Relationship of the Temperature-Humidity Index (THI) with Ovarian Responses and Embryo Production in Superovulated Thai-Holstein Crossbreds under Tropical Climate Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Superovulation Treatment and Embryo Recovery
2.3. Ultrasound Examination
2.4. Temperature-Humidity Index (THI)
2.5. Statistical Analysis
3. Results
3.1. Correlation between THI during Periods of Heat Stress Relative to Insemination Day (9, 21 and 42 Days before Insemination) with the Superovulatory Responses
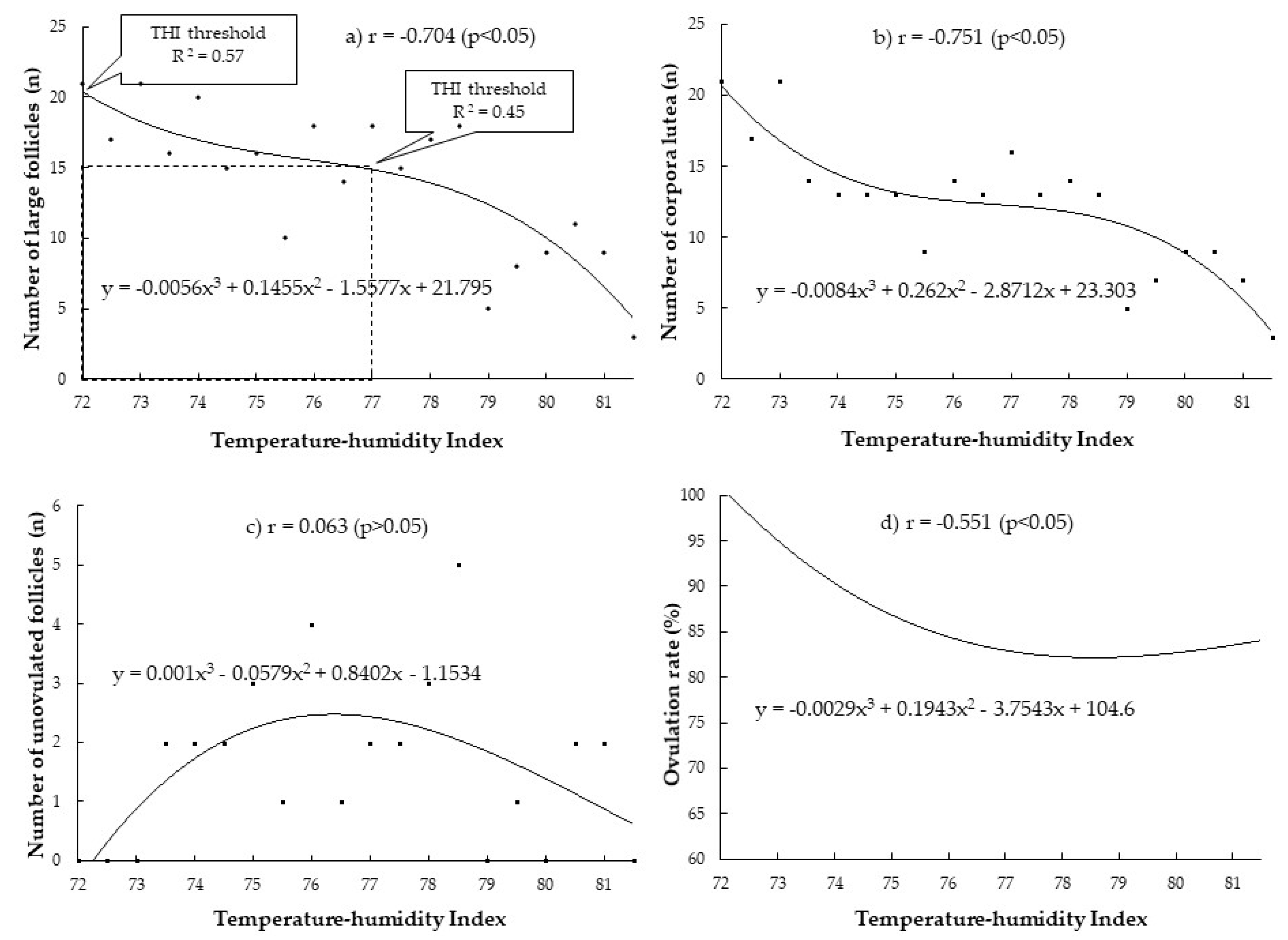
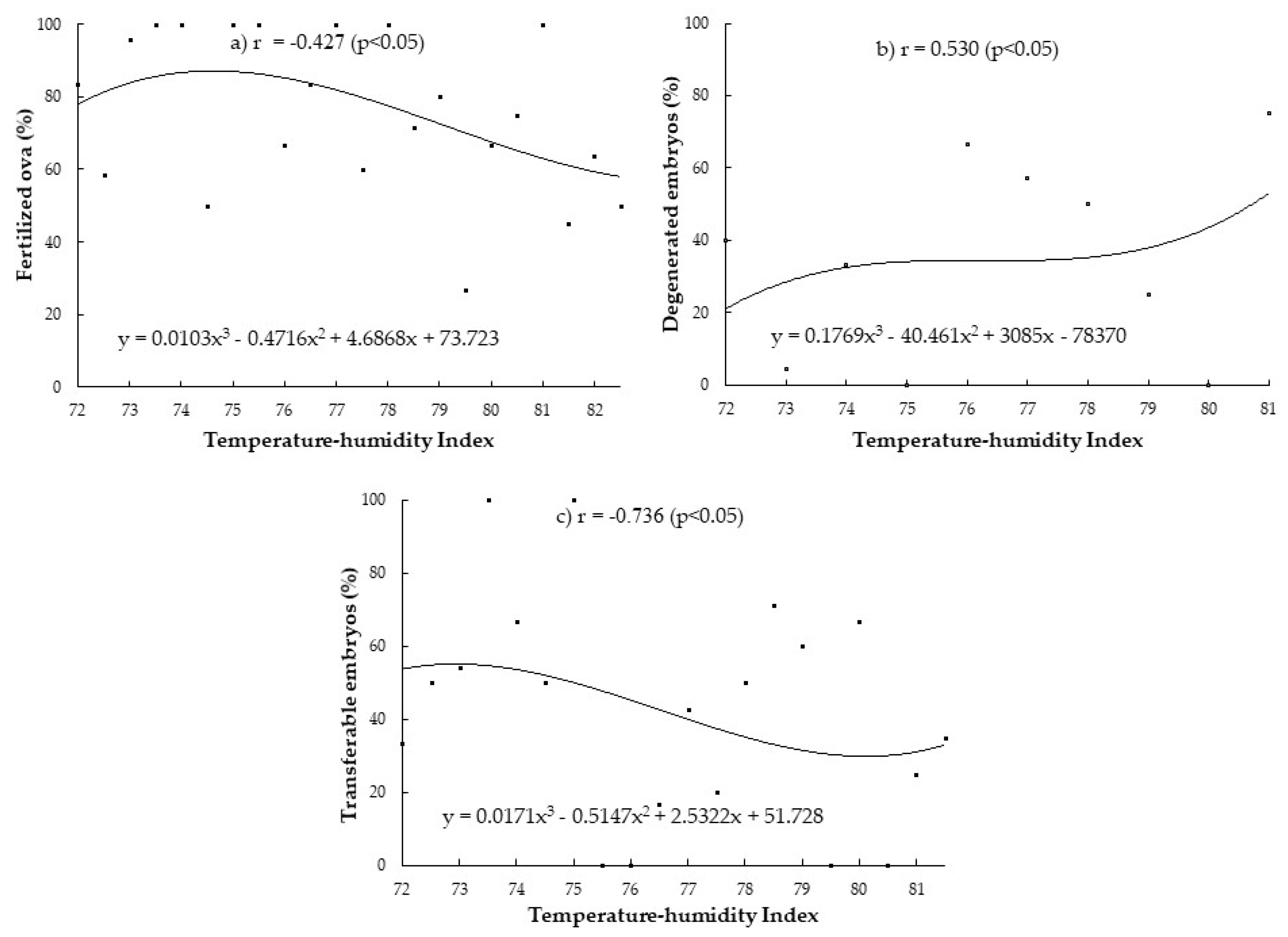
3.2. Determination of Heat-Stress Threshold and Relationship between THI and Superovulatory Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Jordan, E.R. Effects of heat stress on reproduction. J. Dairy Sci. 2003, 86, 104–144. [Google Scholar] [CrossRef]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Vet.-World 2016, 9, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–33. [Google Scholar] [CrossRef]
- Badinga, L.; Thatcher, W.W.; Diaz, T.; Drost, M.; Wolfenson, D. Effect of environmental heat stress on follicular development and steroidogenesis in lactating Holstein cows. Theriogenology 1993, 39, 797–810. [Google Scholar] [CrossRef]
- Payton, R.R.; Romar, R.; Coy, P.; Saxton, A.M.; Lawrence, J.L.; Edwards, J.L. Susceptibility of bovine germinal vesicle-stage oocytes from antral follicles to direct effects of heat stress in vitro. Biol. Reprod. 2004, 71, 1303–1308. [Google Scholar] [CrossRef] [Green Version]
- Sakatani, M.; Yamanaka, K.; Balboula, A.Z.; Takenouchi, N.; Takahashi, M. Heat stress during in vitro fertilization decreases fertilization success by disrupting anti-polyspermy systems of the oocytes. Mol. Reprod. Dev. 2015, 82, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Hansen, P.J. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol. Reprod. Dev. 1997, 46, 138–145. [Google Scholar] [CrossRef]
