Clinicopathological and Fecal Proteome Evaluations in 16 Dogs Presenting Chronic Diarrhea Associated with Lymphangiectasia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Intestinal Markers
2.3. Histopathology
2.4. Fecal Proteomics
2.5. Statistical Analysis
3. Results
3.1. CIBDAI Score
3.2. Intestinal Markers
3.3. Histopathology
3.4. Fecal Proteomics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossi, G.; Cerquetella, M.; Antonelli, E.; Pengo, G.; Magi, G.E.; Villanacci, V.; Rostami-Nejad, M.; Spaterna, A.; Bassotti, G. The importance of histologic parameters of lacteal involvement in cases of canine lymphoplasmacytic enteritis. Gastroenterol. Hepatol. Bed Bench 2015, 8, 33–41. [Google Scholar]
- Okanishi, H.; Yoshioka, R.; Kagawa, Y.; Watari, T. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J. Vet. Intern. Med. 2014, 28, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Simmerson, S.M.; Armstrong, P.J.; Wünschmann, A.; Jessen, C.R.; Crews, L.J.; Washabau, R.J. Clinical features, intestinal histopathology, and outcome in protein-losing enteropathy in Yorkshire Terrier dogs. J. Vet. Intern. Med. 2014, 28, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerquetella, M.; Spaterna, A.; Laus, F.; Tesei, B.; Rossi, G.; Antonelli, E.; Villanacci, V.; Bassotti, G. Inflammatory bowel disease in the dog: Differences and similarities with humans. World J. Gastroenterol. 2010, 16, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- García-Sancho, M.; Sainz, A.; Villaescusa, A.; Rodríguez, A.; Rodríguez-Franco, F. White spots on the mucosal surface of the duodenum in dogs with lymphocytic plasmacytic enteritis. J. Vet. Sci. 2011, 12, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, R.M.; Parnell, N.K.; Grützner, N.; Mansell, J.; Berghoff, N.; Schellenberg, S.; Reusch, C.E.; Suchodolski, J.S.; Steiner, J.M. Serum and fecal canine α1-proteinase inhibitor concentrations reflect the severity of intestinal crypt abscesses and/or lacteal dilation in dogs. Vet. J. 2016, 207, 131–139. [Google Scholar] [CrossRef]
- Craven, M.D.; Washabau, R.J. Comparative pathophysiology and management of protein-losing enteropathy. J. Vet. Intern. Med. 2019, 33, 383–402. [Google Scholar] [CrossRef]
- Cerquetella, M.; Rossi, G.; Suchodolski, J.S.; Schmitz, S.S.; Allenspach, K.; Rodríguez-Franco, F.; Furlanello, T.; Gavazza, A.; Marchegiani, A.; Unterer, S.; et al. Proposal for rational antibacterial use in the diagnosis and treatment of dogs with chronic diarrhoea. J. Small Anim. Pract. 2020, 61, 211–215. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Turner, J.R. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008, 181, 683–695. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Goel, R.; Kim, S.; Richards, E.M.; Carter, C.S.; Pepine, C.J.; Raizada, M.K.; Buford, T.W. Intestinal Permeability Biomarker Zonulin is Elevated in Healthy Aging. J. Am. Med. Dir. Assoc. 