The Embryotoxic Effects of in Ovo Administered Sunset Yellow FCF in Chick Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Eggs, Embryos, and Treatment Groups
2.2. SY Dosing and Incubation
2.3. Macroscopic Evaluation of Embryos
2.4. Collection and Processing of Tissue Samples
2.5. Evaluation of the Stained Tissue Samples
2.6. Statistical Analysis
3. Results
3.1. Developmental Changes
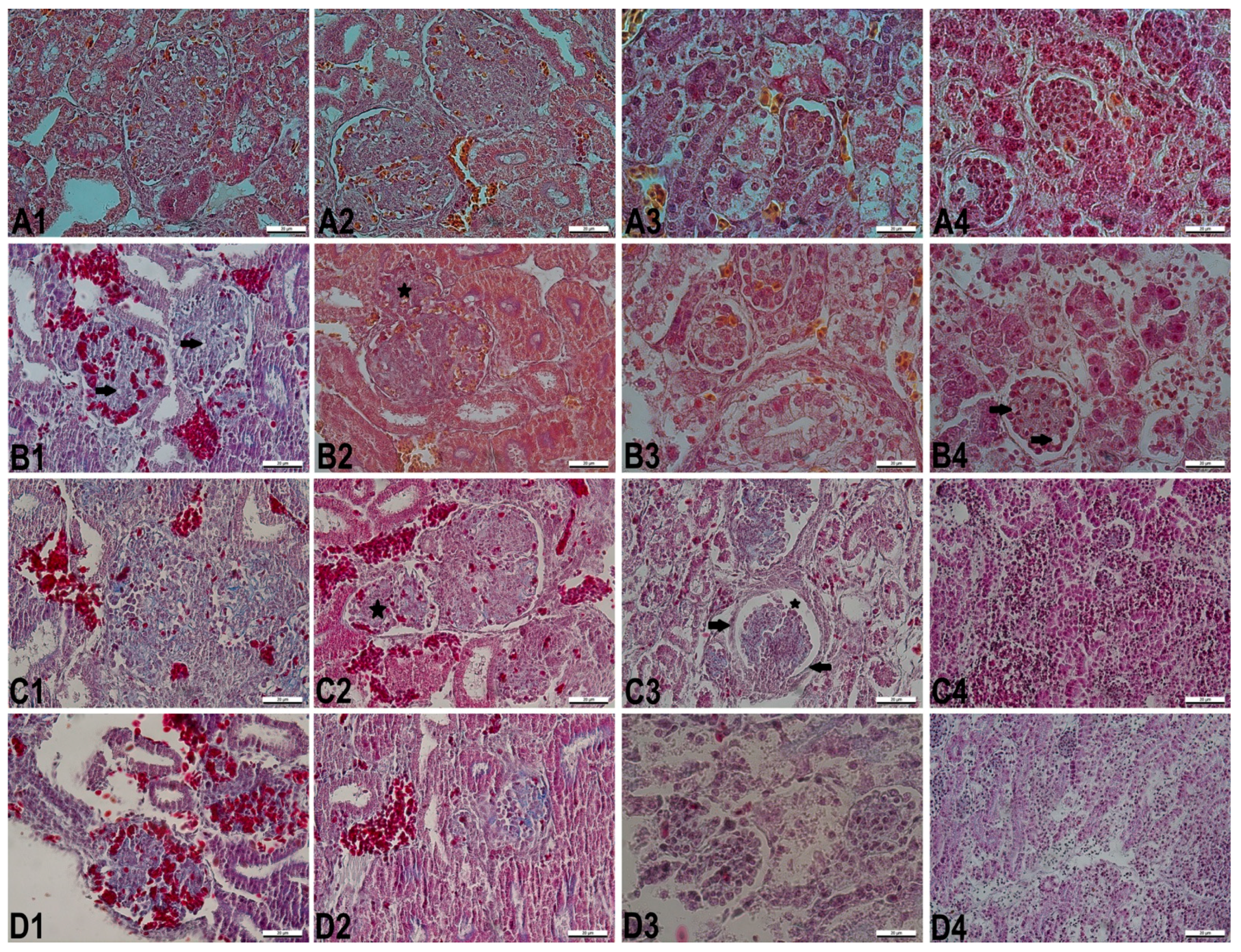
3.2. Histopathological Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çalışır, E.Z.; Çalışkan, D. Gıda katkı maddeleri ve insan sağlığı üzerine etkileri. Ankara Ecz. Fak. Derg. 2003, 32, 206–207. [Google Scholar]
- Bhattacharjee, M. Evaluation of mitodepressive effect of Sunset yellow using Allium sativum assay. IJSET 2014, 3, 1120–1130. [Google Scholar]
- Rus, C.; Gherman, V.; Miclaus, A.; Mihalca, A.; Nadaş, G.C. Comparative toxicity of food dyes on liver and kidney in Guinea pigs histopathological study. Ann. RSCB 2010, 15, 161–165. [Google Scholar]
- Poul, M.; Jarry, G.; Elhkim, M.O.; Poul, J.M. Lack of genotoxic effect of food dyes amaranth, sunset yellow and tartrazine and their metabolites in the gut micronucleus assay in mice. Food Chem. Toxicol. 2009, 47, 443–448. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, A.; Tripathi, A.; Das, M. Sunset yellow FCF, a permitted food dye, alters functional responses of splenocytes at non-cytotoxic dose. Toxicol. Lett. 2013, 217, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Sulekova, M.; Smrcova, M.; Hudak, A.; Hezelova, M.; Fedorova, M. Organic colouring agents in the pharmaceutical industry. Folia Vet. 2017, 61, 32–46. [Google Scholar] [CrossRef]
- Sardi, M.; Haldemann, Y.; Nordmann, H.; Bottex, B.; Safford, B.; Smith, B.; Jasti, P.R. Use of retailer fidelity card schemes in the assessment of food additive intake: Sunset yellow a case study. Food Addit. Contam. 2010, 27, 1507–1515. [Google Scholar] [CrossRef]
- FAO. FAO/WHO Expert Committee of Food Additives (JECFA); 74th Report; FAO: Roma, Italy, 2011; pp. 129–132. [Google Scholar]
- Jonnalagadda, P.R.; Rao, P.; Bhat, R.V.; Naidu, N. Type, extend and use of colours in ready-to-eat foods prepared in the nonindustrial sector: A case study from Hyderabad. India. IJFST 2004, 39, 125–131. [Google Scholar]
- Olsen, R.; Kudirkiene, E.; Thøfner, I.; Pors, S.; Karlskov-Mortensen, P.; Li, L.; Christensen, J. Impact of egg disinfection of hatching eggs on the eggshell microbiome and bacterial load. Poult. Sci. 2017, 96, 3901–3911. [Google Scholar] [CrossRef]
