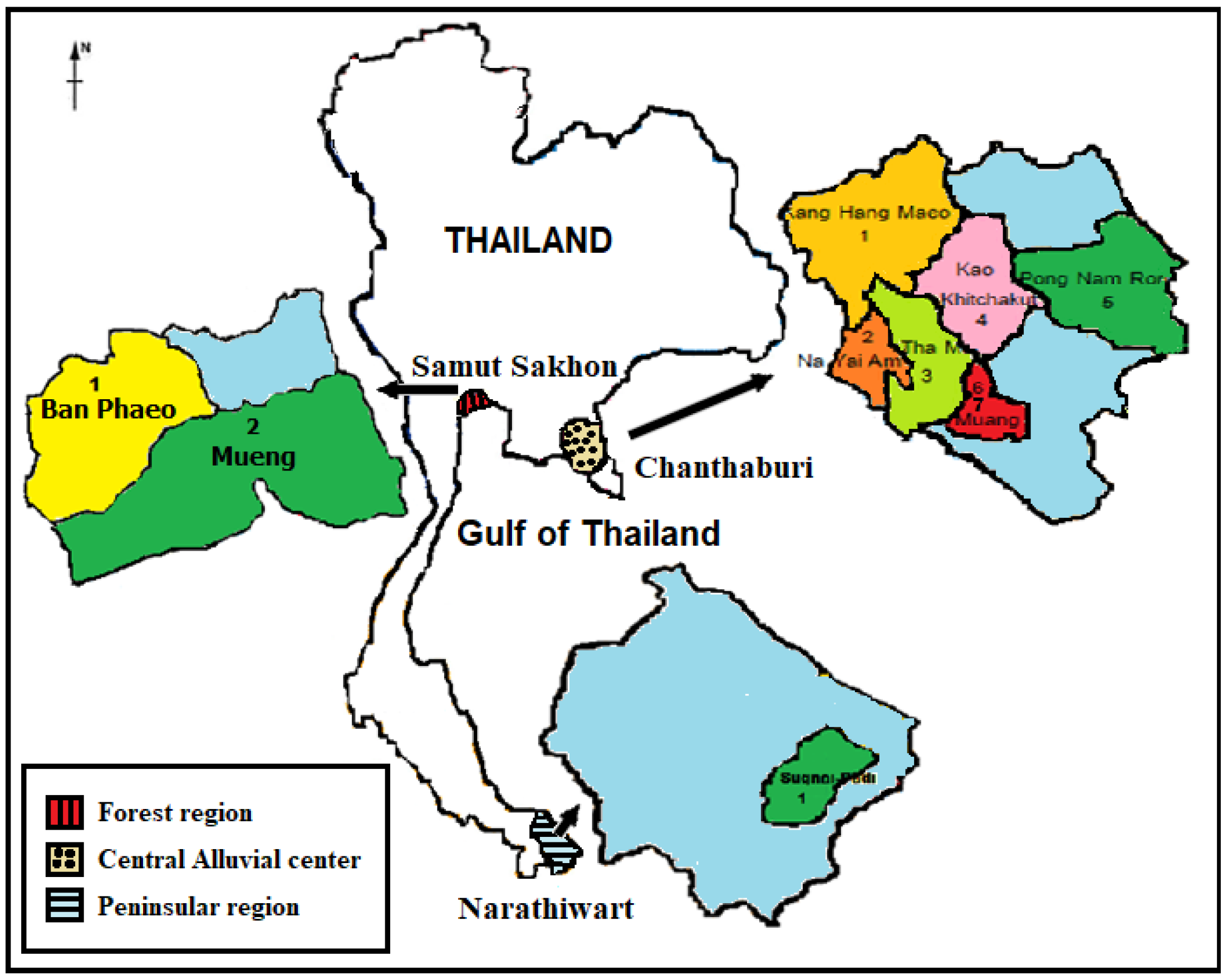
2.2. Study Area
2.3. Study Population
2.4. Microfilariae Detection Using Microfluidic Device
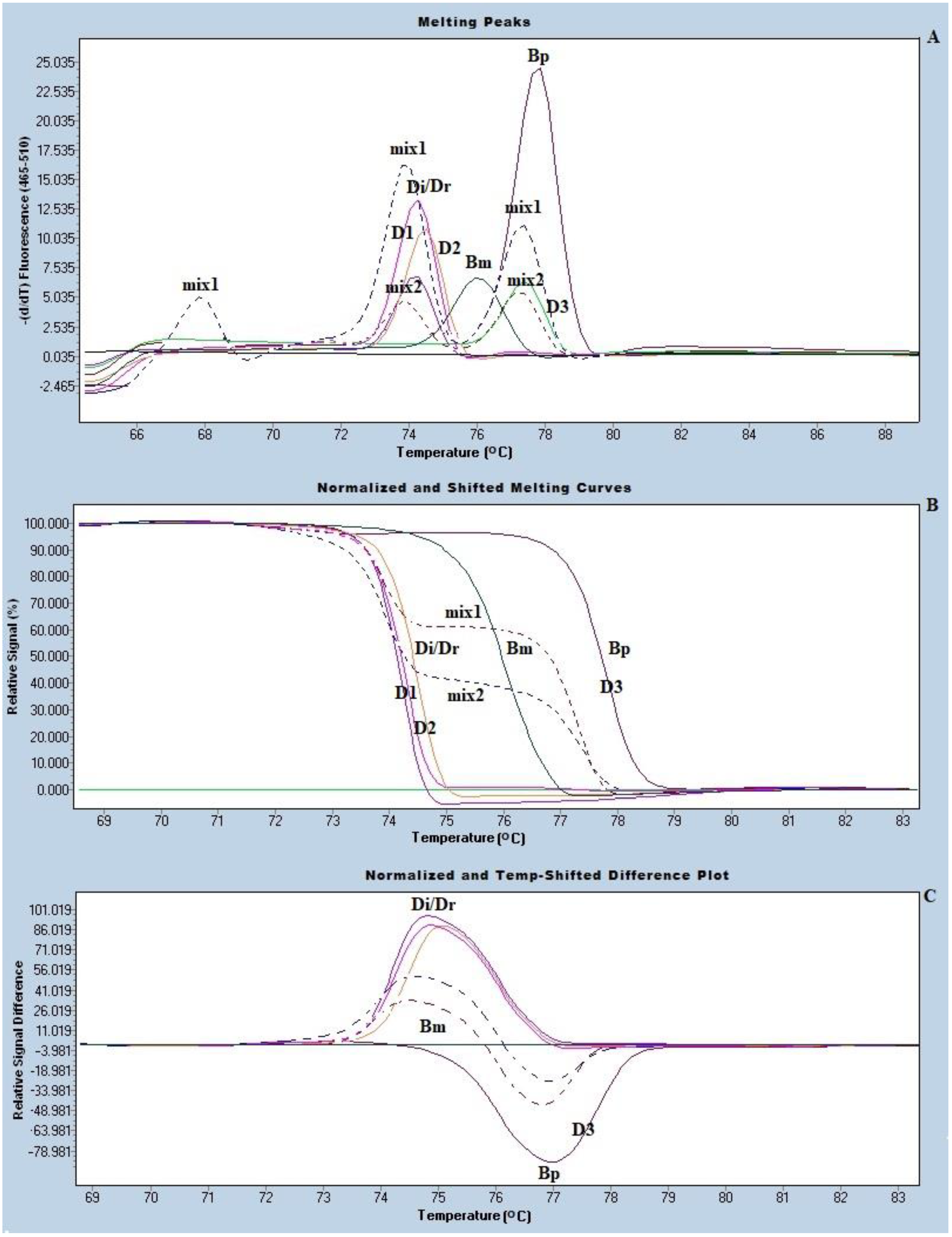
2.5. Species Identification of the Trapped Microfilariae by Real-Time PCR with HRM Analysis
2.6. DNA Sequencing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Lymphatic Filariasis. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 30 November 2020).