2017, 18, 810.e1–810.e4. [Google Scholar] [CrossRef]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, M.C.; Madsen, K.; Doyle, J.; Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2009, 58, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caviglia, G.P.; Dughera, F.; Ribaldone, D.G.; Rosso, C.; Abate, M.L.; Pellicano, R.; Bresso, F.; Smedile, A.; Saracco, G.M.; Astegiano, M. Serum zonulin in patients with inflammatory bowel disease: A pilot study. Minerva Med. 2019, 110, 95–100. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Sabater, M.; Ortega, F.; Ricart, W.; Fernández-Real, J.M. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE 2012, 7, e37160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbaro, M.R.; Cremon, C.; Caio, G.; Bellacosa, L.; Volta, U.; Stanghellini, V.; Barbara, G. The role of zonulin in non-celiac gluten sensitivity and irritable bowel syndrome. United Euro Gastroenterol. J. 2015, 3, A87. [Google Scholar]
- Tarko, A.; Suchojad, A.; Michalec, M.; Majcherczyk, M.; Brzozowska, A.; Maruniak-Chudek, I. Zonulin: A Potential Marker of Intestine Injury in Newborns. Dis. Markers 2017, 2017, 2413437. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Caio, G.; Tovoli, F.; De Giorgio, R. Non-celiac gluten sensitivity: Questions still to be answered despite increasing awareness. Cell. Mol. Immunol. 2013, 10, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Vanuytsel, T.; Vermeire, S.; Cleynen, I. The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers 2013, 1, e27321. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Cerquetella, M.; Rossi, G.; Spaterna, A.; Tesei, B.; Gavazza, A.; Marchegiani, A.; Pengo, G.; Scortichini, L.; Felicioli, A.; Sagratini, G.; et al. Proteomics of canine feces from healthy Boxer dogs: A pilot study. Research Communications of the 28th ECVIM-CA Congress. J. Vet. Int. Med. 2019, 33, 1015–1101. [Google Scholar]
- Cerquetella, M.; Rossi, G.; Spaterna, A.; Tesei, B.; Gavazza, A.; Pengo, G.; Pucciarelli, S.; Scortichini, L.; Sagratini, G.; Ricciutelli, M.; et al. Fecal Proteomic Analysis in Healthy Dogs and in Dogs Suffering from Food Responsive Diarrhea. Sci. World J. 2019, 2019, 2742401. [Google Scholar] [CrossRef] [PubMed]
- Cerquetella, M.; Marchegiani, A.; Mangiaterra, S.; Rossi, G.; Gavazza, A.; Tesei, B.; Spaterna, A.; Sagratini, G.; Ricciutelli, M.; Polzonetti, V.; et al. Fecal proteome in clinically healthy dogs and cats: Findings in pooled faeces from 10 cats and 10 dogs. Vet. Rec. Open 2021, 8, e9. [Google Scholar] [CrossRef]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.J.; Evans, R. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef]
- Rossi, G.; Cerquetella, M.; Berardi, S.; Galosi, L.; Mari, S.; Pengo, G.; Gavazza, A. Evaluation of some potential new serological and faecal markers in canine lymphangiectasia: Correlation with mucosal morphology and histological score. ESVP, ECVCP and ESVCP Proceedings 2019. J. Comp. Pathol. 2020, 174, 173. [Google Scholar] [CrossRef]
- Thomas, H. Nondetects and Data Analysis: Statistics for Censored Environmental Data. Vadose Zone J. 2006, 5, 508–509. [Google Scholar]
