In Situ Hybridization of PRRSV-1 Combined with Digital Image Analysis in Lung Tissues of Pigs Challenged with PRRSV-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics Approval
2.2. Sample Collection
2.3. Tissue Processing and Routine Histology
2.4. RNA-Based In Situ Hybridization—RNAscope
2.5. Section Scanning and Software Analysis
2.6. PRRSV qRT-PCR
2.7. Statistical Analyses
3. Results
3.1. Histopathology and In Situ Hybridization
3.2. qRT-PCR
3.3. Digital Image Analysis
3.4. Beta Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holtkamp, D.J.; Kliebenstein, J.B.; Neumann, E.J.; Zimmerman, J.J.; Rotto, H.F.; Yoder, T.K.; Wang, C.; Yeske, P.E.; Mowrer, C.L.; Haley, C.A. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. 2013, 21, 72–84. [Google Scholar]
- Lunney, J.K.; Benfield, D.A.; Rowland, R.R. Porcine reproductive and respiratory syndrome virus: An update on an emerging and re-emerging viral disease of swine. Virus Res. 2010, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carvajal, J.M.; Rodríguez-Gómez, I.M.; Ruedas-Torres, I.; Larenas-Muñoz, F.; Díaz, I.; Revilla, C.; Mateu, E.; Domínguez, J.; Martín-Valls, G.; Barranco, I.; et al. Activation of pro- and anti-inflammatory responses in lung tissue injury during the acute phase of PRRSV-1 infection with the virulent strain Lena. Vet. Microbiol. 2020, 246, 108744. [Google Scholar] [CrossRef] [PubMed]
- Balka, G.; Podgórska, K.; Brar, M.S.; Bálint, Á.; Cadar, D.; Celer, V.; Dénes, L.; Dirbakova, Z.; Jedryczko, A.; Márton, L.; et al. Genetic diversity of PRRSV 1 in Central Eastern Europe in 1994–2014: Origin and evolution of the virus in the region. Sci. Rep. 2018, 8, 7811. [Google Scholar] [CrossRef]