- Sakatani, M.; Alvarez, N.V.; Takahashi, M.; Hansen, P.J. Consequences of physiological heat shock beginning at the zygote stage on embryonic development and expression of stress response genes in cattle. J. Dairy Sci. 2012, 95, 3080–3091. [Google Scholar] [CrossRef]
- Hansen, P.J. To be or not to be--determinants of embryonic survival following heat shock. Theriogenology 2007, 68, 40–44. [Google Scholar] [CrossRef]
- Hansen, P.J. The incompletely fulfilled promise of embryo transfer in cattle-why aren’t pregnancy rates greater and what can we do about it? J. Anim. Sci. 2020, 98, 1–20. [Google Scholar] [CrossRef]
- Zubor, T.; Holló, G.; Pósa, R.; Nagy-Kiszlinger, H.; Vigh, Z.; Húth, B. Effect of rectal temperature on efficiency of artificial insemination and embryo transfer technique in dairy cattle during hot season. Czech J. Anim. Sci. 2020, 65, 295–302. [Google Scholar] [CrossRef]
- Drost, M.; Ambrose, J.D.; Thatcher, M.J.; Cantrell, C.K.; Wolfsdorf, K.E.; Hasler, J.F.; Thatcher, W.W. Conception rates after artificial insemination or embryo transfer in lactating dairy cows during summer in Florida. Theriogenology 1999, 52, 1161–1167. [Google Scholar] [CrossRef]
- Schuller, L.K.; Burfeind, O.; Heuwieser, W. Impact of heat stress on conception rate of dairy cows in the moderate climate considering different temperature humidity index thresholds, periods relative to breeding, and heat load indices. Theriogenology 2014, 81, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.M.; Tranter, W.P.; Mayer, D.G.; Jonsson, N.N. Effect of environmental heat on conception rates in lactating dairy cows: Critical periods of exposure. J. Dairy Sci. 2007, 90, 2271–2278. [Google Scholar] [CrossRef]