- Kemper, F.H.; Luepke, N.P. Toxicity testing by the hen’s egg test (HET). Food Chem. Toxicol. 1986, 24, 647–648. [Google Scholar] [CrossRef]
- Prelusky, D.B.; Hamilton, R.M.; Foster, B.C.; Trenholm, H.L.; Thompson, B.K. Optimization of chick embryotoxicity bioassay for testing toxicity potential of fungal metabolites. JAOAC 1987, 70, 1049–1055. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Selçuk, M.L.; Tıpırdamaz, S. A morphological and stereological study on brain, cerebral hemispheres and cerebellum of New Zealand rabbits. Anat. Histol. Embryol. 2019, 49, 1–7. [Google Scholar] [CrossRef]
- John, D. The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques; Bancroft, J.D., Suvarna, K., Layton, C., Eds.; ElsevierHealth: Amsterdam, The Netherlands, 2018; pp. 126–138. [Google Scholar]
- Bolat, D.; Bahar, S.; Sur, E.; Selçuk, M.L.; Tıpırdamaz, S. Selective gray and white matter staining of the horse spinal cord. Kafkas Üniv. Vet. Fak. Derg. 2012, 18, 249–254. [Google Scholar]
- Colakoglu, F.; Donmez, H.H. Effects of aflatoxin on AgNOR activity of cells in different hepatic zones of liver, and protective effectiveness of esterified glucomannan in ram. Dicle Üniv. Vet. Fak. Derg. 2016, 2, 54–60. [Google Scholar]
- Colakoglu, F.; Donmez, H.H. Effects of Aflatoxin on AgNOR Activity of Cells in Different Regions of Kidney, and Protective Effectiveness of Esterified Glucomannan in Ram. Kafkas Vet. Derg. 2013, 19, 505–509. [Google Scholar]
- Selcuk, M.L.; Colakoglu, F.; Tipirdamaz, S. Stereological and Histomorphological Assessment of New Zealand Rabbit Kidneys. Kafkas Üniv. Vet. Fak. Derg. 2020, 26, 121–126. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Sakr, S.M. Validation of replacement of the synthetic food dye sunset yellow-ınduced hepatotoxicity and genotoxicity with the nutraceutical curcumin in mice. Merit Res. J. Med. Med. Sci. 2016, 4, 25–50. [Google Scholar]
- Feng, J.; Cerniglia, C.E.; Chen, H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci. 2012, 4, 568–586. [Google Scholar] [CrossRef]
- Balbani, A.P.; Stelzer, L.B.; Montovani, J.C. Pharmaceutical excipients and the information on drug labels. Braz. J. Otorhinolaryngol. 2006, 72, 400–406. [Google Scholar] [CrossRef]
- Joshi, V.; Pancharatma, K. Food colorant Sunset yellow (E110) intervenes developmental profile of Zebrafish (Danio rerio). J. Appl. Toxicol. 2019, 39, 571–581. [Google Scholar] [CrossRef]
- Özparlak, H.; Çelik, B.; Balta, D. Yumurtaya verilen siklofosfamid ve C vitamini’nin tavuk embriyoları üzerindeki bazı etkileri. S.Ü. Fen Fak. Fen Derg. 2018, 44, 157–173. [Google Scholar]