- Whitworth, J.; Hewitt, K. Filariasis. Medicine 2005, 33, 61–64. [Google Scholar] [CrossRef]
- Centers for Disease Control. Lymphatic Filariasis—Resources for Health Professionals—Guidance for Evaluation and Treatment. Available online: https://www.cdc.gov/parasites/lymphaticfilariasis/health_professionals/dxtx.html (accessed on 30 November 2020).
- Sirbhen, K.; Kaewmokul, S.; Kasemsant, N.; Sribhen, C. Blood chemistry profile and cardiac troponin T concentration in Thai stray dogs infected with heartworms. Kasetsart. J. (Nat. Sci.) 1999, 33, 251–257. [Google Scholar]
- Kaikuntod, M.; Thongkorn, K.; Tiwananthagorn, S.; Boonyapakorn, C. Filarial worms in dogs in Southeast Asia. Vet. Integr. Sci. 2018, 16, 1–17. [Google Scholar]
- Tan, L.; Fong, M.; Mahmud, R.; Muslim, A.; Lau, Y.; Kamarulzaman, A. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City, Malaysia. Parasitol. Int. 2011, 60, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Jariya, P.; Sucharit, S. Dirofilaria repens from the eyelid of a woman in Thailand. Am. J. Trop. Med. Hyg. 1983, 32, 1456–1457. [Google Scholar] [CrossRef]
- Pradatsundarasar, A. Dirofilaria infection in man: Report of a case. J. Med. Assoc. Thai. 1995, 38, 378–379. [Google Scholar]
- Sukudom, P.; Phumee, A.; Siriyasatien, P. First report on subconjunctival dirofilariasis in Thailand caused by a Dirofilaria sp. closely related to D. hongkongensis. Acad. J. Sci. Res. 2018, 6, 114–116. [Google Scholar]
- Iamsa-ard, W.; Waewwab, P.; Pukdeeprayoon, S.; Wiriyaalongkorn, W.; Songklin, P.; Thamcharoen, T. An outbreak investigation of autochthonous lymphatic filariasis in Wangchan district, Rayong, Thailand, December 2013–July 2014. Wkly. Epidemiol. Surveille Rep. 2015, 46, 385–392. [Google Scholar]
- Choochote, W.; Keha, P.; Sukhavat, K.; Khamboonruang, C.; Sukontason, K. Aedes (Finlaya) togoi Theobald 1907, Chanthaburi strain, a laboratory vector in studies of filariasis in Thailand. Southeast Asian J. Trop. Med. Public Health 1987, 18, 259–260. [Google Scholar]
- Jittapalapong, S.; Nongnuch, P.; Wissanuwat, C.; Burin, N.; Sinsamutt, S.; Pachratorn, S.; Gunn, K. Prevalence of heartworm infection of stray dogs and cats in Bangkok metropolitan areas. Witthayasan Kasetsart 2005, 39, 30–34. [Google Scholar]
- Kamyingkird, K.; Junsiri, W.; Chimnoi, W.; Kengradomkij, C.; Saengow, S.; Sangchuto, K.; Kajeerum, W.; Pangjai, D.; Nimsuphan, B.; Inpankeaw, T.; et al. Prevalence and risk factors associated with Dirofilaria immitis infection in dogs and cats in Songkhla and Satun provinces, Thailand. Agric. Nat. Resour. 2017, 51, 299–302. [Google Scholar] [CrossRef]
- Simonsen, P.; Mwakitalu, M. Urban lymphatic filariasis. Parasitol. Res. 2013, 112, 35–44. [Google Scholar] [CrossRef]
- Paniz Mondolfi, A.; Sordillo, E. Invited editorial: Zoonotic lymphatic filariasis in the Americas: Trends in epidemiology, diagnosis and treatment, with special emphasis on brugian filariasis. Recent Pat. Antiinfect Drug Discov. 2014, 9, 161–163. [Google Scholar] [CrossRef]
- Phuakrod, A.; Sripumkhai, W.; Jeamsaksiri, W.; Pattamang, P.; Juntasaro, E.; Thienthong, T.; Foongladda, S.; Brindley, P.J.; Wongkamchai, S. Diagnosis of feline filariasis assisted by a novel semi-automated microfluidic device in combination with high resolution melting real-time PCR. Parasites Vectors 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Wongkamchai, S.; Monkong, N.; Mahannol, P.; Taweethavonsawat, P.; Loymak, S.; Foongladda, S. Rapid Detection and Identification of Brugia malayi, B. pahangi, and Dirofilaria immitis by High-Resolution Melting Assay. Vector Borne Zoonotic Dis. 2013, 13, 31–36. [Google Scholar] [CrossRef]