- Shi, M.; Lam, T.T.; Hon, C.C.; Hui, R.K.; Faaberg, K.S.; Wennblom, T.; Murtaugh, M.P.; Stadejek, T.; Leung, F.C. Molecular epidemiology of PRRSV: A phylogenetic perspective. Virus Res. 2010, 154, 7–17. [Google Scholar] [CrossRef]
- Bordet, E.; Maisonnasse, P.; Renson, P.; Bouguyon, E.; Crisci, E.; Tiret, M.; Descamps, D.; Bernelin-Cottet, C.; Urien, C.; Lefèvre, F.; et al. Porcine Alveolar Macrophage-like cells are pro-inflammatory Pulmonary Intravascular Macrophages that produce large titers of Porcine Reproductive and Respiratory Syndrome Virus. Sci. Rep. 2018, 8, 10172. [Google Scholar] [CrossRef]
- Gómez-Laguna, J.; Salguero, F.J.; Barranco, I.; Pallarés, F.J.; Rodríguez-Gómez, I.M.; Bernabé, A.; Carrasco, L. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J. Comp. Pathol. 2010, 142, 51–60. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Nauwynck, H.J.; Van Gorp, H.; Vanhee, M.; Karniychuk, U.; Geldhof, M.; Cao, A.; Verbeeck, M.; Van Breedam, W. Micro-dissecting the pathogenesis and immune response of PRRSV infection paves the way for more efficient PRRSV vaccines. Transbound. Emerg. Dis. 2012, 59, 50–54. [Google Scholar] [CrossRef]
- Burkard, C.; Opriessnig, T.; Mileham, A.J.; Stadejek, T.; Ait-Ali, T.; Lillico, S.G.; Whitelaw, C.B.A.; Archibald, A.L. Pigs Lacking the Scavenger Receptor Cysteine-Rich Domain 5 of CD163 Are Resistant to Porcine Reproductive and Respiratory Syndrome Virus 1 Infection. J. Virol. 2018, 92, e00415-18. [Google Scholar] [CrossRef] [Green Version]
- Whitworth, K.M.; Rowland, R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef]