- Day, M.J.; Bilzer, T.; Mansell, J.; Wilcock, B.; Hall, E.J.; Jergens, A.; Minami, T.; Willard, M.; Washabau, R. World Small Animal Veterinary Association Gastrointestinal Standardization Group. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 2008, 138 (Suppl. S1), S1–S43. [Google Scholar]
- Karp, N.A.; Lilley, K.S. Investigating sample pooling strategies for DIGE experiments to address biological variability. Proteomics 2009, 9, 388–397. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Pucciarelli, S.; Huang, Y.; Ricciutelli, M.; Lambertucci, C.; Volpini, R.; Scuppa, G.; Soverchia, L.; Ubaldi, M.; Polzonetti, V. Biomarkers mapping of neuropathic pain in a nerve chronic constriction injury mice model. Biochimie 2019, 158, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Felici, A.; Ciarrocchi, G.; Pucciarelli, S.; Ricciutelli, M.; Ariani, A.; Polzonetti, V.; Polidori, P. Comparative proteomic analysis of two clam species: Chamelea gallina and Tapes philippinarum. Food Chem. 2017, 219, 223–229. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Steiner, J.M. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J. Vet. Intern. Med. 2018, 32, 1495–1508. [Google Scholar] [CrossRef]
- Kull, P.A.; Hess, R.S.; Craig, L.E.; Saunders, H.M.; Washabau, R.J. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996–1998). J. Am. Vet. Med. Assoc. 2001, 219, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczepaniak, W.S.; Zhang, Y.; Hagerty, S.; Crow, M.T.; Kesari, P.; Garcia, J.G.; Choi, A.M.; Simon, B.A.; McVerry, B.J. Sphingosine 1-phosphate rescues canine LPS-induced acute lung injury and alters systemic inflammatory cytokine production in vivo. Transl. Res. 2008, 152, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauf, S.; de la Fuente, G.; Newbold, C.J.; Salas-Mani, A.; Torre, C.; Abecia, L.; Castrillo, C. Effect of dietary fat to starch content on fecal microbiota composition and activity in dogs. J. Anim. Sci. 2018, 96, 3684–3698. [Google Scholar] [CrossRef] [PubMed]
- Church, R.J.; Watkins, P.B. The transformation in biomarker detection and management of drug-induced liver injury. Liver Int. 2017, 37, 1582–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerou-Ferriani, M.; Allen, R.; Noble, P.M.; German, A.J.; Caldin, M.; Batchelor, D.J. Determining optimal therapy of dogs with chronic enteropathy by measurement of serum citrulline. J. Vet. Intern. Med. 2018, 32, 993–998. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Atherly, T.; Guard, B.; Rossi, G.; Wang, C.; Mosher, C.; Webb, C.; Hill, S.; Ackermann, M.; Sciabarra, P.; et al. Randomized, controlled trial evaluating the effect of multi-strain probiotic on the mucosal microbiota in canine idiopathic inflammatory bowel disease. Gut Microbes 2017, 8, 451–466. [Google Scholar] [CrossRef] [Green Version]