- Rishniw, M.; Barr, S.C.; Simpson, K.W.; Frongillo, M.F.; Franz, M.; Alpizar, J.L.D. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet. Parasitol. 2006, 135, 303–314. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673. [Google Scholar] [CrossRef]
- World Health Organization. Bench Aids for the Diagnosis of Filarial Infections; World Health Organization: Geneva, Switzerland, 1997; ISBN 978-92-4-154489-4. [Google Scholar]
- Southgate, B. Studies of filariasis in the Pacific. 1. A field trial of a counting-chamber technique for the determination of microfilarial rates and densities. Southeast Asian J. Trop. Med. Public Health 1973, 4, 172–178. [Google Scholar]
- Dobrowolski, S.F.; Gray, J.; Miller, T.; Sears, M. Identifying sequence variants in the human mitochondrial genome using high resolution melt (HRM) profiling. Hum. Mutat. 2009, 30, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, L.; Palais, R.; Pryor, R.; Wittwer, C.T. High-resolution DNA melting analysis for simultaneous mutation scanning and genotyping in solution. Clin. Chem. 2005, 51, 1770–1777. [Google Scholar] [PubMed]
- Liotta, J.L.; Sandhu, G.K.; Rishniw, M.; Bowman, D.D. Differentiation of the microfilariae of Dirofilaria immitis and Dirofilaria repens in stained blood films. J. Parasitol. 2013, 99, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Nunthanid, P.; Roongruanchai, K.; Wongkamchai, S.; Sarasombath, P. Case report: Periorbital filariasis caused by brugia malayi. Am. J. Trop. Med. Hyg. 2020, 37, 652–655. [Google Scholar] [CrossRef]
- Thongpiya, J.; Sa-Nguanraksa, D.; Samarnthai, N.; Sarasombath, P. Filariasis of the breast caused by Brugia pahangi: A concomitant finding with invasive ductal carcinoma. Parasitol. Int. 2020, 80, 102203. [Google Scholar] [CrossRef] [PubMed]
- Apiwathnasorn, C.; Samung, Y.; Prummongkol, S.; Panasoponkul, C.; Loymek, S. Mosquito fauna of “Toh Daeng” swamp forest, Thailand. Southeast Asian. J. Trop. Med. Public. Health 2009, 40, 720–726. [Google Scholar] [PubMed]
- Kanjanopas, K.; Choochote, W.; Jitpakdi, A.; Suvannadabba, S.; Loymak, S.; Chungpivat, S.; Nithiuthai, S. Brugia malayi in a naturally infected cat from Narathiwat Province, southern Thailand. Southeast Asian J. Trop. Med. Public Health 2001, 32, 585–587. [Google Scholar]
- Kobasa, T.; Thammapalo, S.; Suvannadabba, S.; Armesombun, A.; Loymak, S.; Leemingsawat, S.; Choochote, W. Identification of Brugia malayi-like microfilariae in naturally-infected cats from Narathiwat Province, Southern Thailand. Trop. Med. Parasitol. 2004, 27, 21–25. [Google Scholar]
- Wikipedia. Narathiwat Province, Thailand. Available online: https://en.wikipedia.org/wiki/Narathiwat_Province (accessed on 4 December 2020).
- Wongkamchai, S.; Nochote, H.; Foongladda, S.; Dekumyoy, P.; Thammapalo, S.; Boitano, J.; Choochote, W. A high resolution melting real time PCR for mapping of filaria infection in domestic cats living in brugian filariosis-endemic areas. Vet. Parasitol. 2014, 201, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia. Chanthaburi Province, Thailand. Available online: https://en.wikipedia.org/wiki/Chanthaburi_Province (accessed on 4 December 2020).
