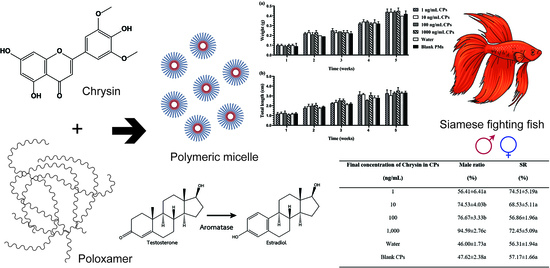
Masculinizing Effects of Chrysin-Loaded Poloxamer Micelles on Siamese Fighting Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fish and Rearing Conditions
2.3. Development of the Polymeric Micelles Containing Chrysin
2.4. HPLC Analysis
2.5. Characterization of the CPs
2.6. In Vivo Toxicity Study
2.7. In Vivo Masculinization Effect
2.8. Statistical Analysis
3. Results and Discussion
3.1. Development and Characterization of CPs
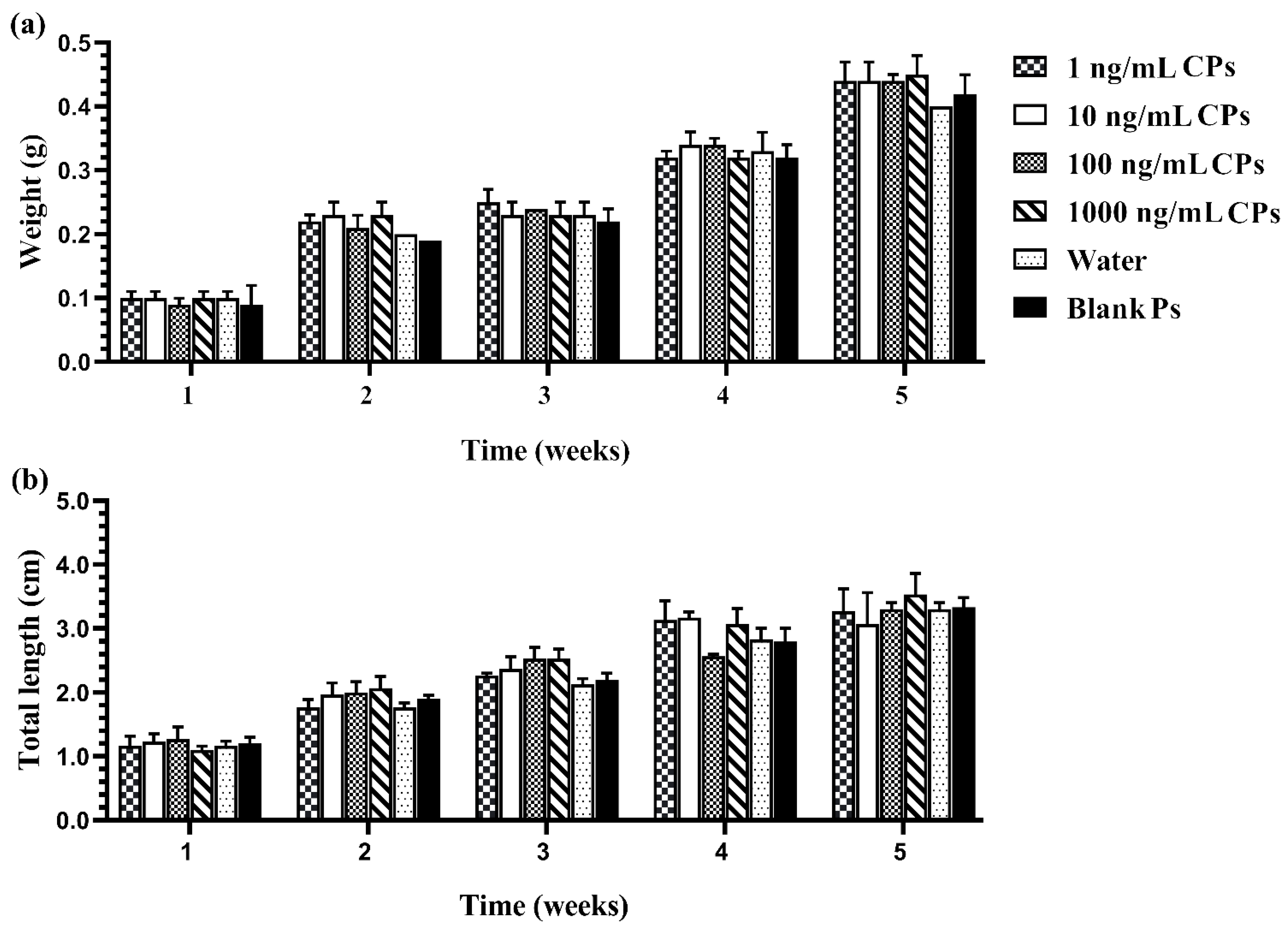
3.2. In Vivo Toxicity of the Developed CPs
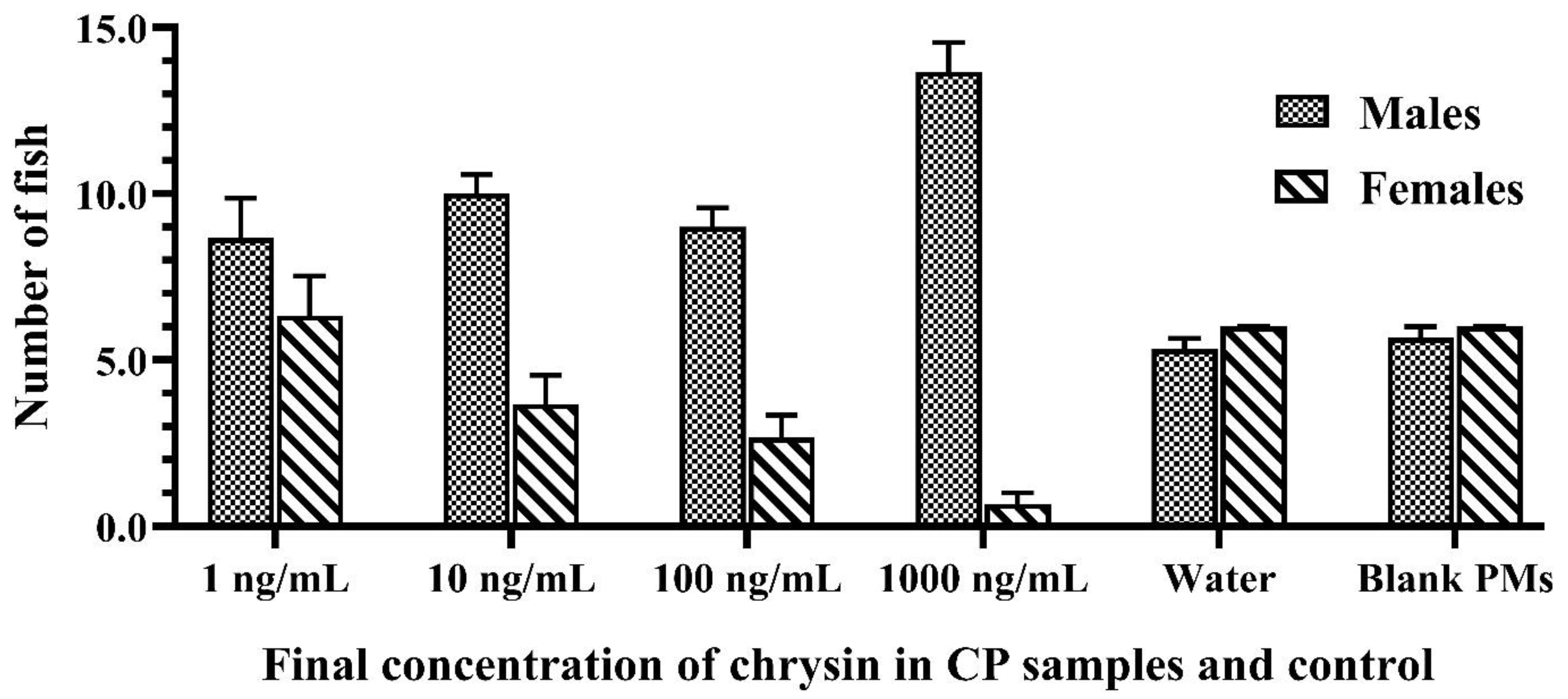
3.3. In Vivo Masculinization Effect of the Developed CPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Meliska, C.J.; Meliska, J.A.; Peeke, H.V.S. Threat displays and combat aggression in Betta splendens following visual exposure to conspecifics and one-way mirrors. Behav. Neural Biol. 1980, 28, 473–486. [Google Scholar] [CrossRef]
- Chapman, F.A.; Fitz-Coy, S.A.; Thunberg, E.M.; Adams, C.M. United States of America trade in ornamental fish. J. World Aquac. Soc. 1997, 28, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Sun, F.; Wan, Z.Y.; Ye, B.; Wen, Y.; Liu, H.; Yang, Z.; Pang, H.; Meng, Z.; Fan, B.; et al. Genomic Basis of Striking Fin Shapes and Colors in the Fighting Fish. Mol. Biol. Evol. 2021, 38, 3383–3396. [Google Scholar] [CrossRef]
- Sermwatanakul, A. Capacitating the local farmers to enhance global marketing of Thailand’s national aquatic animal, the Siamese fighting fish. Fish People 2019, 17, 42–48. [Google Scholar]
- Saekhow, S.; Thongprajukaew, K.; Phromkunthong, W.; Sae-khoo, H. Minimal water volume for intensively producing male Siamese fighting fish (Betta splendens Regan, 1910). Fish Physiol. Biochem. 2018, 44, 1075–1085. [Google Scholar] [CrossRef]
- Baroiller, J.F.; Guiguen, Y.; Fostier, A. Endocrine and environmental aspects of sex differentiation fish. Cell. Mol. Life Sci. 1999, 55, 910–931. [Google Scholar] [CrossRef]
- Baroiller, J.F.; Guiguen, Y. Endocrine and environmental aspects of sex differentiation in gonochoristic fish. Genes Mech. Vertebr. Sex Determ. 2001, 91, 177–201. [Google Scholar] [CrossRef]
