Evaluation of Infrared Thermography for the Detection of Footrot and White Line Disease Lesions in Dairy Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm and Animal Selection
2.2. Thermal Imaging and Data Recording
2.3. Statistical Analyses
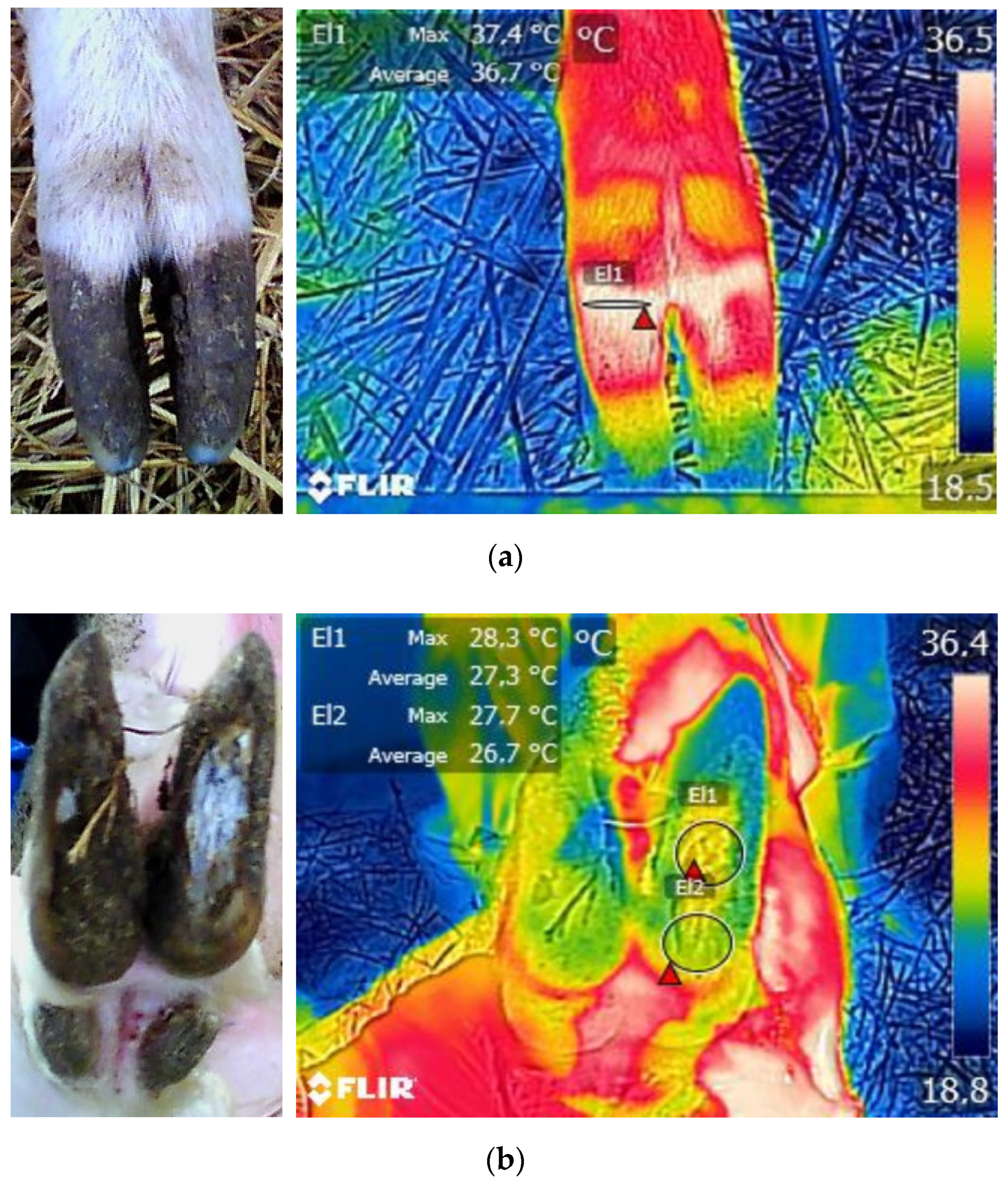
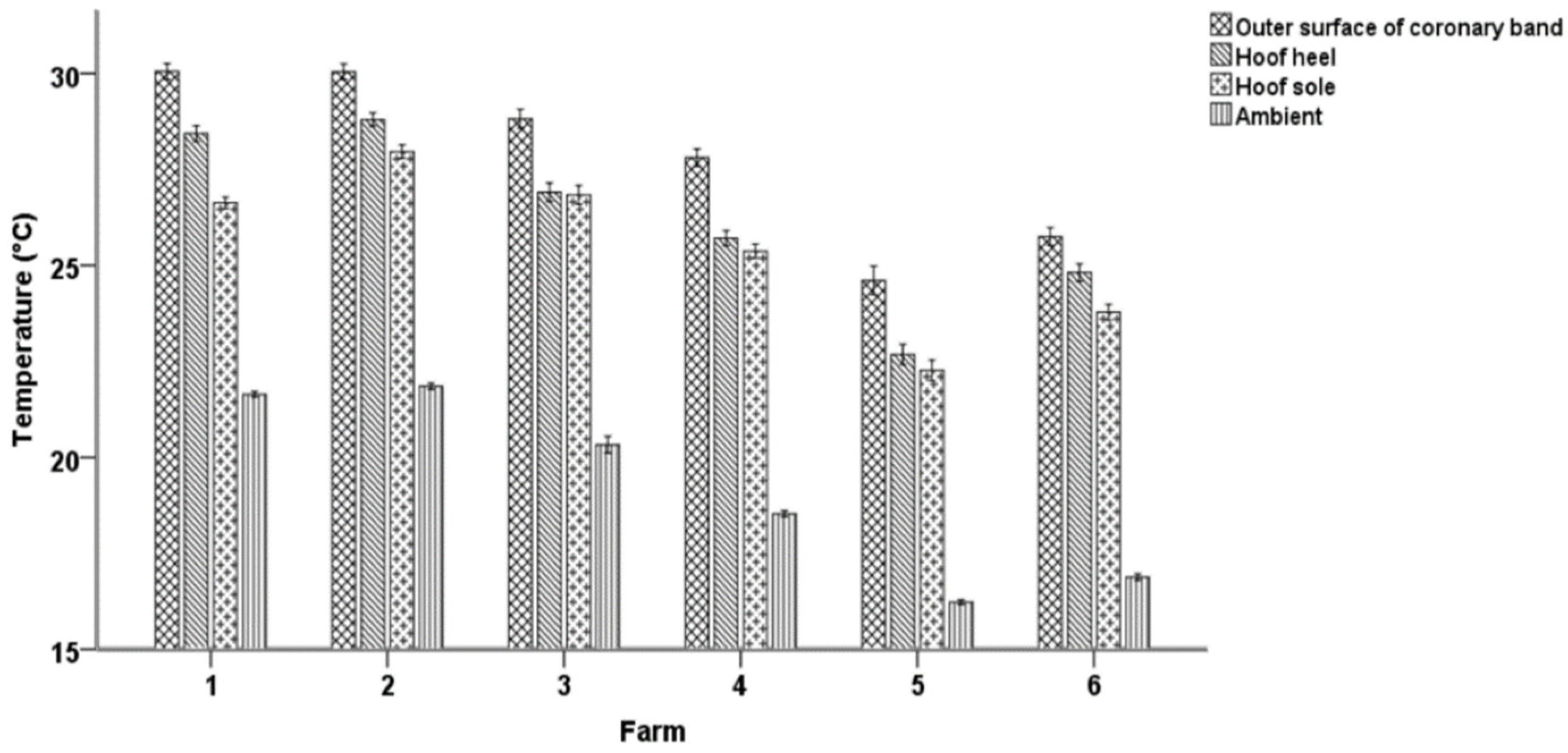
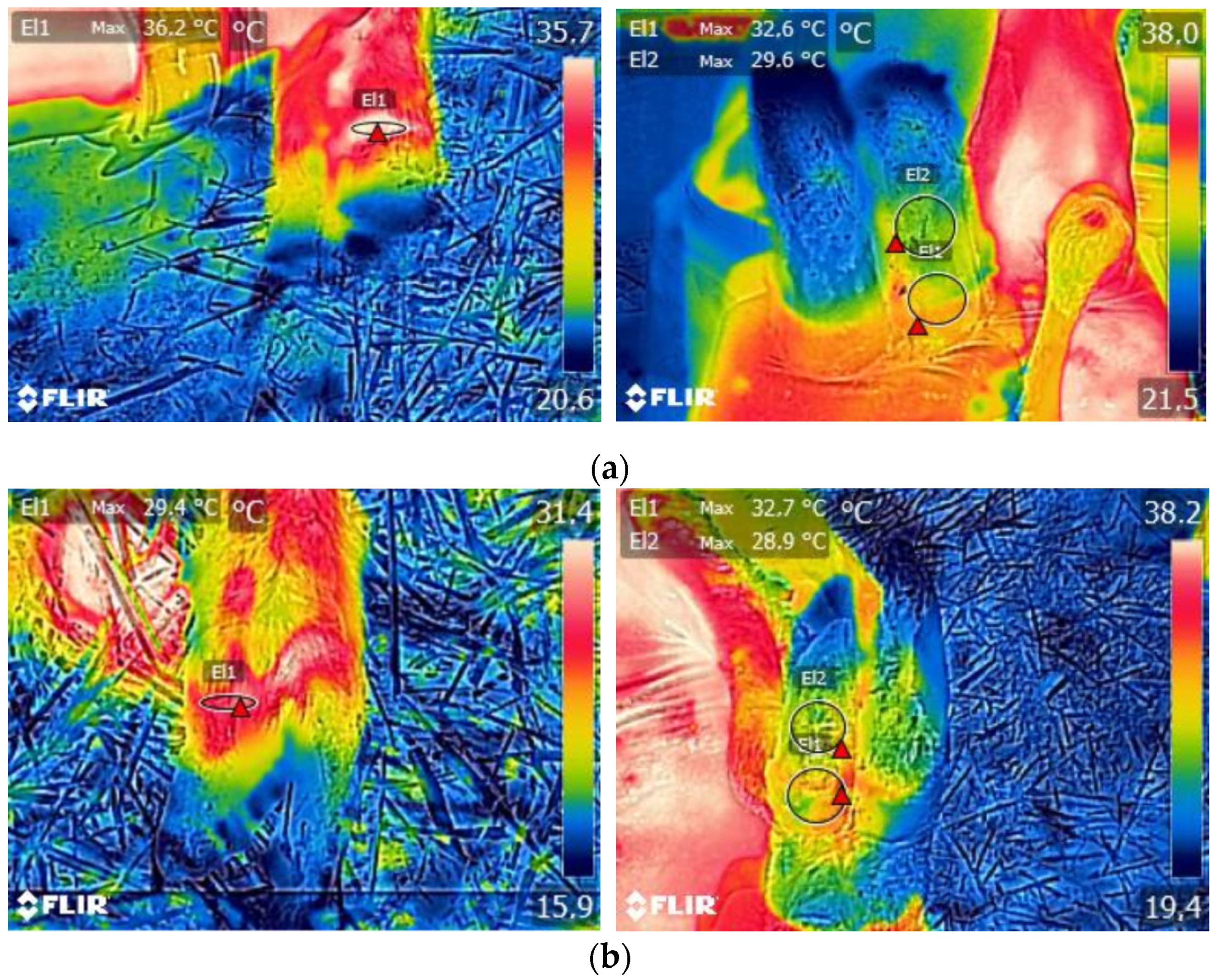
3. Results
3.1. Descriptive Statistics
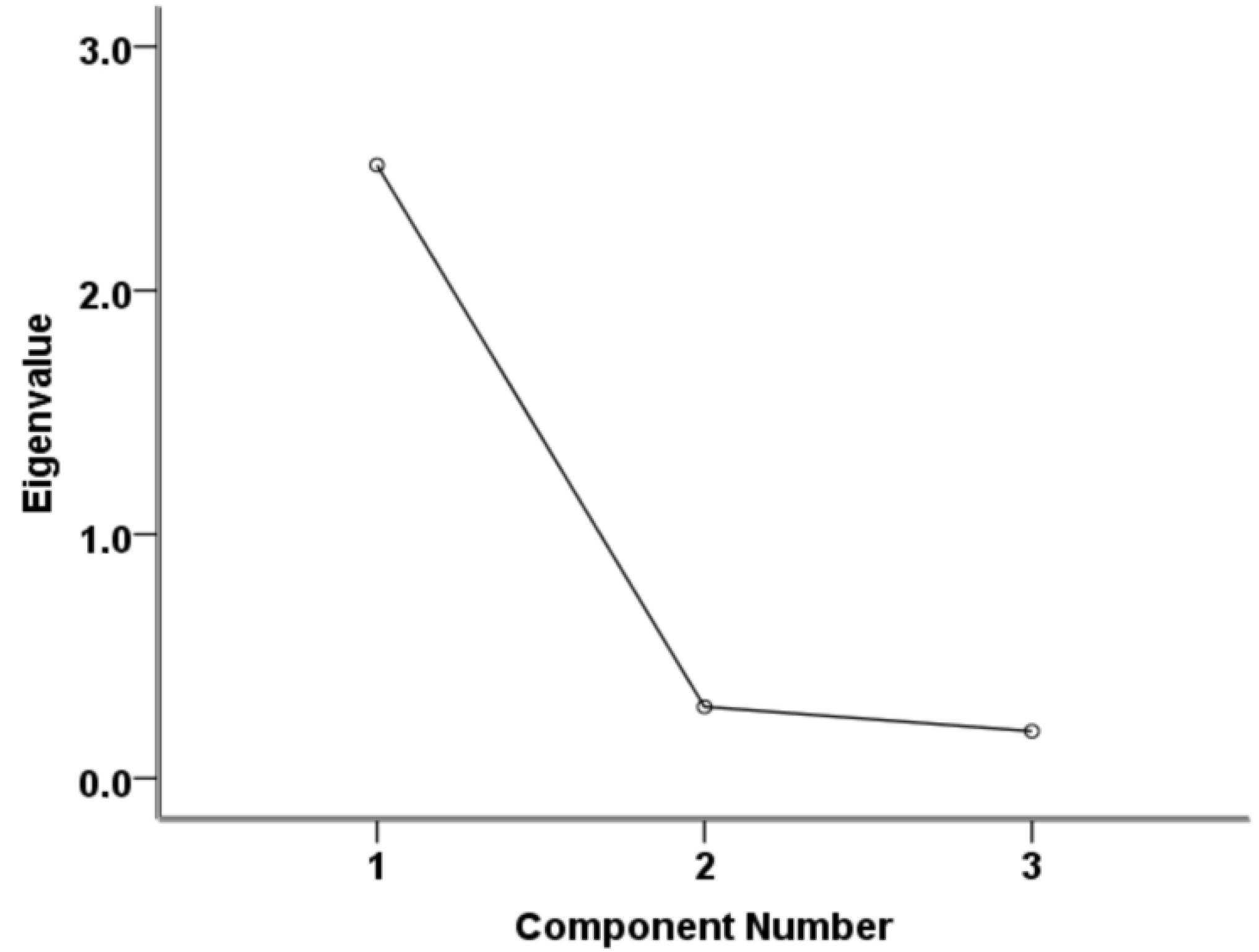
3.2. Consistency of IRT
3.3. Goodness-of-Fit and Performance of the Models for the Diagnosis of Footrot and WLD
4. Discussion
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gelasakis, A.I.; Kalogianni, A.I.; Bossis, I. Aetiology, risk factors, diagnosis and control of foot-related lameness in dairy sheep. Animals 2019, 9, 509. [Google Scholar] [CrossRef]
- Best, C.M.; Roden, J.; Phillips, K.; Pyatt, A.Z.; Behnke, M.C. Prevalence and temporal dynamics of white line disease in sheep: An exploratory investigation into disease distribution and associated risk factors. Vet. Sci. 2021, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Gelasakis, A.I.; Arsenos, G.; Valergakis, G.E.; Banos, G. Association of lameness with milk yield and lactation curves in Chios dairy ewes. J. Dairy Res. 2015, 82, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gelasakis, A.I.; Oikonomou, G.; Bicalho, R.C.; Valergakis, G.E.; Fthenakis, G.S.; Arsenos, G. Clinical characteristics of lameness and potential risk factors in intensive and semi-intensive dairy sheep flocks in Greece. J. Hell. Vet. Med. Soc. 2013, 64, 123–130. [Google Scholar] [CrossRef]