- Lussier, J.G.; Matton, P.; Dufour, J.J. Growth rates offollicles in the ovary of the cow. J. Reprod. Fertil. 1987, 81, 301–307. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient, Requirements of Dairy Cattl; Paperback; NRC: Washington, DC, USA, 2001; pp. 258–281.
- Ratsiri, T.; Ratchamak, R.; Chumchai, R.; Boonkum, W.; Vongpralub, T.; Chankitisakul, V. A novel route of follicle-stimulating hormone administration with a split-single ischiorectal fossa in Thai-Holstein crossbred superovulation programs under heat stress conditions. Anim. Sci. J. 2021, 92, 1–10. [Google Scholar] [CrossRef]
- Lindner, G.M.; Wright, R.W., Jr. Bovine embryo morphology and evaluation. Theriogenology 1983, 20, 407–416. [Google Scholar] [CrossRef]
- Chankitisakul, V.; Pitchayapipatkul, J.; Chuawongboon, P.; Rakwongrit, D.; Sakhong, D.; Boonkum, W.; Vongpralub, T. Comparison of three superovulation protocols with or without GnRH treatment at the time of artificial insemination on ovarian response and embryo quality in Thai native heifers. Trop. Anim. Health Prod. 2017, 49, 633–639. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Hackbart, K.S.; Bender, R.W.; Baez, G.M.; Dresch, A.R.; Guenther, J.N.; Souza, A.H.; Fricke, P.M. Use of a single injection of long-acting recombinant bovine FSH to superovulate Holstein heifers: A preliminary study. Theriogenology 2014, 82, 481–489. [Google Scholar] [CrossRef] [PubMed]
- National Oceanic and Atmospheric Administration. Livestock Hot Weather Stress; Operations Manual Letter C-31-76; Department of Commerce, NOAA, National Weather Service Central Region: Kansas City, MO, USA, 1976.