- Rossow, K.D. Porcine reproductive and respiratory syndrome. Vet. Pathol. 1998, 35, 1–20. [Google Scholar] [CrossRef]
- Schulze, M.; Revilla-Fernández, S.; Schmoll, F.; Grossfeld, R.; Griessler, A. Effects on boar semen quality after infection with porcine reproductive and respiratory syndrome virus: A case report. Acta. Vet. Scand. 2013, 55, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balka, G.; Hornyák, A.; Bálint, A.; Benyeda, Z.; Rusvai, M. Development of a one-step real-time quantitative PCR assay based on primer-probe energy transfer for the detection of porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2009, 158, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Toplak, I.; Rihtarič, D.; Hostnik, P.; Grom, J.; Stukelj, M.; Valenčak, Z. Identification of a genetically diverse sequence of porcine reproductive and respiratory syndrome virus in Slovenia and the impact on the sensitivity of four molecular tests. J. Virol. Methods 2012, 179, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.J.; Dee, S.A.; Holtkamp, D.J.; Murtaugh, M.P.; Stadejek, T.; Stevenson, G.W.; Torremorell, M.; Yang, H.; Zhang, J. Porcine Reproductive and Respiratory Syndrome Viruses (Porcine Arteriviruses). In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; pp. 696–697. [Google Scholar]
- Maes, R.K.; Langohr, I.M.; Wise, A.G.; Smedley, R.C.; Thaiwong, T.; Kiupel, M. Beyond H&E: Integration of nucleic acid-based analyses into diagnostic pathology. Vet. Pathol. 2014, 51, 238–256. [Google Scholar] [PubMed] [Green Version]