- Moschovas, M.; Kalogianni, A.I.; Simitzis, P.; Pavlatos, G.; Petrouleas, S.; Bossis, I.; Gelasakis, A.I. A Cross-Sectional Epizootiological Study and Risk Assessment of Foot-Related Lesions and Lameness in Intensive Dairy Sheep Farms. Animals 2021, 11, 1614. [Google Scholar] [CrossRef]
- Bennett, G.N.; Hickford, J.G.H. Ovine footrot: New approaches to an old disease. Vet. Microbiol. 2011, 148, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.C. Lameness in sheep. Small Rumin. Res. 2008, 76, 149–153. [Google Scholar] [CrossRef]
- Bennett, G.; Hickford, J.; Sedcole, R.; Zhou, H. Dichelobacter nodosus, Fusobacterium necrophorum and the epidemiology of footrot. Anaerobe 2009, 15, 173–176. [Google Scholar] [CrossRef]
- Witcomb, L.A.; Green, L.E.; Kaler, J.; Ul-Hassan, A.; Calvo-Bado, L.A.; Medley, G.F.; Grogono-Thomas, R.; Wellington, E.M.H. A longitudinal study of the role of Dichelobacter nodosus and Fusobacterium necrophorum load in initiation and severity of footrot in sheep. Prev. Vet. Med. 2014, 115, 48–55. [Google Scholar] [CrossRef]
- Raadsma, H.W.; Egerton, J.R. A review of footrot in sheep: Aetiology, risk factors and control methods. Livest. Sci. 2013, 156, 106–114. [Google Scholar] [CrossRef]
- Winter, A.; Arsenos, G. Diagnosis of white line lesions in sheep. In Pract. 2009, 31, 17–21. [Google Scholar] [CrossRef]
- Nieuwhof, G.J.; Bishop, S.C.; Hill, W.G.; Raadsma, H.W. The effect of footrot on weight gain in sheep. Animal 2008, 2, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Gelasakis, A.I.; Arsenos, G.; Valergakis, G.E.; Fortomaris, P.; Banos, G. Effect of lameness on milk production in a flock of dairy sheep. Vet. Rec. 2010, 167, 533–534. [Google Scholar] [CrossRef]
- Zanolari, P.; Dürr, S.; Jores, J.; Steiner, A.; Kuhnert, P. Ovine footrot: A review of current knowledge. Vet. J. 2021, 271, 105647. [Google Scholar] [CrossRef]