- Romanoff, A.L. Life in twenty-one days. Extention Bull. 1997, 205. [Google Scholar]
- El-Malky, W.A.; Khiralla, G.M.; Salem, S.A. Nutritional study of some coloring agents on experimental rats. Int. J. Nutr. Food Sci. 2014, 3, 538–544. [Google Scholar] [CrossRef][Green Version]
- Elbanna, K.; Sarhan, O.M.; Khider, M.; Elmogy, M.; Abulreesh, H.H.; Shaaban, M.R. Microbiological, histological, and biochemical evidence for the adverse effects of food azo dyes on rats. JFDA 2017, 25, 667–680. [Google Scholar] [CrossRef]
- Saxena, B.; Sharma, S. Food color induced hepatotoxicity in Swiss Albino rats, Rattus norvegicus. Toxicol. Int. 2015, 22, 152–157. [Google Scholar] [CrossRef]
- Khayyat, L.I.; Essawy, A.E.; Sorour, J.M.; Soffar, A. Sunset Yellow and Allura Red modulate Bcl2 and COX2 expression levels and confer oxidative stress-mediated renal and hepatic toxicity in male rats. PeerJ 2018, 6, e5689. [Google Scholar] [CrossRef]
- Francés, D.E.; Ingaramo, P.I.; Ronco, M.T.; Carnovale, C.E. Diabetes, an inflammatory process: Oxidative stress and TNF-alpha involved in hepatic complication. J. Biomed. Eng. 2013, 6, 645–653. [Google Scholar] [CrossRef]
- Ali, F.A.; Abdelgayed, S.A.S.; EL-Tawil, O.S.; Bakeer, M.A. Toxicological and histopathological studies on the effect of tartrazine in male albino rats. Int. J. Pharm. Pharm. Sci. 2016, 10, 527–532. [Google Scholar]
- Alsolami, M.A. Effect of a food additive on certain haematological and biochemical parameters in male albino rat. IJZR 2017, 7, 1–10. [Google Scholar]
- Cemek, M.; Buyukokuroglu, M.E.; Sertkaya, F.; Alpdağtaş, S.; Hazini, A.; Önül, A.; Göneş, S. Effects of food color additive antioxidant functions and bioelement contents of liver, kidney and brain tissues in rats. J. Food Nutr. Res. 2014, 2, 686–691. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Jarrar, B.M. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis. 2011, 10, 166. [Google Scholar] [CrossRef]
- Soltan, S.A.; Shehata, M.N. The effects of using color foods of children on immunity properties and liver, kidney on rats. Food Nutr. Sci. 2012, 3, 897–904. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Abdel-Ghaffar, A.; Al-Othman, Z.A.; Habila, M.A.; Al-Sheikh, Y.A.; Ghneim, H.K.; Giesy, J.P.; Aboul-Soud, M.A. Curcumin protects against tartrazine-mediated oxidative stress and hepatotoxicity in male rats. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 635–645. [Google Scholar]