- Larochelle, R.; Mardassi, H.; Dea, S.; Magar, R. Detection of porcine reproductive and respiratory syndrome virus in cell cultures and formalin-fixed tissues by in situ hybridization using a digoxigenin-labeled probe. J. Vet. Diagn. Investig. 1996, 8, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingham, V.; McIlreavey, L.; Greene, C.; O’Doherty, E.; Clarke, R.; Craig, S.; Salto-Tellez, M.; McQuaid, S.; Lewis, C.; James, J. RNAscope in situ hybridization confirms mRNA integrity in formalin-fixed, paraffin-embedded cancer tissue samples. Oncotarget 2017, 8, 93392–93403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, M.X.; Su, N.; Wang, L.C.; Wu, X.; Bui, S.; Nielsen, A.; Vo, H.T.; Nguyen, N.; Luo, Y.; et al. RNAscope for in situ detection of transcriptionally active human papillomavirus in head and neck squamous cell carcinoma. J. Vis. Exp. 2014, 85, 51426. [Google Scholar] [CrossRef] [Green Version]
- Resende, T.P.; Marthaler, D.G.; Vannucci, F.A. A novel RNA-based in situ hybridization to detect Seneca Valley virus in neonatal piglets and sows affected with vesicular disease. PLoS ONE 2017, 12, e0173190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boros, Á.; Albert, M.; Pankovics, P.; Bíró, H.; Pesavento, P.A.; Phan, T.G.; Delwart, E.; Reuter, G. Outbreaks of Neuroinvasive Astrovirus Associated with Encephalomyelitis, Weakness, and Paralysis among Weaned Pigs, Hungary. Emerg. Infect. Dis. 2017, 23, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Arruda, B.; Piñeyro, P.; Derscheid, R.; Hause, B.; Byers, E.; Dion, K.; Long, D.; Sievers, C.; Tangen, J.; Williams, T.; et al. PCV3-associated disease in the United States swine herd. Emerg. Microbes Infect. 