- Winter, J.R.; Kaler, J.; Ferguson, E.; KilBride, A.L.; Green, L.E. Changes in prevalence of, and risk factors for, lameness in random samples of English sheep flocks: 2004-2013. Prev. Vet. Med. 2015, 122, 121–128. [Google Scholar] [CrossRef]
- Olasehinde, O. Infrared Thermography and Machine Learning in Livestock Production. Int. J. Adv. Res. Rev. 2021, 6, 38–57. [Google Scholar]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary applications of infrared thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.L.; Van Hoogmoed, L.M.; Snyder, J.R. The role of thermography in the management of equine lameness. Vet. J. 2001, 162, 172–181. [Google Scholar] [CrossRef]
- Turner, T.A. Thermography as an aid to the clinical lameness evaluation. Vet. Clin. N. Am. Equine Pract. 1991, 7, 311–338. [Google Scholar] [CrossRef]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Alsaaod, M.; Schaefer, A.L.; Büscher, W.; Steiner, A. The role of infrared thermography as a non-invasive tool for the detection of lameness in cattle. Sensors 2015, 15, 14513–14525. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.J.; Kennedy, A.D.; Scott, S.L.; Kyle, B.L.; Schaefer, A.L. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: Potential for mastitis detection. Can. J. Anim. Sci. 2003, 83, 687–693. [Google Scholar] [CrossRef]
- Pérez de Diego, A.C.; Sánchez-Cordón, P.J.; Pedrera, M.; Martínez-López, B.; Gómez-Villamandos, J.C.; Sánchez-Vizcaíno, J.M. The use of infrared thermography as a non-invasive method for fever detection in sheep infected with bluetongue virus. Vet. J. 2013, 198, 182–186. [Google Scholar] [CrossRef]