- Neyrinck, A. Modulation of Kupffer cell activity: Physio-pathological consequences on hepatic metabolism. Bull. Mem. Acad. R. Med. Belg. 2004, 159, 358–366. [Google Scholar] [PubMed]
- Pandey, G.; Srivastava, D.N.; Madhuri, S. A standard hepatotoxic model produced by paracetamol in rat. Toxicol. Int. 2008, 15, 69–70. [Google Scholar]
- Issaeva, N. p53 Signaling in cancers. Cancers 2019, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Toufektchan, E.; Toledo, F. The guardian of the genome revisited: p53 downregulates genes required for telomere maintenance, DNA repair, and centromere structure. Cancers 2018, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Colakoglu, F.; Donmez, H.H. Effects of aflatoxin on kidney and protective effectiveness of esterified glucomannan in ram. Van Vet. Derg. 2017, 28, 37–40. [Google Scholar]
- Miller, L.M.; Gal, A. Pathologic Basis of Veterinary Disease; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ramos, C.A.F.; Sá, R.C.S.; Alves, M.F.; Benedito, R.B.; de Sousa, D.P.; Diniz, M.F.F.M.; Araujo, M.S.T.; de Almeida, R.N. Histopathological and biochemical assessment of d -limonene-induced liver injury in rats. Toxicol. Rep. 2015, 2, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Louei Monfared, A. Histological, ultrastructural and biochemical studies on the kidney of mice treated with Carthamus tinctorius L. extract. Avicenna J. Phytomed. 2013, 3, 272–278. [Google Scholar] [PubMed]
- Yu, J.; Mao, S.; Zhang, Y.; Gong, W.; Jia, Z.; Huang, S.; Zhang, A. MnTBAP therapy attenuates renal fibrosis in mice with 5/6 nephrectomy. Oxid. Med. Cell. Longev. 2016, 2016, 10. [Google Scholar] [CrossRef]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative stress and renal fibrosis: Recent insights for the development of novel therapeutic strategies. Front. Physiol. 2018, 2, 105. [Google Scholar] [CrossRef]
- Jagruti, B. Evaluation of azo dye toxicity using some haematological and histopathological alterations in fish (Catla catla). Int. J. Environ. Ecol. Eng. 2015, 9, 458–461. [Google Scholar]
- Greaves, P. Histopathology of Preclinical Toxicity Studies; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]

| Day | Group | First Egg Weight (g) | Pre-hatching Egg Weight (g) | Embryo Weight (g) | Relative Embryo Weight (%) | Daily Egg Weight Loss (%) | Vitellus Sac Weight (g) |
|---|---|---|---|---|---|---|---|
| 10th | C | 60.71 ± 2.58 | 56.51 ± 2.61 | 3.11 ± 0.09 | 5.50 | 0.69 | 1.91 ± 0.16 |
| SY200 | 57.68 ± 1.54 | 54.61 ± 1.28 | 3.10 ± 0.17 | 5.68 | 0.53 | 1.82 ± 0.17 | |