2019, 8, 684–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dénes, L.; Ruedas-Torres, I.; Szilasi, A.; Balka, G. Detection and localization of atypical porcine pestivirus in the testicles of naturally infected, CT-affected piglets. Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Stålhammar, G.; Robertson, S.; Wedlund, L.; Lippert, M.; Rantalainen, M.; Bergh, J.; Hartman, J. Digital image analysis of Ki67 in hot spots is superior to both manual Ki67 and mitotic counts in breast cancer. Histopathology 2018, 72, 974–989. [Google Scholar] [CrossRef]
- Sinn, L.J.; Klingler, E.; Lamp, B.; Brunthaler, R.; Weissenböck, H.; Rümenapf, T.; Ladinig, A. Emergence of a virulent porcine reproductive and respiratory syndrome virus (PRRSV) 1 strain in Lower Austria. Porcine Health Manag. 2016, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Balka, G.; Ladinig, A.; Ritzmann, M.; Saalmüller, A.; Gerner, W.; Käser, T.; Jakab, C.; Rusvai, M.; Weißenböck, H. Immunohistochemical characterization of type II pneumocyte proliferation after challenge with type I porcine reproductive and respiratory syndrome virus. J. Comp. Pathol. 2013, 149, 322–330. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Egli, C.; Thür, B.; Liu, L.; Hofmann, M.A. Quantitative TaqMan RT-PCR for the detection and differentiation of European and North American strains of porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2001, 98, 63–75. [Google Scholar] [CrossRef]
- Staroscik, A. Science Primer. Available online: http://scienceprimer.com/copy-number-calculator-for-realtime-pcr (accessed on 24 March 2021).