- Stewart, M.; Wilson, M.T.; Schaefer, A.L.; Huddart, F.; Sutherland, M.A. The use of infrared thermography and accelerometers for remote monitoring of dairy cow health and welfare. J. Dairy Sci. 2017, 100, 3893–3901. [Google Scholar] [CrossRef]
- Montanholi, Y.R.; Odongo, N.E.; Swanson, K.C.; Schenkel, F.S.; McBride, B.W.; Miller, S.P. Application of infrared thermography as an indicator of heat and methane production and its use in the study of skin temperature in response to physiological events in dairy cattle (Bos taurus). J. Therm. Biol. 2008, 33, 468–475. [Google Scholar] [CrossRef]
- Cândido, M.G.L.; Tinôco, I.F.F.; Albino, L.F.T.; Freitas, L.C.S.R.; Santos, T.C.; Cecon, P.R.; Gates, R.S. Effects of heat stress on pullet cloacal and body temperature. Poult. Sci. 2020, 99, 2469–2477. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Rainwater-Lovett, K.; Pacheco, J.M.; Packer, C.; Rodriguez, L.L. Detection of foot-and-mouth disease virus infected cattle using infrared thermography. Vet. J. 2009, 180, 317–324. [Google Scholar] [CrossRef]
- Castells, E.; Lacasta, D.; Climent, M.; Pérez, M.; Sanromán, F.; Jiménez, C.; Ferrer, L.M. Diagnostic imaging techniques of the respiratory tract of sheep. Small Rumin. Res. 2019, 180, 112–126. [Google Scholar] [CrossRef]
- Menzel, A.; Beyerbach, M.; Siewert, C.; Gundlach, M.; Hoeltig, D.; Graage, R.; Seifert, H.; Waldmann, K.H.; Verspohl, J.; Hennig-Pauka, I. Actinobacillus pleuropneumoniae challenge in swine: Diagnostic of lung alterations by infrared thermography. BMC Vet. Res. 2014, 10, 1–13. [Google Scholar] [CrossRef]
- Yanmaz, L.E.; Okumus, Z.; Dogan, E. Instrumentation of Thermography and its Applications in Horses. J. Anim. Vet. Adv. 2007, 6, 858–862. [Google Scholar]