| SY1000 | 58.54 ± 1.96 | 54.78 ± 4.62 | 3.26 ± 0.14 | 5.95 | 0.64 | 1.77 ± 0.12 | |
| SY2000 | 57.82 ± 2.40 | 54.46 ± 2.36 | 3.21 ± 0.16 | 5.89 | 0.58 | 2.27 ± 0.38 | |
| 13th | C | 58.03 ± 2.25 | 53.06 ± 2.12 | 8.91 ± 0.19 | 16.79 | 0.65 | 1.23 ± 0.04 |
| SY200 | 58.44 ± 0.87 | 53.94 ± 2.19 | 8.79 ± 0.56 | 16.30 | 0.59 | 1.52 ± 0.18 | |
| SY1000 | 59.36 ± 1.19 | 54.38 ± 1.19 | 8.98 ± 0.37 | 16.51 | 0.64 | 1.48 ± 0.16 | |
| SY2000 | 58.03 ± 1.37 | 53.61 ± 1.58 | 8.96 ± 0.49 | 16.71 | 0.58 | 1.58 ± 0.12 | |
| 16th | C | 58.58 ± 1.13 | 52.78 ± 1.57 | 22.74 ± 1.11 | 43.08 a | 0.61 | 2.13 ± 0.18 |
| SY200 | 56.48 ± 1.82 | 50.59 ± 1.98 | 24.68 ± 1.38 | 48.78 ab | 0.65 | 2.13 ± 0.36 | |
| SY1000 | 55.44 ± 0.84 | 49.50 ± 0.98 | 26.12 ± 0.53 | 52.77 b | 0.66 | 2.60 ± 0.31 | |
| SY2000 | 56.81 ± 2.04 | 50.88 ± 1.82 | 25.39 ± 0.82 | 49.90 b | 0.65 | 2.63 ± 0.34 | |
| 21st | C | 56.07 ± 1.53 | 49.01 ± 0.91 | 41.18 ± 0.92 | 84.02 | 0.60 | 2.18 ± 0.32 |
| SY200 | 60.67 ± 1.42 | 53.05 ± 1.65 | 44.89 ± 1.89 | 84.62 | 0.60 | 2.89 ± 0.66 | |
| SY1000 | 57.67 ± 1.48 | 50.67 ± 1.77 | 37.85 ± 2.96 | 74.70 | 0.58 | 3.39 ± 0.65 | |
| SY2000 | 58.00 ± 1.49 | 50.59 ± 1.49 | 40.76 ± 1.42 | 80.57 | 0.61 | 4.35 ± 0.74 |
| Day | Group | Nuclear Areas of Hepatocytes (µm2) | Nuclear Areas of Glomeruli (µm2) | Nuclear Areas of Proximal Tubules (µm2) | Nuclear Areas of Distal Tubules (µm2) |
|---|---|---|---|---|---|
| 10th | C | 20.40 ± 0.61 c | 23.97 ± 0.74 c | 21.61 ± 0.42 | 20.05 ± 0.36 b |
| SY200 | 16.25 ± 0.52 b | 24.31 ± 0.85 c | 20.21 ± 0.99 | 18.33 ± 0.49 ab | |
| SY1000 | 14.10 ± 0.52 b | 19.39 ± 1.81 ab | 20.49 ± 0.49 | 18.19 ± 0.85 ab | |
| SY2000 | 9.70 ± 0.66 a | 17.68 ± 1.06 a | 19.18 ± 0.22 | 16.58 ± 0.61 a | |
| 13th | C | 22.30 ± 0.49 b | 21.07 ± 1.19 | 22.37 ± 0.48 | 21.74 ± 1.18 b |
| SY200 | 17.20 ± 1.01 b | 17.67 ± 3.14 | 20.99 ± 0.22 | 17.71 ± 0.85 a | |
| SY1000 | 15.45 ± 0.52 b | 16.32 ± 1.64 | 21.39 ± 2.32 | 16.82 ± 0.58 a | |
| SY2000 | 8.40 ± 0.62 a | 16.40 ± 1.63 | 18.47 ± 1.11 | 16.79 ± 0.18 a | |
| 16th | C | 19.55 ± 0.58 c | 9.92 ± 0.48 | 14.87 ± 0.31 | 13.39 ± 0.12 |
| SY200 | 15.25 ± 0.55 b | 10.06 ± 0.39 | 15.64 ± 0.22 | 13.74 ± 0.66 | |
| SY1000 | 16.00 ± 0.67 b | 8.52 ± 0.70 | 15.50 ± 0.46 | 13.35 ± 0.69 | |
| SY2000 | 13.10 ± 0.44 a | 9.56 ± 1.02 | 15.12 ± 1.18 | 13.09 ± 1.25 | |
| 21st | C | 21.45 ± 0.77 b | 7.43 ± 0.72 b | 12.18 ± 0.56 b | 10.55 ± 1.21 b |
| SY200 | 16.05 ± 0.77 a | 8.69 ± 0.64 b | 12.31 ± 1.27 b | 11.54 ± 0.91 b | |