- Han, K.; Seo, H.W.; Oh, Y.; Kang, I.; Park, C.; Kang, S.H.; Kim, S.H.; Lee, B.H.; Kwon, B.; Chae, C. Evaluation of monoclonal antibody-based immunohistochemistry for the detection of European and North American Porcine reproductive and respiratory syndrome virus and a comparison with in situ hybridization and reverse transcription polymerase chain reaction. J. Vet. Diagn. Investig. 2012, 24, 719–724. [Google Scholar]
- Sánchez-Carvajal, J.M.; Ruedas-Torres, I.; Carrasco, L.; Pallarés, F.J.; Mateu, E.; Rodríguez-Gómez, I.M.; Gómez-Laguna, J. Activation of regulated cell death in the lung of piglets infected with virulent PRRSV-1 Lena strain occurs earlier and mediated by cleaved Caspase-8. Vet. Res. 2021, 52, 12. [Google Scholar] [CrossRef] [PubMed]
- Van Alstine, W.G.; Popielarczyk, M.; Albregts, S.R. Effect of formalin fixation on the immunohistochemical detection of PRRS virus antigen in experimentally and naturally infected pigs. J. Vet. Diagn. Investig. 2002, 14, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trang, N.T.; Hirai, T.; Ngan, P.H.; Lan, N.T.; Fuke, N.; Toyama, K.; Yamamoto, T.; Yamaguchi, R. Enhanced detection of Porcine reproductive and respiratory syndrome virus in fixed tissues by in situ hybridization following tyramide signal amplification. J. Vet. Diagn. Investig. 2015, 27, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.M.; Li, J.N.; Wang, G.; He, X.J.; Weng, C.J. Detection of porcine reproductive and respiratory syndrome virus RNA using RNAscope in situ hybridization. Chin. J. Prev. Vet. Med. 2018, 40, 596–600. [Google Scholar]