- Kipouros, K.; Paschos, I.; Gouva, E.; Ergolavou, A.; Perdikaris, C. Masculinization of the ornamental Siamese fighting fish with oral hormonal administration. Sci. Asia 2011, 37, 277–280. [Google Scholar] [CrossRef]
- Kavumpurath, S.; Pandian, T.J. Masculinization of fighting fish, Betta splendens Regan, using synthetic or natural androgens. Aquac. Res. 1994, 25, 373–381. [Google Scholar] [CrossRef]
- Malekinejad, H.; Rezabakhsh, A. Hormones in Dairy Foods and Their Impact on Public Health—A Narrative Review Article. Iran. J. Public Health 2015, 44, 742–758. [Google Scholar] [PubMed]
- Desvages, G.; Pieau, C. Aromatase activity in gonads of turtle embryos as a function of the incubation temperature of eggs. J. Steroid Biochem. Mol. Biol. 1992, 41, 851–853. [Google Scholar] [CrossRef]
- Young, G.; Kagawa, H.; Nagahama, Y. Evidence for a Decrease in Aromatase Activity in the Ovarian Granulosa Cells of Amago Salmon (Oncorhynchus rhodurus) Associated with Final Oocyte Maturation. Biol. Reprod. 1983, 29, 310–315. [Google Scholar] [CrossRef]
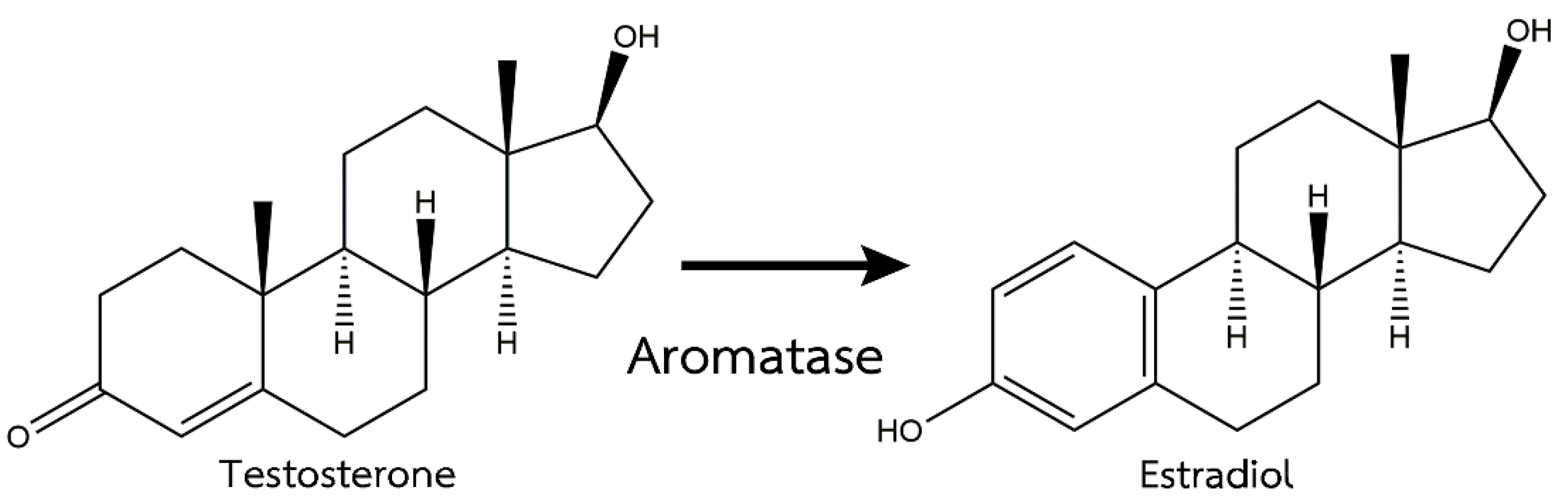
- Cheshenko, K.; Pakdel, F.; Segner, H.; Kah, O.; Eggen, R.I.L. Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish. Gen. Comp. Endocrinol. 2008, 155, 31–62. [Google Scholar] [CrossRef]
- Kitano, T.; Takamune, K.; Nagahama, Y.; Abe, S. Aromatase inhibitor and 17α-methyltestosterone cause sex-reversal from genetical females to phenotypic males and suppression of P450 aromatase gene expression in Japanese flounder (Paralichthys olivaceus). Mol. Reprod. Dev. Inc. Gamete Res. 2000, 56, 1–5. [Google Scholar] [CrossRef]
- Vizziano, D.; Baron, D.; Randuineau, G.; Mahè, S.; Cauty, C.; Guiguen, Y. Rainbow trout gonadal masculinization induced by inhibition of estrogen synthesis is more physiological than masculinization induced by androgen supplementation. Biol. Reprod. 2008, 78, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Séralini, G.-E.; Moslemi, S. Aromatase inhibitors: Past, present, and future. Mol. Cell. Endocrinol. 2001, 178, 117–131. [Google Scholar] [CrossRef]