- Guilbault, L.A.; Grasso, F.; Lussier, J.G.; Rouillier, P.; Matton, P. Decreased superovulatory responses in heifers superovulated in the presence of a dominant follicle. J. Reprod. Fert. 1991, 91, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfenson, D.; Lew, B.J.; Thatcher, W.W.; Graber, Y.; Meidan, R. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 1997, 47, 9–19. [Google Scholar] [CrossRef]
- Armstrong, D.V. Symposium: Nutrition and Heat Stress: Heat Stress Interaction with Shade and Cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Moran, J.B. Tropical Dairy Farming: Feeding Management for Small Holder Dairy Farmers in the Humid Tropics; CSIRO Publishing: Clayton, Australia, 2005; pp. 223–228. [Google Scholar]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Behera, R.; Upadhyay, A.; Shivahre, P.R. Determination of critical heat stress zone for fertility traits using temperature humidity index in Murrah buffaloes. Indian J. Anim. Sci. 2014, 84, 1181–1184. [Google Scholar]
- West, J.W. Effects of Heat-Stress on Production in Dairy Cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonkum, W.; Misztal, I.; Duangjinda, M.; Pattarajinda, V.; Tumwasorn, S.; Buaban, S. Genetic effects of heat stress on days open for Thai Holstein crossbreds. J. Dairy Sci. 2011, 94, 1592–1596. [Google Scholar] [CrossRef] [Green Version]
- Boonkum, W.; Duangjinda, M. Estimation of genetic parameters for heat stress, including dominance gene effects, on milk yield in Thai Holstein dairy cattle. Anim. Sci. J. 2015, 86, 245–250. [Google Scholar] [CrossRef]
- Chokcharoen, T.; Boonkum, W.; Chankitisakul, V. Association of temperature humidity index with days open of crossbred Thai-Holstein dairy cattle under tropical climate in Thailand. Khon Kaen AGR J. 2017, 45, 425–432. [Google Scholar]
- Kaewlamun, W.; Chayaratanasin, R.; Virakul, P.; Andrew, A.P.; Humblot, P.; Suadsong, S.; Tummaruk, P.; Techakumphu, M. Differences of periods of calving on days open of dairy cows in different regions and months of Thailand. Thai J. Vet. Med. 2011, 41, 315–320. [Google Scholar]
- Ravagnolo, O.; Misztal, I. Effect of heat stress on nonreturn rate in Holsteins: Fixed-model analyses. J. Dairy Sci. 2002, 85, 3101–3106. [Google Scholar] [CrossRef]
- García-Ispierto, I.; López-Gatius, F.; Bech-Sabat, G.; Santolaria, P.; Yániz, J.L.; Nogareda, C.; De Rensis, F.; López-Béjar, M. Climate factors affecting conception rate of high producing dairy cows in northeastern Spain. Theriogenology 2007, 67, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Oseni, S.; Misztal, I.; Tsuruta, S.; Rekaya, R. Genetic components of days open under heat stress. J. Dairy Sci. 2004, 87, 3022–3028. [Google Scholar] [CrossRef] [Green Version]
- Kaim, M.; Bloch, A.; Wolfenson, D.; Braw-Tal, R.; Rosenberg, M.; Voet, H.; Folman, Y. Effects of GnRH administered to cows at the onset of estrus on timing of ovulation, endocrine responses, and conception. J. Dairy Sci. 2003, 86, 2012–2021. [Google Scholar] [CrossRef]
- López-Gatius, F.; Santolaria, P.; Martino, A.; Delétang, F.; De Rensis, F. The effects of GnRH treatment at the time of AI and 12 days later on reproductive performance of high producing dairy cows during the warm season in northeastern Spain. Theriogenology 2006, 65, 820–830. [Google Scholar] [CrossRef]