- Song, J.; Gao, P.; Kong, C.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. The nsp2 Hypervariable Region of Porcine Reproductive and Respiratory Syndrome Virus Strain JXwn06 Is Associated with Viral Cellular Tropism to Primary Porcine Alveolar Macrophages. J. Virol. 2019, 93, e01436-19. [Google Scholar] [CrossRef]
- Acs, B.; Salgado, R.; Hartman, J. What do we still need to learn on digitally assessed biomarkers? EBioMedicine 2021, 70, 103520. [Google Scholar] [CrossRef]
- Sun, P.; He, J.; Chao, X.; Chen, K.; Xu, Y.; Huang, Q.; Yun, J.; Li, M.; Luo, R.; Kuang, J.; et al. Computational Tumor-Infiltrating Lymphocyte Assessment Method Comparable with Visual Reporting Guidelines for Triple-Negative Breast Cancer. EBioMedicine 2021, 70, 103492. [Google Scholar] [CrossRef]
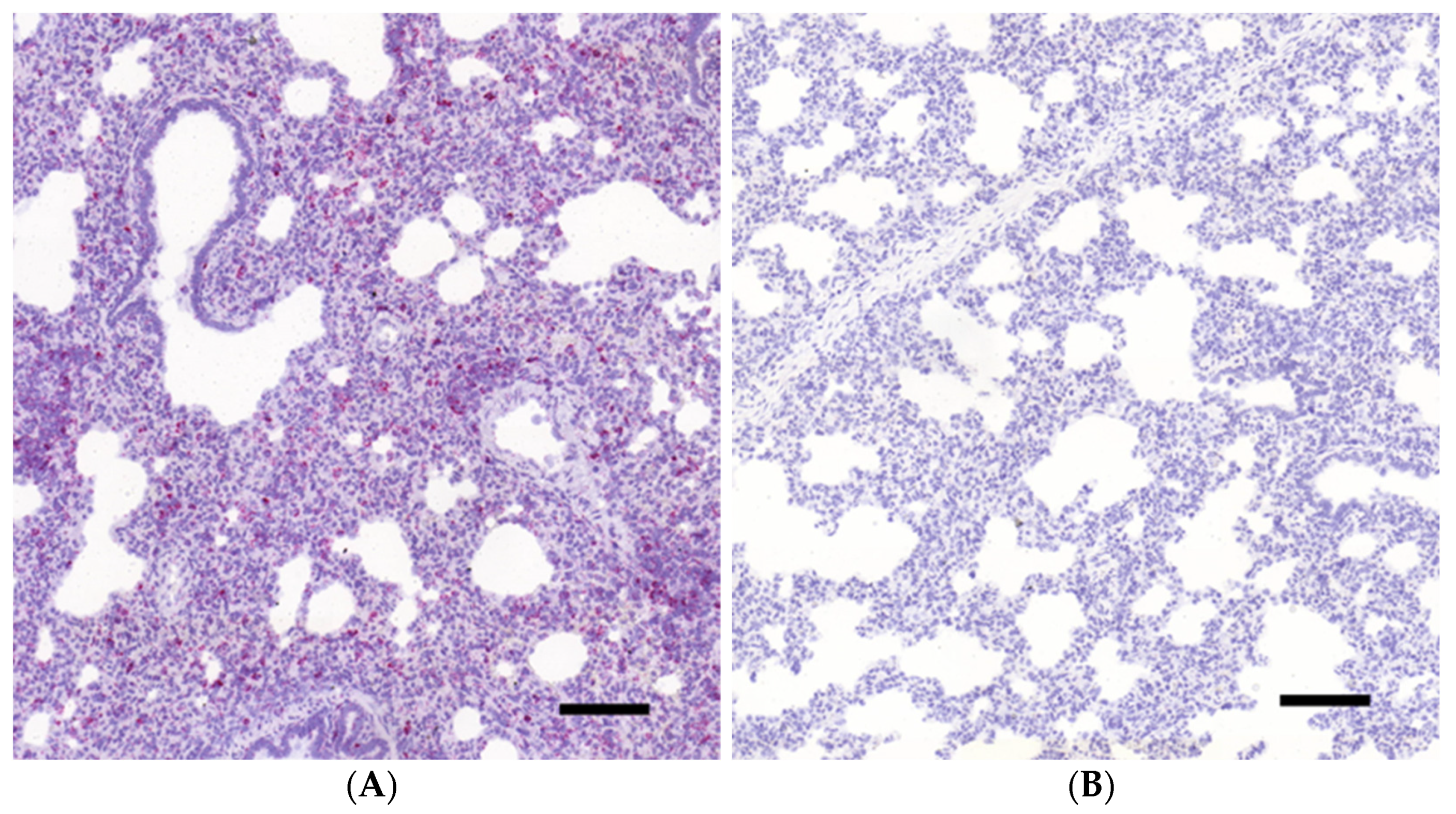
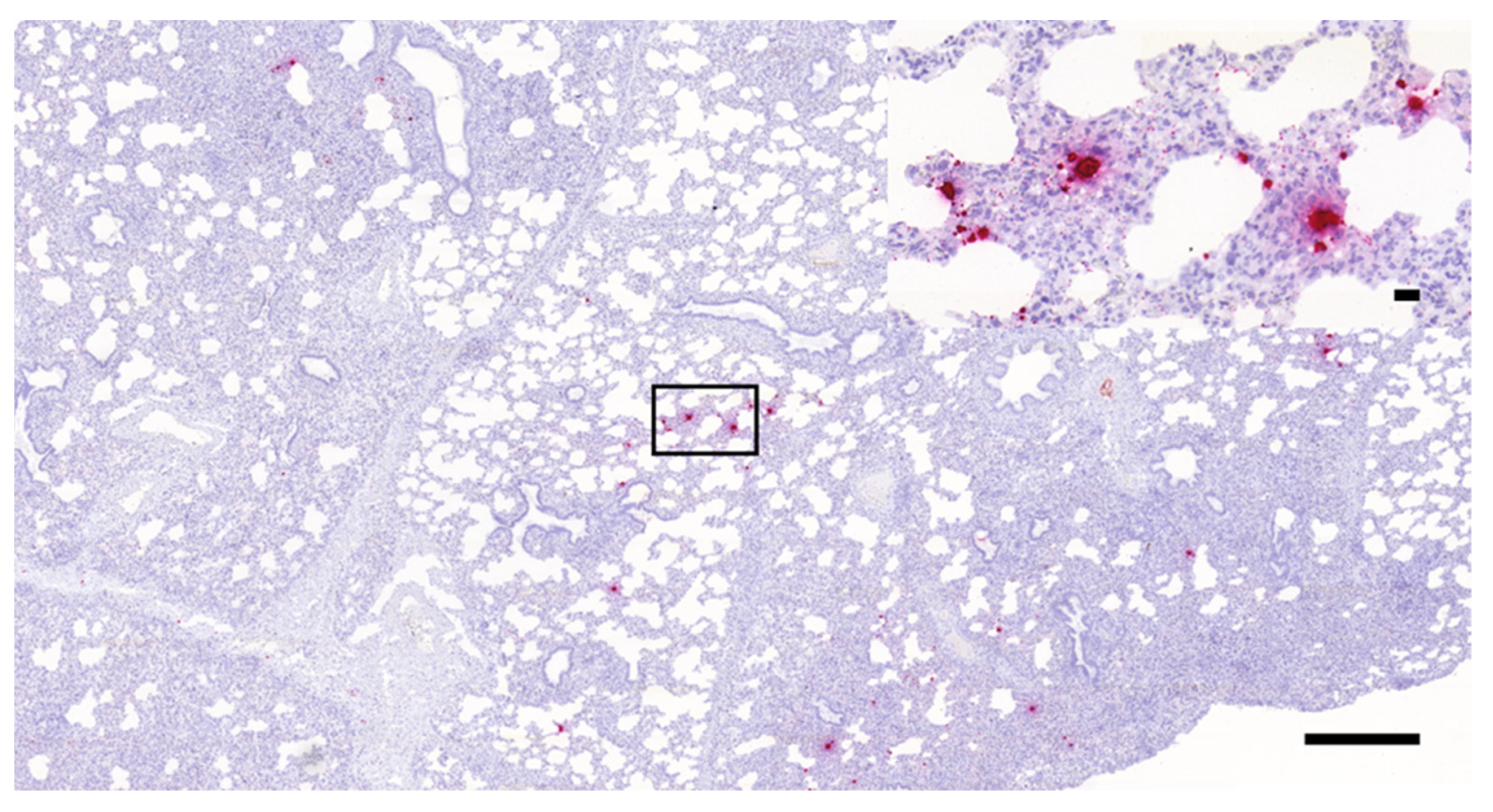
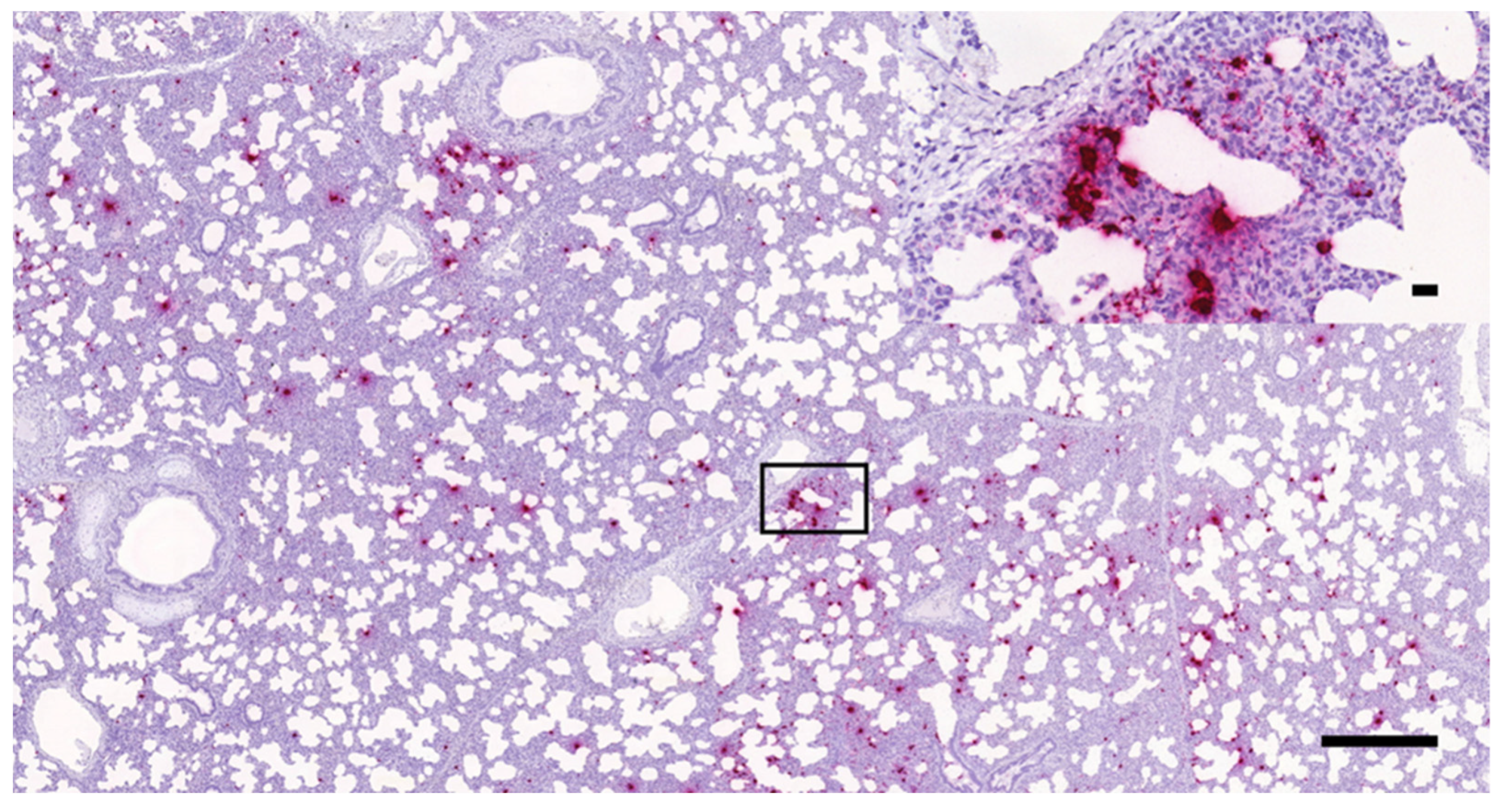
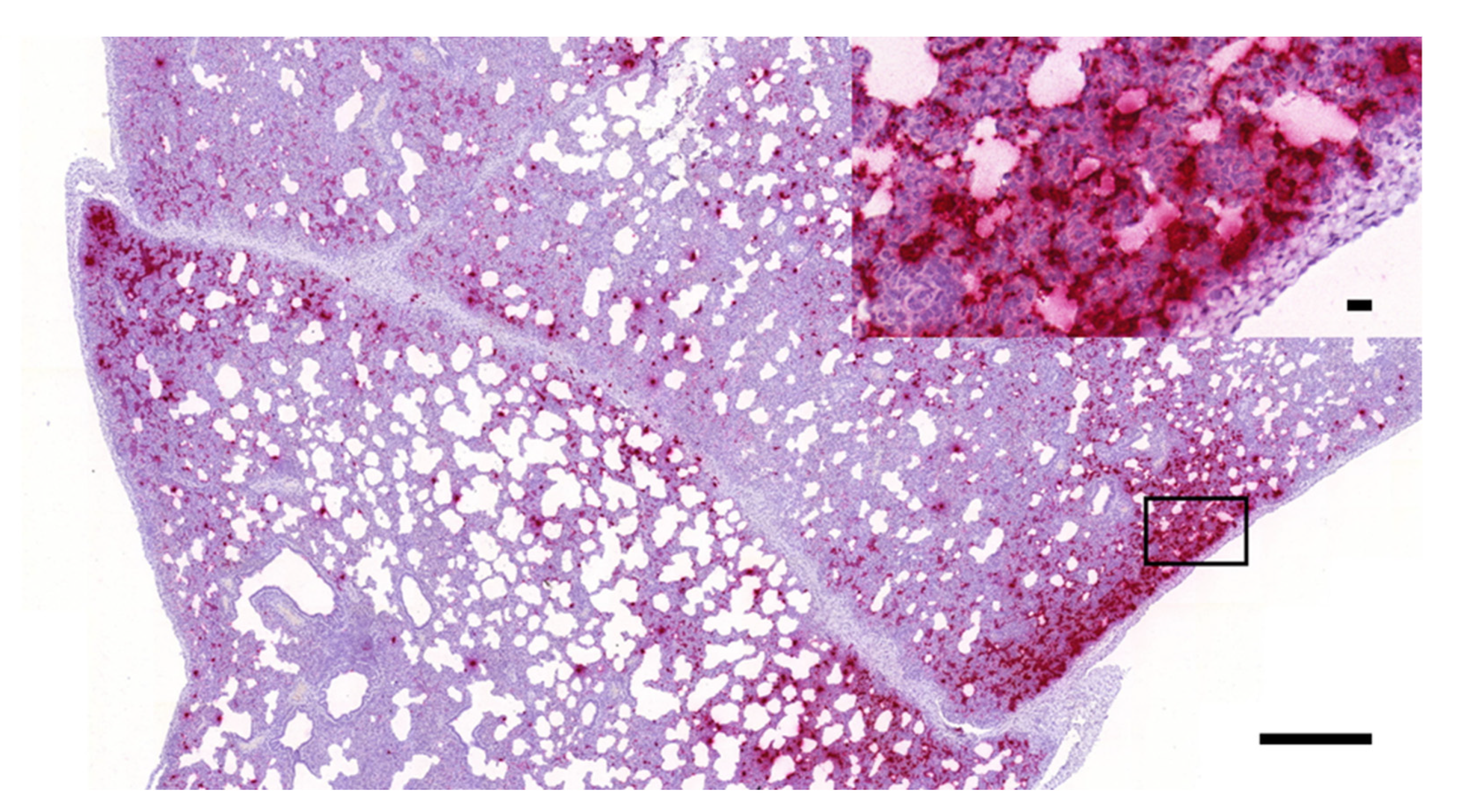
| Animal No. | Proportion Of Infected Cells (%) | log10 Genome Copies/g | L. Med. Severity a | Overall Severity b |
|---|---|---|---|---|
| 19 | 0.00 | 0.00 | 0 | 0 |
| 9 | 0.00 | 0.00 | 0 | 0 |
| 7 | 0.00 | 0.00 | 0 | 0 |
| 4 | 0.00 | 0.00 | 0 | 0 |
| 2 | 0.00 | 0.00 | 0 | 0 |
| 23 | 0.16 | 10.09 | 17 | 68 |
| 22 | 0.19 | 10.95 | 20 | 143 |
| 14 | 0.20 | 8.79 | 10 | 72 |
| 25 | 0.41 | 9.80 | 12 | 67 |
| 21 | 0.72 | 9.71 | 17 | 101 |
| 20 | 1.04 | 10.41 | 16 | 77 |
| 17 | 1.04 | 8.18 | 12 | 56 |
| 5 | 1.86 | 10.65 | 26 | 180 |
| 6 | 1.88 | 10.68 | 19 | 157 |
| 12 | 2.73 | 11.46 | 20 | 135 |
| 3 | 2.93 | 10.98 | 17 | 101 |
| 18 | 3.86 | 10.73 | 23 | 139 |
| 13 | 3.97 | 10.66 | 12 | 65 |