| SY1000 | 14.10 ± 0.84 a | 8.33 ± 1.28 b | 10.69±1.93 ab | 7.89 ± 1.55 ab | |
| SY2000 | 14.05 ± 0.55 a | 4.74 ± 0.29 a | 6.82±0.15 a | 6.13 ± 0.35 a |
| Day | Group | Areas of Glomeruli (µm2) | Areas of Renal Corpuscules (µm2) | Diameters of Proximal Tubules (µm) | Diameters of Distal Tubules (µm) |
|---|---|---|---|---|---|
| 10th | C | 10911.18 ± 358.62 | 15508.41 ± 595.25 | 52.85 ± 1.73 | 36.39 ± 3.93 |
| SY200 | 9928.72 ± 1184.10 | 13476.77 ± 1407.93 | 55.83 ± 1.83 | 38.62 ± 1.65 | |
| SY1000 | 8201.13 ± 1294.55 | 12401.27 ± 1354.53 | 54.11 ± 1.18 | 44.65 ± 3.26 | |
| SY2000 | 7965.46 ± 883.15 | 11306.88 ± 1741.95 | 56.12 ± 1.41 | 47.14 ± 2.58 | |
| 13th | C | 15411.71 ± 534.25 b | 21981.23 ± 890.81 b | 58.39 ± 2.61 | 37.43 ± 3.42 |
| SY200 | 10670.66 ± 1171.59 a | 14507.59 ± 1501.94 a | 57.87 ± 5.78 | 42.53 ± 2.71 | |
| SY1000 | 10188.73 ±1190.86 a | 13945.97 ± 1468.99 a | 52.03 ± 1.97 | 42.91 ± 1.66 | |
| SY2000 | 9644.33 ± 1121.13 a | 13529.14 ± 1260.89 a | 63.19 ± 0.91 | 47.76 ± 2.31 | |
| 16th | C | 2131.26 ± 1218.45 | 3738.86 ± 2267.49 | 19.93 ± 1.57 a | 18.53 ± 0.84 a |
| SY200 | 1927.21 ± 1089.76 | 3390.74 ± 2054.15 | 27.98 ± 1.65 b | 21.74 ± 4.18 ab | |
| SY1000 | 840.98 ± 84.23 | 1384.33 ± 129.65 | 34.39 ± 1.69 bc | 28.43 ± 3.22 ab | |
| SY2000 | 814.75 ± 74.96 | 1326.86 ± 106.88 | 36.99 ± 2.83 c | 30.67 ± 2.27 b | |
| 21st | C | 1154.01 ± 63.77 b | 1960.04 ± 119.33 b | 27.98 ± 1.25 b | 21.22 ± 1.05 a |
| SY200 | 888.64 ± 92.24 a | 1448.81 ± 90.06 a | 26.59 ± 1.64 b | 24.65 ± 0.73 a | |
| SY1000 | 897.31 ± 126.11 a | 1357.56 ± 94.44 a | 25.79 ± 1.53 ab | 26.26 ± 0.53 ab | |
| SY2000 | 863.39 ± 68.45 a | 1294.52 ± 165.85 a | 28.73 ± 2.05 a | 29.48 ± 1.97 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colakoglu, F.; Selcuk, M.L. The Embryotoxic Effects of in Ovo Administered Sunset Yellow FCF in Chick Embryos. Vet. Sci. 2021, 8, 31. https://doi.org/10.3390/vetsci8020031
Colakoglu F, Selcuk ML. The Embryotoxic Effects of in Ovo Administered Sunset Yellow FCF in Chick Embryos. Veterinary Sciences. 2021; 8(2):31. https://doi.org/10.3390/vetsci8020031
Chicago/Turabian StyleColakoglu, Fatma, and Muhammet Lutfi Selcuk. 2021. "The Embryotoxic Effects of in Ovo Administered Sunset Yellow FCF in Chick Embryos" Veterinary Sciences 8, no. 2: 31. https://doi.org/10.3390/vetsci8020031
APA StyleColakoglu, F., & Selcuk, M. L. (2021). The Embryotoxic Effects of in Ovo Administered Sunset Yellow FCF in Chick Embryos. Veterinary Sciences, 8(2), 31. https://doi.org/10.3390/vetsci8020031