- Lo’pez-Gatiusa, F.; Lo’pez-Be’jarb, M.; Fenechb, M.; Huntera, R.H.F. Ovulation failure and double ovulation in dairy cattle: Risk factors and effects. Theriogenology 2005, 63, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Roth, Z.; Meidan, R. Impaired reproduction in heat- stressed cattle: Basic and applied aspects. Anim. Reprod. Sci. 2000, 60–61, 535–547. [Google Scholar] [CrossRef]
- Sartori, R.; Sartor-Bergfelt, R.; Mertens, S.A.; Guenther, J.N.; Parrish, J.J.; Wiltbank, M.C. Fertilization and early embryonic development in heifers and lactating cows in summer and lactating and dry cows in winter. J. Dairy Sci. 2002, 85, 2803–2812. [Google Scholar] [CrossRef]
- Edwards, J.L.; Saxton, A.M.; Lawrence, J.L.; Payton, R.R.; Dunlap, J.R. Exposure to a physiologically relevant elevated temperature hastens in vitro maturation in bovine oocytes. J. Dairy Sci. 2005, 88, 4326–4333. [Google Scholar] [CrossRef]
- Roth, Z. Physiology and endocrinology symposium: Cellular and molecular mechanisms of heat stress related to bovine ovarian function. J. Anim. Sci. 2015, 93, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.B.; Choi, S.A.; Sim, B.W.; Kim, J.S.; Mun, S.E.; Jeong, P.S.; Yang, H.J.; Lee, Y.; Park, Y.H.; Song, B.S.; et al. Developmental competence of bovine early embryos depends on the coupled response between oxidative and endoplasmic reticulum stress. Biol. Reprod. 2014, 90, 104. [Google Scholar] [CrossRef] [PubMed]
- Putney, D.J.; Drost, M.; Thatcher, W.W. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between Days 1 to 7 post insemination. Theriogenology 1988, 30, 195–209. [Google Scholar] [CrossRef]


| Periods of Heat Stress before Insemination | Large Follicles (Number) | Corpora Lutea (Number) | Unovulated Follicles (Number) | Ovulation Rate (%) |
|---|---|---|---|---|
| THI 9-day | −0.38 | −0.40 | −0.27 | 0.15 |
| p-value | 0.04 * | 0.03 * | 0.21 | 0.46 |
| THI 21-day | −0.32 | −0.35 | −0.32 | 0.14 |
| p-value | 0.09 | 0.07 | 0.14 | 0.50 |
| THI 42-day | −0.24 | −0.27 | −0.30 | 0.16 |
| p-value | 0.21 | 0.16 | 0.16 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratchamak, R.; Ratsiri, T.; Chumchai, R.; Boonkum, W.; Chankitisakul, V. Relationship of the Temperature-Humidity Index (THI) with Ovarian Responses and Embryo Production in Superovulated Thai-Holstein Crossbreds under Tropical Climate Conditions. Vet. Sci. 2021, 8, 270. https://doi.org/10.3390/vetsci8110270
Ratchamak R, Ratsiri T, Chumchai R, Boonkum W, Chankitisakul V. Relationship of the Temperature-Humidity Index (THI) with Ovarian Responses and Embryo Production in Superovulated Thai-Holstein Crossbreds under Tropical Climate Conditions. Veterinary Sciences. 2021; 8(11):270. https://doi.org/10.3390/vetsci8110270
Chicago/Turabian StyleRatchamak, Ruthaiporn, Thanaporn Ratsiri, Rujira Chumchai, Wuttigrai Boonkum, and Vibuntita Chankitisakul. 2021. "Relationship of the Temperature-Humidity Index (THI) with Ovarian Responses and Embryo Production in Superovulated Thai-Holstein Crossbreds under Tropical Climate Conditions" Veterinary Sciences 8, no. 11: 270. https://doi.org/10.3390/vetsci8110270
APA StyleRatchamak, R., Ratsiri, T., Chumchai, R., Boonkum, W., & Chankitisakul, V. (2021). Relationship of the Temperature-Humidity Index (THI) with Ovarian Responses and Embryo Production in Superovulated Thai-Holstein Crossbreds under Tropical Climate Conditions. Veterinary Sciences, 8(11), 270. https://doi.org/10.3390/vetsci8110270