- Talukder, S.; Gabai, G.; Celi, P. The use of digital infrared thermography and measurement of oxidative stress biomarkers as tools to diagnose foot lesions in sheep. Small Rumin. Res. 2015, 127, 80–85. [Google Scholar] [CrossRef]
- Stelletta, C.; Gianesella, M.; Vencato, J.; Fiore, E.; Morgante, M. Thermographic Applications in Veterinary Medicine. Infrared Thermogr. 2012. [Google Scholar] [CrossRef]
- Sykes, D.J.; Couvillion, J.S.; Cromiak, A.; Bowers, S.; Schenck, E.; Crenshaw, M.; Ryan, P.L. The use of digital infrared thermal imaging to detect estrus in gilts. Theriogenology 2012, 78, 147–152. [Google Scholar] [CrossRef]
- Stokes, J.E.; Leach, K.A.; Main, D.C.J.; Whay, H.R. An investigation into the use of infrared thermography (IRT) as a rapid diagnostic tool for foot lesions in dairy cattle. Vet. J. 2012, 193, 674–678. [Google Scholar] [CrossRef]
- Alsaaod, M.; Büscher, W. Detection of hoof lesions using digital infrared thermography in dairy cows. J. Dairy Sci. 2012, 95, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Alsaaod, M.; Syring, C.; Dietrich, J.; Doherr, M.G.; Gujan, T.; Steiner, A. A field trial of infrared thermography as a non-invasive diagnostic tool for early detection of digital dermatitis in dairy cows. Vet. J. 2014, 199, 281–285. [Google Scholar] [CrossRef]
- Gianesella, M.; Arfuso, F.; Fiore, E.; Giambelluca, S.; Giudice, E.; Armato, L.; Piccione, G. Infrared thermography as a rapid and non-invasive diagnostic tool to detect inflammatory foot diseases in dairy cows. Pol. J. Vet. Sci. 2018, 21, 299–305. [Google Scholar] [CrossRef]