- Atherly, T.; Rossi, G.; White, R.; Seo, Y.J.; Wang, C.; Ackermann, M.; Breuer, M.; Allenspach, K.; Mochel, J.P.; Jergens, A.E. Glucocorticoid and dietary effects on mucosal microbiota in canine inflammatory bowel disease. PLoS ONE 2019, 14, e0226780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef] [Green Version]
- Di Pierro, M.; Lu, R.; Uzzau, S.; Wang, W.; Margaretten, K.; Pazzani, C.; Maimone, F.; Fasano, A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001, 276, 19160–19165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: Living life on the edge of the wall. Am. J. Pathol. 2008, 173, 1243–1252. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113 Pt 24, 4435–4440. [Google Scholar] [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef] [Green Version]
- Ramezani Ahmadi, A.; Sadeghian, M.; Alipour, M.; Ahmadi Taheri, S.; Rahmani, S.; Abbasnezhad, A. The Effects of Probiotic/Synbiotic on Serum Level of Zonulin as a Biomarker of Intestinal Permeability: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2020, 49, 1222–1231. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, F1000 Faculty Rev-69. [Google Scholar] [CrossRef]
- Schmidt, E.; Eckersall, P. Acute Phase Proteins as Markers of Infectious Diseases in small Animals/Proteini Akutne Faze Kao Markeri Infektivnih Bolesti Malih Životinja. Acta Vet. 2015, 65, 149–161. [Google Scholar] [CrossRef]
- Henze, A.; Homann, T.; Serteser, M.; Can, O.; Sezgin, O.; Coskun, A.; Unsal, I.; Schweigert, F.J.; Ozpinar, A. Post-translational modifications of transthyretin affect the triiodonine-binding potential. J. Cell. Mol. Med. 2015, 19, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Obici, L.; Suhr, O.B. Diagnosis and treatment of gastrointestinal dysfunction in hereditary TTR amyloidosis. Clin. Auton. Res. 2019, 29 (Suppl. S1), 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.; Thomsson, K.A.; Hansson, G.C. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J. Proteome Res. 2009, 8, 3549–3557. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Iijima, S.; Kobayashi, K.; Yoshida, T.; Brown, W.R.; Hibi, T.; Oshima, A.; Morikawa, M. Human IgGFc binding protein (FcgammaBP) in colonic epithelial cells exhibits mucin-like structure. J. Biol. Chem. 1997, 272, 15232–15241. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Blaser, M.J.; Brown, W.R. Identification of a unique IgG Fc binding site in human intestinal epithelium. J. Immunol. 1989, 143, 2567–2574. [Google Scholar]
- Gomis-Rüth, F.X.; Gómez-Ortiz, M.; Vendrell, J.; Ventura, S.; Bode, W.; Huber, R.; Avilés, F.X. Cutting at the right place—The importance of selective limited proteolysis in the activation of proproteinase E. Eur. J. Biochem. 1998, 251, 839–844. [Google Scholar] [CrossRef]
- Szabó, A.; Pilsak, C.; Bence, M.; Witt, H.; Sahin-Tóth, M. Complex Formation of Human Proelastases with Procarboxypeptidases A1 and A2. J. Biol. Chem. 2016, 291, 17706–17716. [Google Scholar] [CrossRef] [Green Version]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Alpers, D.H.; Tedesco, F.J. The possible role of pancreatic proteases in the turnover of intestinal brush border proteins. Biochim. Biophys. Acta BBA Biomembr. 1975, 401, 28–40. [Google Scholar] [CrossRef]