- Balam, F.H.; Ahmadi, Z.S.; Ghorbani, A. Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): A systematic review. Heliyon 2020, 6, e03557. [Google Scholar] [CrossRef]
- Oliveira, G.A.R.; Ferraz, E.R.A.; Souza, A.O.; Lourenço, R.A.; Oliveira, D.P.; Dorta, D.J. Evaluation of the Mutagenic Activity of Chrysin, a Flavonoid Inhibitor of the Aromatization Process. J. Toxicol. Environ. Health Part A 2012, 75, 1000–1011. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham-Ul-Haq; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Raghu, A.V.; George, S.; Krishna, V.R.; Sindhu, K.K. Bioactive properties of phenolics present in Oroxylum indicum—A review. J. Pharmacogn. Phytochem. 2013, 2, 23–27. [Google Scholar]
- Stompor-Gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef] [PubMed]
- Khumpirapang, N.; von Gersdorff Jørgensen, L.; Müllertz, A.; Rades, T.; Okonogi, S. Formulation optimization, anesthetic activity, skin permeation, and transportation pathway of Alpinia galanga oil SNEDDS in zebrafish (Danio rerio). Eur. J. Pharm. Biopharm. 2021, 165, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; van Steenbergen, M.J.; Torano, J.S.; Okonogi, S.; Hennink, W.E. A Kinetic Degradation Study of Curcumin in Its Free Form and Loaded in Polymeric Micelles. AAPS J. 2016, 18, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Phumat, P.; Khongkhunthian, S.; Suttiat, K.; Chaijareenont, P. Denture-soaking solution containing Piper betle extract-loaded polymeric micelles; inhibition of Candida albicans, clinical study, and effects on denture base resin. Antibiotics 2021, 10, 440. [Google Scholar] [CrossRef]
- Tima, S.; Okonogi, S.; Ampasavate, C.; Berkland, C.; Anuchapreeda, S. FLT3-specific curcumin micelles enhance activity of curcumin on FLT3-ITD overexpressing MV4-11 leukemic cells. Drug Dev. Ind. Pharm. 2019, 45, 498–505. [Google Scholar] [CrossRef]