- Tsukamoto, M.; Miyagi, I.; Toma, T.; Sucharit, S.; Tumrasvin, W.; Khamboonruang, C.; Choochote, W.; Phanthumachinda, B.; Phan-Urai, P. The mosquito fauna of Thailand (Diptera: Culicidae): An annotated checklist. Jpn. J. Trop. Med. Hyg. 1987, 15, 291–326. [Google Scholar] [CrossRef][Green Version]
- Riyong, D.; Choochote, W.; Jitpakdi, A.; Suvannadabba, S.; Leemingsawat, S.; Chaithong, U. Autogenous Aedes togoi sub-colony (Chanthaburi, Thailand strain), an efficient laboratory vector in study of filariasis. Southeast Asian J. Trop. Med. Public Health 2000, 31, 246–251. [Google Scholar]
- Soi Dog Foundation. Soi Dog Foundation|Ending the suffering of animals in Asia. Available online: https://www.soidog.org/ (accessed on 4 December 2020).

| Study Site | Total Number of Samples (%) | mf Positive (%) | Species of mf | ||
|---|---|---|---|---|---|
| Bp (%) | Di (%) | Mix Bp + Di (%) | |||
| Chanthaburi | 278 (100) | 45 (162) | 34 (12.25) | 7 (2.5) | 4 (1.46) |
| Samut Sakhon | 217 (100) | 12 (5.5) | 5 (2.3) | 6 (2.7) | 1 (0.5) |
| Narathiwat | 273 (100) | 62 (22.7) | 0 (0) | 62 (22.7) | 0 (0) |
| Total | 768 (100) | 119 (15.5) | 39 (5.1) | 75 (9.) | 5 (0.7) |
| Study Site | Age in Years | |||||
|---|---|---|---|---|---|---|
| ≤1 | 2–5 | >5 | ||||
| Total Number of Samples (%) | mf Positive (%) | Total Number of Samples (%) | mf Positive (%) | Total Number of Samples (%) | mf Positive (%) | |
| Chanthaburi | 66 (100) | 3 (1.9) | 177 (100) | 39 (22) | 35 (100) | 3 (8.6) |
| Samut Sakhon | 31 (100) | 0 (0) | 169 (100) | 12 (7.1) | 17 (100) | 0 (0) |
| Narathiwat | 63 (100) | 0 (0) | 192 (100) | 57 (29.7) | 18 (100) | 5 (27) |
| Total (%) | 160 (100) | 3 (1.9) | 538 (100) | 108 (20) | 70 (100) | 8 (11.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loymek, S.; Phuakrod, A.; Zaelai, K.; Sripumkhai, W.; Vongjaroensanti, P.; Wongkamchai, S. Investigation on the Prevalence of Canine Microfilaremia in Thailand Using a Novel Microfluidic Device in Combination with Real-Time PCR. Vet. Sci. 2021, 8, 39. https://doi.org/10.3390/vetsci8030039
Loymek S, Phuakrod A, Zaelai K, Sripumkhai W, Vongjaroensanti P, Wongkamchai S. Investigation on the Prevalence of Canine Microfilaremia in Thailand Using a Novel Microfluidic Device in Combination with Real-Time PCR. Veterinary Sciences. 2021; 8(3):39. https://doi.org/10.3390/vetsci8030039
Chicago/Turabian StyleLoymek, Sumas, Achinya Phuakrod, Kati Zaelai, Witsaroot Sripumkhai, Prapakorn Vongjaroensanti, and Sirichit Wongkamchai. 2021. "Investigation on the Prevalence of Canine Microfilaremia in Thailand Using a Novel Microfluidic Device in Combination with Real-Time PCR" Veterinary Sciences 8, no. 3: 39. https://doi.org/10.3390/vetsci8030039
APA StyleLoymek, S., Phuakrod, A., Zaelai, K., Sripumkhai, W., Vongjaroensanti, P., & Wongkamchai, S. (2021). Investigation on the Prevalence of Canine Microfilaremia in Thailand Using a Novel Microfluidic Device in Combination with Real-Time PCR. Veterinary Sciences, 8(3), 39. https://doi.org/10.3390/vetsci8030039