- Byrne, D.T.; Berry, D.P.; Esmonde, H.; McGovern, F.; Creighton, P.; McHugh, N. Infrared thermography as a tool to detect hoof lesions in sheep. Transl. Anim. Sci. 2019, 3, 577–588. [Google Scholar] [CrossRef]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Harris-Bridge, G.; Young, L.; Handel, I.; Farish, M.; Mason, C.; Mitchell, M.A.; Haskell, M.J. The use of infrared thermography for detecting digital dermatitis in dairy cattle: What is the best measure of temperature and foot location to use? Vet. J. 2018, 237, 26–33. [Google Scholar] [CrossRef]
- Nikkhah, A.; Plaizier, J.C.; Einarson, M.S.; Berry, R.J.; Scott, S.L.; Kennedy, A.D. Short communication: Infrared thermography and visual examination of hooves of dairy cows in two stages of lactation. J. Dairy Sci. 2005, 88, 2749–2753. [Google Scholar] [CrossRef]
- Orman, A.; Endres, M.I. Use of thermal imaging for identification of foot lesions in dairy cattle. Acta Agric. Scand. A Anim. Sci. 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Fabbri, G.; Fiore, E.; Piccione, G.; Giudice, E.; Gianesella, M.; Morgante, M.; Armato, L.; Bonato, O.; Giambelluca, S.; Arfuso, F. Detection of digital and interdigital dermatitis in holstein friesian dairy cows by means of infrared thermography. Large Anim. Rev. 2020, 26, 113–116. [Google Scholar]
- Wood, S.; Lin, Y.; Knowles, T.G.; Main, D.C.J. Infrared thermometry for lesion monitoring in cattle lameness. Vet. Rec. 2015, 176, 308. [Google Scholar] [CrossRef] [PubMed]
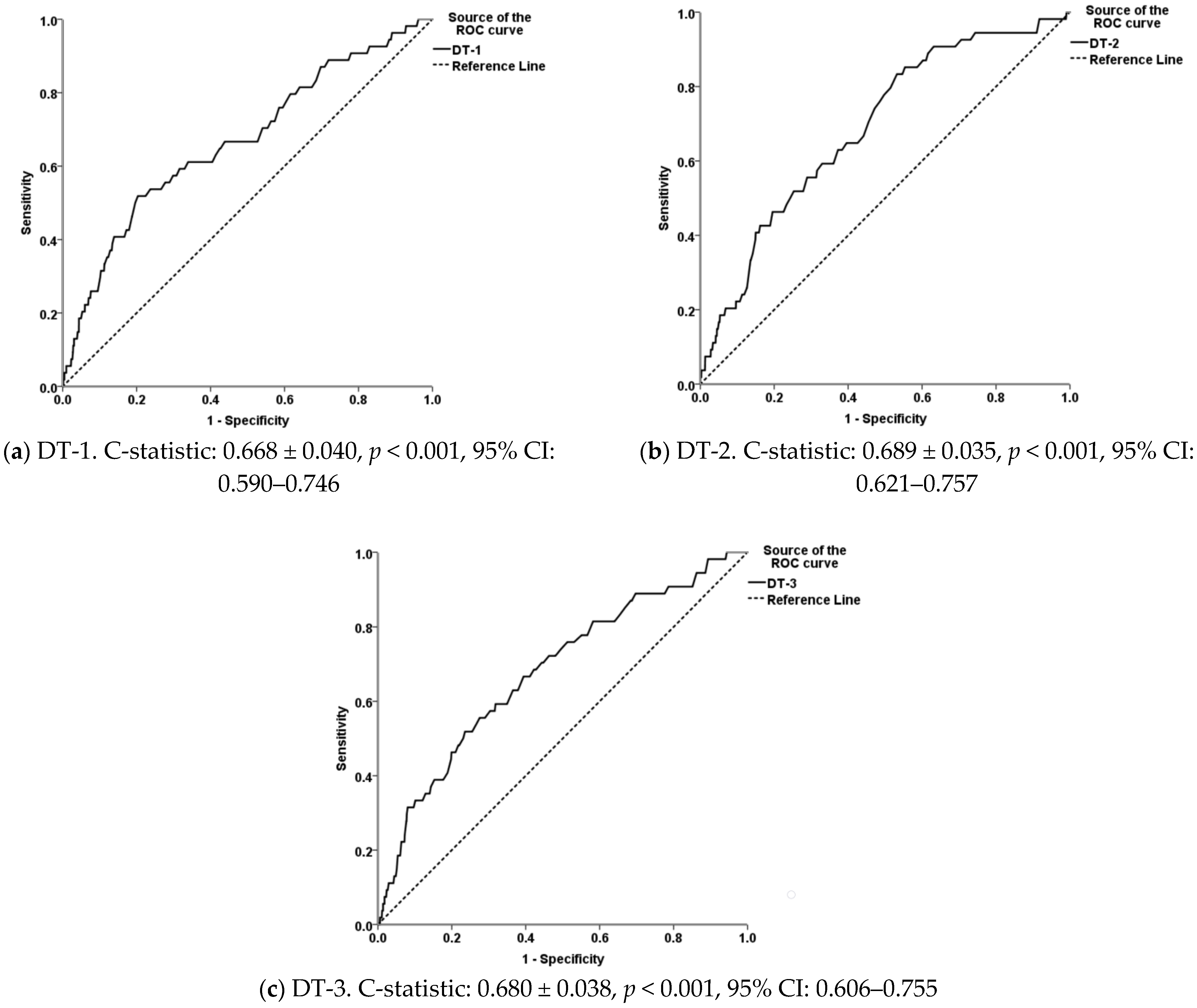
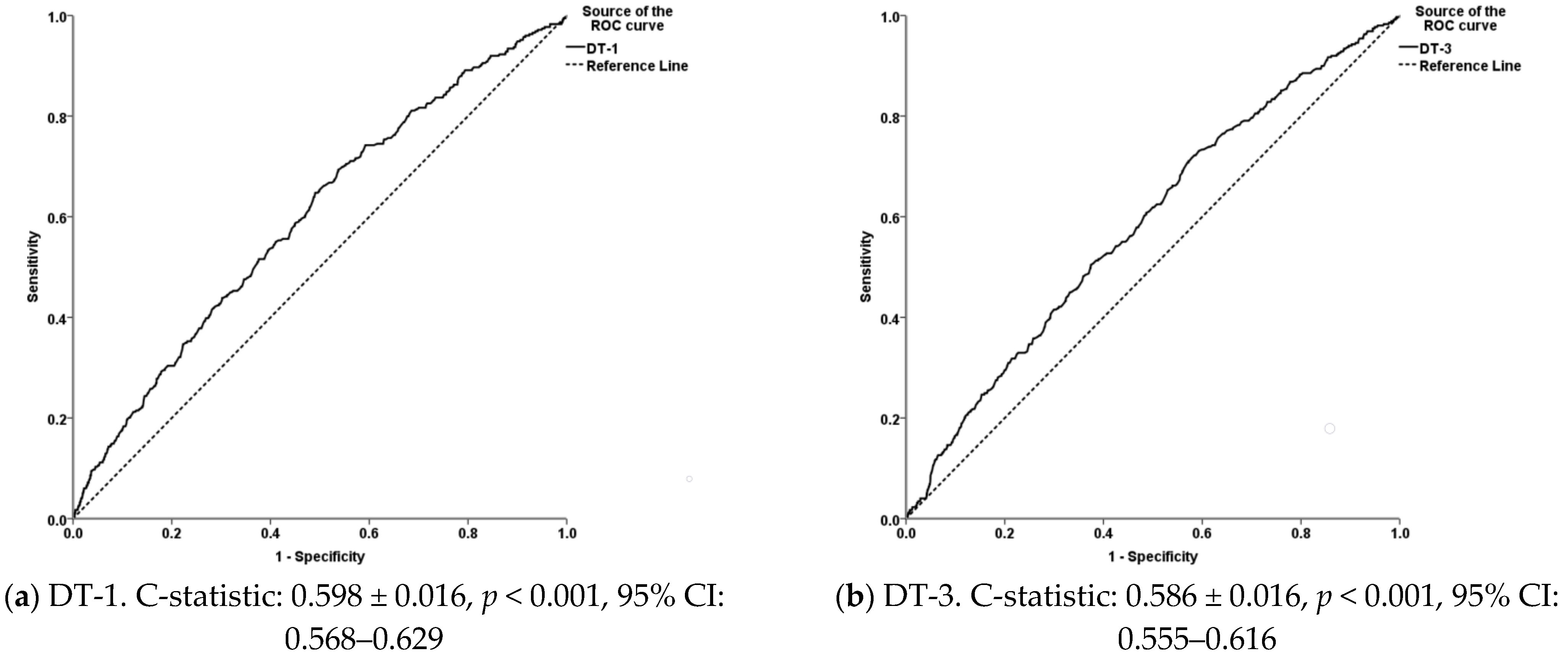
| Hosmer and Lemeshow Test | Omnibus Test of Model Coefficients | Nagelkerke R2 | |||||
|---|---|---|---|---|---|---|---|
| Χ2 | df | p | Χ2 | df | p | ||
| Footrot | |||||||
| DT-1 | 7.24 | 8 | 0.511 | 11.20 | 1 | 0.001 | 0.043 |