- Okonogi, S.; Phumat, P.; Khongkhunthian, S. Enhancement of aqueous solubility and antibiofilm activity of 4-allylpyrocatechol by polymeric micelles. Bioprocess Biosyst. Eng. 2021, 44, 1289–1300. [Google Scholar] [CrossRef]
- Naksuriya, O.; Shi, Y.; Van Nostrum, C.F.; Anuchapreeda, S.; Hennink, W.E.; Okonogi, S. HPMA-based polymeric micelles for curcumin solubilization and inhibition of cancer cell growth. Eur. J. Pharm. Biopharm. 2015, 94, 501–512. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Okonogi, S. Encapsulation of Sesbania grandiflora extract in polymeric micelles to enhance its solubility, stability, and antibacterial activity. J. Microencapsul. 2017, 34, 73–81. [Google Scholar] [CrossRef]
- Kolašinac, N.; Kachrimanis, K.; Homšek, I.; Grujić, B.; Đurić, Z.; Ibrić, S. Solubility enhancement of desloratadine by solid dispersion in poloxamers. Int. J. Pharm. 2012, 436, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Jindal, N.; Mehta, S.K. Nevirapine loaded Poloxamer 407/Pluronic P123 mixed micelles: Optimization of formulation and in vitro evaluation. Coll. Surf. B Biointerfaces 2015, 129, 100–106. [Google Scholar] [CrossRef]
- Sassa-deepaeng, T.; Pikulkaew, S.; Okonogi, S. Development of chrysin loaded poloxamer micelles and toxicity evaluation in fish embryos. Drug Discov. Ther. 2016, 10, 150–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simpson, M.J.A. The Display of the Siamese Fighting Fish, Betta splendens. Anim. Behav. Monogr. 1968, 1, 1–73. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Aboud, H.M.; Mahmoud, M.O.; Abdeltawab Mohammed, M.; Shafiq Awad, M.; Sabry, D. Preparation and appraisal of self-assembled valsartan-loaded amalgamated Pluronic F127/Tween 80 polymeric micelles: Boosted cardioprotection via regulation of Mhrt/Nrf2 and Trx1 pathways in cisplatin-induced cardiotoxicity. J. Drug Target. 2020, 28, 282–299. [Google Scholar] [CrossRef]
- Tănase, M.A.; Raducan, A.; Oancea, P.; Diţu, L.M.; Stan, M.; Petcu, C.; Scomoroşcenco, C.; Ninciuleanu, C.M.; Nistor, C.L.; Cinteza, L.O. Mixed pluronic—Cremophor polymeric micelles as nanocarriers for poorly soluble antibiotics—The influence on the antibacterial activity. Pharmaceutics 2021, 13, 435. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 116797. [Google Scholar] [CrossRef]