- Ahn, S.B.; Sharma, S.; Mohamedali, A.; Mahboob, S.; Redmond, W.J.; Pascovici, D.; Wu, J.X.; Zaw, T.; Adhikari, S.; Vaibhav, V.; et al. Potential early clinical stage colorectal cancer diagnosis using a proteomics blood test panel. Clin. Proteom. 2019, 16, 34. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.L.; Sun, Y.C.; Chang, P.Y.; Tsao, K.C.; Sun, C.F.; Wu, J.T. Establishment of ELISA on 384-well microplate for AFP, CEA, CA 19-9, CA 15-3, CA 125, and PSA-ACT: Higher sensitivity and lower reagent cost. J. Clin. Lab. Anal. 2003, 17, 241–246. [Google Scholar] [CrossRef]
- Geyer, P.E.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017, 13, 942. [Google Scholar] [CrossRef]
| Case (ILG) Mean Value ± DS | Control (CG) Mean Value ± DS | * p | |
|---|---|---|---|
| CIBDAI | 12.31 ± 2.75 | 1 ± 0.81 | 0.007 |
| Serum | |||
| Albumin | 17.32 ± 4.23 | 32.14 ± 3.84 | <0.0001 |
| Cholesterol | 110.75 ± 39.89 | 212.28 ± 18.81 | <0.0001 |
| CRP | 26.83 ± 16.98 | 1.21 ± 0.6 | 0.0009 |
| LPS | 0.52 ± 0.30 | 0.1 ± 0.05 | 0.0001 |
| cCK18 | 365.81 ± 104.92 | 148.71 ± 74.68 | 0.0001 |
| Citrulline | 4.56 ± 1.59 | 2.9 ± 3.10 | 0.14 |
| Zonulin | 77.27 ± 32.38 | 38.91 ± 19.10 | 0.0181 |
| Feces | |||
| Zonulin | 523.20 ± 156.42 | 141.56 ± 72.67 | <0.0001 |
| Biopsies | |||
| Histological score | 13.5 ± 2.25 | 1.85 ± 1.21 | <0.0001 |
| Villous height | 623.31 ± 17.14 | 809.57 ± 26.78 | <0.0001 |
| Villous width | 277.25 ± 11.03 | 151.42 ± 11.68 | <0.0001 |
| Villous h/w ratio | 2.25 ± 0.11 | 5.37 ± 0.45 | <0.0001 |
| Lymphatic vessels (chyliferous ducts) height | 551.18 ± 24.03 | 658.71 ± 33.50 | <0.0001 |
| Lymphatic vessels (chyliferous ducts) width | 107.50 ± 42.68 | 23.85 ±2.47 | <0.0001 |
| Lymphatic vessels (chyliferous ducts) h/w ratio | 5.95 ± 2.32 | 27.79 ± 3.56 | <0.0001 |
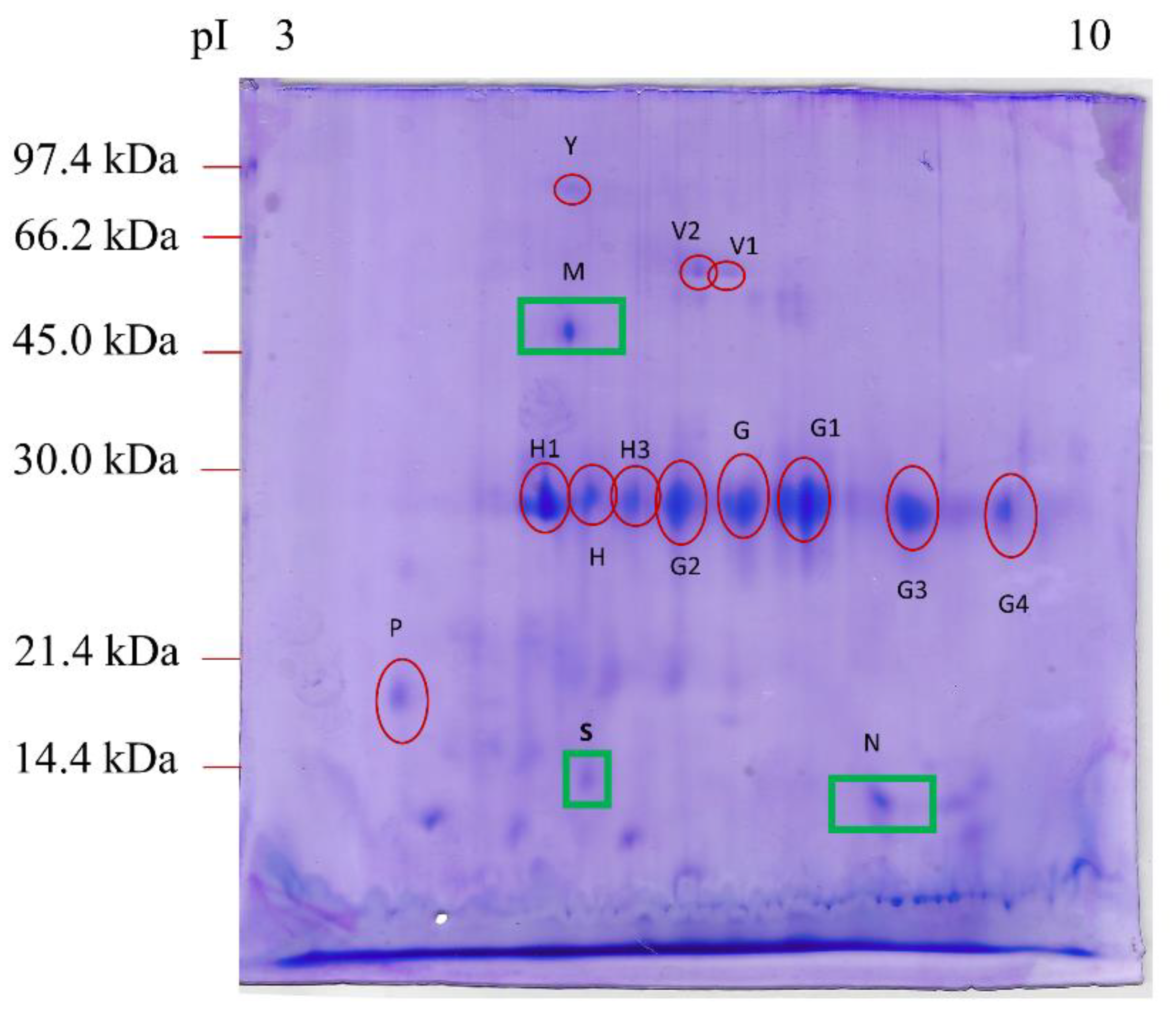
| SPOT ID a | Protein Name b | Score c | Mr (kDa)/pI d | Mr (kDa)/pI e | Sequence | NQ e (×103) Healthy * | NQ (×103) IL |
|---|---|---|---|---|---|---|---|
| Y | Serum albumin isoform X1 (Canis lupus familiaris) | 56 | 68.6/5.51 | 72 ± 4.6/5.3 ± 0.06 | LVAAAQAALV | 115 ± 47 | 71 ± 42 |
| V1 | Alkaline phosphatase (Canis lupus familiaris) | 125 | 68.6/6.47 | 59 ± 1.3/6.6 ± 0.23 | ANYQTIGVSAAAR | 174 ± 108 | 47 ± 26 |
| V2 | Alkaline phosphatase (Canis lupus familiaris) | 117 | 48.3/6.15 | 58 ± 1.6/6.6 ± 0.15 | ANYQTIGVSAAAR | 148 ± 51 | 35 ± 18 |
| H | Chymotrypsin-C-like (Canis lupus dingo) | 49 | 29.1/5.33 | 29 ± 1.0/5.6 ± 0.11 | LAEPVQLSDTIK | 290 ± 93 | 264 ± 27 |
| H1 | Elastase-3B, proteinase E (Canis lupus familiaris) | 40 | 28.8/5.27 | 29 ± 0.8/5.2 ± 0.11 | VSAFNDWIEEVMSSH | 585 ± 139 | 616 ± 351 |
| H3 | Immunoglobulin kappa light chain (Felis catus) | 41 | 26.7/6.10 | 29 ± 1.1/6.3 ± 0.21 | FSGSGSGTDFTLR | 374 ± 248 | 214 ± 32 |