| DT-2 | 9.20 | 8 | 0.326 | 8.26 | 1 | 0.004 | 0.032 |
| DT-3 | 6.82 | 8 | 0.556 | 9.10 | 1 | 0.003 | 0.035 |
| White line disease | |||||||
| DT-1 | 5.23 | 8 | 0.732 | 12.20 | 2 | 0.002 | 0.013 |
| DT-3 | 10.48 | 8 | 0.233 | 11.21 | 2 | 0.004 | 0.012 |
| B | S.E. | p | OR | 95% CI for OR | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Footrot | Constant-1 a | −6.52 | 0.694 | 0.000 | 0.00 | na | na |
| DT-1 | 0.20 | 0.061 | 0.001 | 1.23 | 1.09 | 1.38 | |
| Constant-2 a | −6.01 | 0.590 | 0.000 | 0.00 | na | na | |
| DT-2 | 0.19 | 0.064 | 0.003 | 1.21 | 1.07 | 1.37 | |
| Constant-3 a | −6.01 | 0.591 | 0.000 | 0.00 | na | na | |
| DT-3 | 0.22 | 0.071 | 0.002 | 1.25 | 1.09 | 1.44 | |
| White line disease | Constant-1 a | −1.58 | 0.807 | 0.051 | 0.21 | na | na |
| DT-1 | 0.07 | 0.024 | 0.002 | 1.08 | 1.03 | 1.13 | |
| BCS | −0.57 | 0.286 | 0.046 | 0.57 | 0.32 | 0.99 | |
| Constant-2 | −2.55 | 0.080 | 0.000 | 0.08 | na | na | |
| Constant-3 a | −1.75 | 0.821 | 0.033 | 0.17 | na | na | |
| DT-3 | 0.09 | 0.031 | 0.003 | 1.10 | 1.03 | 1.16 | |
| BCS | −0.50 | 0.287 | 0.084 | 0.61 | 0.35 | 1.07 | |
| Footrot | White Line Disease | ||||
|---|---|---|---|---|---|
| DT-1 | DT-2 | DT-3 | DT-1 | DT-3 | |
| Threshold value of the predicted probability | −4.23 | −4.76 | −4.32 | −2.53 | −2.66 |
| Threshold values of DTs (°C) | 11.3 | 6.5 | 7.9 | 10.0 * | 6.4 * |
| Sensitivity (%) | 51.9 | 83.3 | 51.9 | 64.8 | 73.1 |
| Specificity (%) | 79.7 | 47.8 | 76.4 | 51.0 | 40.7 |
| Threshold value of the predicted probability (t.s.) | −4.23 | −4.68 | −4.44 | −2.54 | −2.66 |
| Threshold value of DTs (°C) (t.s.) | 11.3 | 6.9 | 7.4 | 10.4 * | 6.4 * |
| Sensitivity (%) (t.s.) | 56.0 | 76.0 | 64.0 | 67.5 | 75.9 |