- Serra, H.; Scholze, M.; Altenburger, R.; Busch, W.; Budzinski, H.; Brion, F.; Aït-Aïssa, S. Combined effects of environmental xeno-estrogens within multi-component mixtures: Comparison of in vitro human- and zebrafish-based estrogenicity bioassays. Chemosphere 2019, 227, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Anuchapreeda, S.; Khumpirapang, N.; Chiampanichayakul, S.; Nirachonkul, W.; Saiai, A.; Usuki, T.; Okonogi, S. Characterization and biological properties of zederone and zedoarondiol from rhizomes of En-lueang (Curcuma cf. Amada). Nat. Prod. Commun. 2018, 13, 1615–1618. [Google Scholar] [CrossRef]
- Scholz, S.; Gutzeit, H.O. 17-α-ethinylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquat. Toxicol. 2000, 50, 363–373. [Google Scholar] [CrossRef]
- Pandian, T.J.; Sheela, S.G. Hormonal induction of sex reversal in fish. Aquaculture 1995, 138, 1–22. [Google Scholar] [CrossRef]
- Piferrer, F.; Lim, L.C. Application of sex reversal technology in ornamental fish culture. Aquarium Sci. Conserv. 1997, 1, 113–118. [Google Scholar] [CrossRef]
- Hattori, R.S.; Fernandino, J.I.; Kishii, A.; Kimura, H.; Kinno, T.; Oura, M.; Somoza, G.M.; Yokota, M.; Strüssmann, C.A.; Watanabe, S. Cortisol-Induced Masculinization: Does Thermal Stress Affect Gonadal Fate in Pejerrey, a Teleost Fish with Temperature-Dependent Sex Determination? PLoS ONE 2009, 4, e6548. [Google Scholar] [CrossRef] [PubMed]
- Khater, E.G.; Ali, S.A.; Mohamed, W.E. Effect of water temperature on masculinization and growth of Nile tilapia fish. J. Aquac. Res. Dev. 2017, 8, 507. [Google Scholar] [CrossRef]
- Pattiasina, B.J.; Pattinasarany, M.M.; Manuputty, M.M.D.; Kokmesa, E.R. Masculinization of beta fish larvae Betta splendens through the different treatment immersion of honey solution and larval age. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 797, pp. 1–7. [Google Scholar] [CrossRef]