| 1 | 4.24 | 11.25 | 17 | 107 |
| 15 | 4.29 | 10.91 | 21 | 115 |
| 11 | 5.60 | 11.00 | 11 | 110 |
| 24 | 5.79 | 10.70 | 25 | 144 |
| 8 | 8.25 | 10.93 | 19 | 110 |
| 10 | 9.02 | 10.99 | 20 | 136 |
| 16 | 26.70 | 11.68 | 21 | 137 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dénes, L.; Horváth, D.G.; Duran, O.; Ratkhjen, P.H.; Kraft, C.; Acs, B.; Szász, A.M.; Rümenapf, T.; Papp, M.; Ladinig, A.; et al. In Situ Hybridization of PRRSV-1 Combined with Digital Image Analysis in Lung Tissues of Pigs Challenged with PRRSV-1. Vet. Sci. 2021, 8, 235. https://doi.org/10.3390/vetsci8100235
Dénes L, Horváth DG, Duran O, Ratkhjen PH, Kraft C, Acs B, Szász AM, Rümenapf T, Papp M, Ladinig A, et al. In Situ Hybridization of PRRSV-1 Combined with Digital Image Analysis in Lung Tissues of Pigs Challenged with PRRSV-1. Veterinary Sciences. 2021; 8(10):235. https://doi.org/10.3390/vetsci8100235
Chicago/Turabian StyleDénes, Lilla, Dávid G. Horváth, Oliver Duran, Poul H. Ratkhjen, Christian Kraft, Balazs Acs, Attila M. Szász, Till Rümenapf, Marton Papp, Andrea Ladinig, and et al. 2021. "In Situ Hybridization of PRRSV-1 Combined with Digital Image Analysis in Lung Tissues of Pigs Challenged with PRRSV-1" Veterinary Sciences 8, no. 10: 235. https://doi.org/10.3390/vetsci8100235
APA StyleDénes, L., Horváth, D. G., Duran, O., Ratkhjen, P. H., Kraft, C., Acs, B., Szász, A. M., Rümenapf, T., Papp, M., Ladinig, A., & Balka, G. (2021). In Situ Hybridization of PRRSV-1 Combined with Digital Image Analysis in Lung Tissues of Pigs Challenged with PRRSV-1. Veterinary Sciences, 8(10), 235. https://doi.org/10.3390/vetsci8100235