| Specificity (%) (t.s.) | 80.1 | 52.3 | 69.1 | 49.4 | 39.4 |
| Threshold value of the predicted probability (v.s.) | −4.22 | −4.78 | −4.24 | −2.58 | −2.60 |
| Threshold value of DTs (°C) (v.s.) | 11.3 | 6.4 | 8.3 | 9.9 * | 7.1 * |
| Sensitivity (%) (v.s.) | 51.7 | 89.7 | 51.7 | 72.1 | 62.3 |
| Specificity (%) (v.s.) | 81.0 | 45.5 | 80.2 | 44.7 | 52.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelasakis, A.I.; Kalogianni, A.I.; Moschovas, M.; Tsimpouri, E.; Pnevmatikos, T.; Bossis, I.; Arsenos, G.; Simitzis, P. Evaluation of Infrared Thermography for the Detection of Footrot and White Line Disease Lesions in Dairy Sheep. Vet. Sci. 2021, 8, 219. https://doi.org/10.3390/vetsci8100219
Gelasakis AI, Kalogianni AI, Moschovas M, Tsimpouri E, Pnevmatikos T, Bossis I, Arsenos G, Simitzis P. Evaluation of Infrared Thermography for the Detection of Footrot and White Line Disease Lesions in Dairy Sheep. Veterinary Sciences. 2021; 8(10):219. https://doi.org/10.3390/vetsci8100219
Chicago/Turabian StyleGelasakis, Athanasios I., Aphrodite I. Kalogianni, Marios Moschovas, Eirini Tsimpouri, Theodoros Pnevmatikos, Ioannis Bossis, Georgios Arsenos, and Panagiotis Simitzis. 2021. "Evaluation of Infrared Thermography for the Detection of Footrot and White Line Disease Lesions in Dairy Sheep" Veterinary Sciences 8, no. 10: 219. https://doi.org/10.3390/vetsci8100219
APA StyleGelasakis, A. I., Kalogianni, A. I., Moschovas, M., Tsimpouri, E., Pnevmatikos, T., Bossis, I., Arsenos, G., & Simitzis, P. (2021). Evaluation of Infrared Thermography for the Detection of Footrot and White Line Disease Lesions in Dairy Sheep. Veterinary Sciences, 8(10), 219. https://doi.org/10.3390/vetsci8100219