| G | Immunoglobulin λ-1 light chain (Canis lupus familiaris) | 34 | 25.2/6.88 | 29 ± 0.9/7.1 ± 0.5 | KGTHVTVLGQPK | 644 ± 327 | 570 ± 59 |
| G1 | Immunoglobulin λ-1 light chain (Felis catus) | 39 | 27.8/8.17 | 29 ± 1.2/7.6 ± 0.6 | QSNNKYAASSYL | 555 ± 204 | 232 ± 142 |
| G2 | Immunoglobulin λ-light chain VLJ region (Homo sapiens) | 42 | 29.0/8.14 | 29 ± 1.0/6.6 ± 0.3 | EFGGGTKLTVLGQPK | 642 ± 439 | 321 ± 92 |
| G3 | Immunoglobulin λ-light chain VLJ region (Homo sapiens) | 30 | 29.0/8.14 | 27 ± 1.2/8.4 ± 0.5 | EFGGGTKLTVLGQPK | 395 ± 85 | 483 ± 64 |
| G4 | Immunoglobulin λ-light chain VLJ region (Homo sapiens) | 40 | 29.0/8.14 | 27.7/8.9 | QSNNKYAASSYL | 285 ± 17 | 334 ± 271 |
| M | Fc fragment of IgG binding protein (Homo sapiens) | 23 | 572/5.1 | 49.1 ± 2.2/5.3 ± 0.06 | VLVENEHR | n.d. | 148 ± 25 |
| N | Transthyretin (Canis lupus familiaris) | 31 | 15.8/6.4 | 14.2 ± 1.2/7.8 ± 0.1 | GSPAVNVAVK | n.d. | 62 ± 58 |
| P | Deleted in malignant brain tumors 1 protein isoform X1 (Canis lupus dingo) | 55 | 26/5.2 | 19.8 ± 0.15/4.2 ± 0.3 | FGQGSGPIVLDDVR | 196.5 ± 53.2 | 132 ± 39 |
| S | Proproteinase E (Bos taurus) | 43 | 27.3/5.1 | 14 ± 3.4/5.8 ± 0 | LYTGGPLPDK | n.d. | 103 ± 78 |
| § * Serum albumin isoform X1 (Canis lupus familiaris) | 68.6/5.51 | 63 ± 3.0/5.8 ± 0.15 | ADFAEISK | 79 ± 13 | n.d. | ||
| § * Nuclear pore membrane glycoprotein-210 (Canis lupus familiaris) | 192.4/6.30 | 19.6 ± 1.5/5.8 ± 0.14 | TALLVTASISGSHAPR | 227 ± 69 | n.d. | ||
| § * Cytosol aminopeptidase (Canis lupus familiaris) | 56.2/8.03 | 21.0 ± 1.3/5.7 ± 0.07 | EILNISGPPLK | 125 ± 32 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, G.; Gavazza, A.; Vincenzetti, S.; Mangiaterra, S.; Galosi, L.; Marchegiani, A.; Pengo, G.; Sagratini, G.; Ricciutelli, M.; Cerquetella, M. Clinicopathological and Fecal Proteome Evaluations in 16 Dogs Presenting Chronic Diarrhea Associated with Lymphangiectasia. Vet. Sci. 2021, 8, 242. https://doi.org/10.3390/vetsci8100242
Rossi G, Gavazza A, Vincenzetti S, Mangiaterra S, Galosi L, Marchegiani A, Pengo G, Sagratini G, Ricciutelli M, Cerquetella M. Clinicopathological and Fecal Proteome Evaluations in 16 Dogs Presenting Chronic Diarrhea Associated with Lymphangiectasia. Veterinary Sciences. 2021; 8(10):242. https://doi.org/10.3390/vetsci8100242
Chicago/Turabian StyleRossi, Giacomo, Alessandra Gavazza, Silvia Vincenzetti, Sara Mangiaterra, Livio Galosi, Andrea Marchegiani, Graziano Pengo, Gianni Sagratini, Massimo Ricciutelli, and Matteo Cerquetella. 2021. "Clinicopathological and Fecal Proteome Evaluations in 16 Dogs Presenting Chronic Diarrhea Associated with Lymphangiectasia" Veterinary Sciences 8, no. 10: 242. https://doi.org/10.3390/vetsci8100242
APA StyleRossi, G., Gavazza, A., Vincenzetti, S., Mangiaterra, S., Galosi, L., Marchegiani, A., Pengo, G., Sagratini, G., Ricciutelli, M., & Cerquetella, M. (2021). Clinicopathological and Fecal Proteome Evaluations in 16 Dogs Presenting Chronic Diarrhea Associated with Lymphangiectasia. Veterinary Sciences, 8(10), 242. https://doi.org/10.3390/vetsci8100242