- Soumokil, A.W.; Lumamuly, J.O.; Laimeheriwa, B.M. Masculinization of betta fish (Betta splendens) through natural honey immersion with different concentration. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 584, pp. 1–7. [Google Scholar] [CrossRef]
- Susanto, G.N.; Sutyarso; Widianto, W. Monosex male formation of juvenile redclaw crayfish using natural steroid hormone from gamma sea cucumber and different doses of honeybee. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; Volume 1751, pp. 1–9. [Google Scholar] [CrossRef]
- Mansour, A.T.; Omar, E.A.; Srour, T.M.; Yousef, M.I. Effect of three natural phytochemicals supplementation on growth performance, testosterone level and feed utilization of Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2018, 24, 408–415. [Google Scholar] [CrossRef]
- Tekin, İ.Ö.; Marotta, F. Chapter 22—Polyphenols and Immune System. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 263–276. ISBN 9780128130087. [Google Scholar]
- Domínguez-Delgado, C.L.; Fuentes-Prado, E.; Escobar-Chávez, J.J.; Vidal-Romero, G.; Rodríguez Cruz, I.; Díaz-Torres, R. Chitosan and pluronic® F-127: Pharmaceutical applications. In Encyclopedia of Biomedical Polymers Polymeric Biomaterials; Mishra, M.K., Ed.; Taylor and Francis Group: New York, NY, USA, 2016; pp. 1513–1535. [Google Scholar]
- Cerit, O.; Koc, F. The effects of chrysin on cypermethrin-induced acute intoxication in rainbow trout (Oncorhynchus mykiss). Fish. Aquat. Life 2019, 27, 102–111. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Walle, T. Cytotoxic effects of the dietary flavones chrysin and apigenin in a normal trout liver cell line. Chem. Biol. Interact. 2008, 171, 37–44. [Google Scholar] [CrossRef]

| CP Samples and Control | Particle Size (nm) | Size Distribution |
|---|---|---|
| Blank PMs | 21.3 ± 0.8 a | 0.218 ± 0.011 a |
| 1:1 | 84.7 ± 1.2 b | 0.420 ± 0.018 b |
| 1:2 | 74.2 ± 1.6 c | 0.288 ± 0.012 c |
| 1:3 | 72.5 ± 2.2 c | 0.322 ± 0.019 cd |
| 1:4 | 72.4 ± 2.1 c | 0.286 ± 0.013 c |
| 1:5 | 70.0 ± 2.2 c | 0.279 ± 0.014 ac |
| 1:10 | 68.9 ± 2.6 c | 0.256 ± 0.012 ac |
| 1:15 | 68.3 ± 2.0 c | 0.250 ± 0.019 ac |
| CP Samples and Control (Final Concentration of Chrysin (ng/mL)) | Mortality (%) after Exposure Time | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 1 | 16.00 ± 4.62 a | 16.00 ± 4.62 a | 16.00 ± 4.62 a |
| 10 | 9.33 ± 3.53 a | 9.33 ± 3.53 a | 9.33 ± 3.53 a |
| 100 | 16.00 ± 2.31 a | 16.00 ± 2.31 a | 16.00 ± 2.31 a |
| 1000 | 20.00 ± 2.31 a | 20.00 ± 2.31 a | 26.00 ± 2.31 b |
| 10,000 | 65.33 ± 7.42 b | 73.33 ± 7.42 b | 90.67 ± 5.33 c |
| Water | 9.33 ± 1.33 a | 9.33 ± 1.33 a | 9.33 ± 1.33 a |
| Blank PMs | 9.33 ± 1.33 a | 9.33 ± 1.33 a | 9.33 ± 1.33 a |
| CP Samples and Control (Final Concentration of Chrysin (ng/mL)) | Male Ratio (%) | Survival Rate (%) |
|---|---|---|
| 1 | 56.41 ± 6.41 a | 74.51 ± 5.19 a |
| 10 | 74.53 ± 4.03 b | 68.53 ± 5.11 a |
| 100 | 76.67 ± 3.33 b | 56.86 ± 1.96 a |
| 1000 | 94.59 ± 2.76 c | 72.45 ± 5.09 a |
| Water | 46.00 ± 1.73 a | 56.31 ± 1.94 a |
| Blank PMs | 47.62 ± 2.38 a | 57.17 ± 1.66 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumpirapang, N.; Sassa-deepaeng, T.; Suknuntha, K.; Anuchapreeda, S.; Okonogi, S. Masculinizing Effects of Chrysin-Loaded Poloxamer Micelles on Siamese Fighting Fish. Vet. Sci. 2021, 8, 305. https://doi.org/10.3390/vetsci8120305
Khumpirapang N, Sassa-deepaeng T, Suknuntha K, Anuchapreeda S, Okonogi S. Masculinizing Effects of Chrysin-Loaded Poloxamer Micelles on Siamese Fighting Fish. Veterinary Sciences. 2021; 8(12):305. https://doi.org/10.3390/vetsci8120305
Chicago/Turabian StyleKhumpirapang, Nattakanwadee, Tanongsak Sassa-deepaeng, Krit Suknuntha, Songyot Anuchapreeda, and Siriporn Okonogi. 2021. "Masculinizing Effects of Chrysin-Loaded Poloxamer Micelles on Siamese Fighting Fish" Veterinary Sciences 8, no. 12: 305. https://doi.org/10.3390/vetsci8120305
APA StyleKhumpirapang, N., Sassa-deepaeng, T., Suknuntha, K., Anuchapreeda, S., & Okonogi, S. (2021). Masculinizing Effects of Chrysin-Loaded Poloxamer Micelles on Siamese Fighting Fish. Veterinary Sciences, 8(12), 305. https://doi.org/10.3390/vetsci8120305