Evaluation of the Worldwide Occurrence of Rabies in Dogs and Cats Using a Simple and Homogenous Framework for Quantitative Risk Assessments of Rabies Reintroduction in Disease-Free Areas through Pet Movements
Abstract
1. Introduction
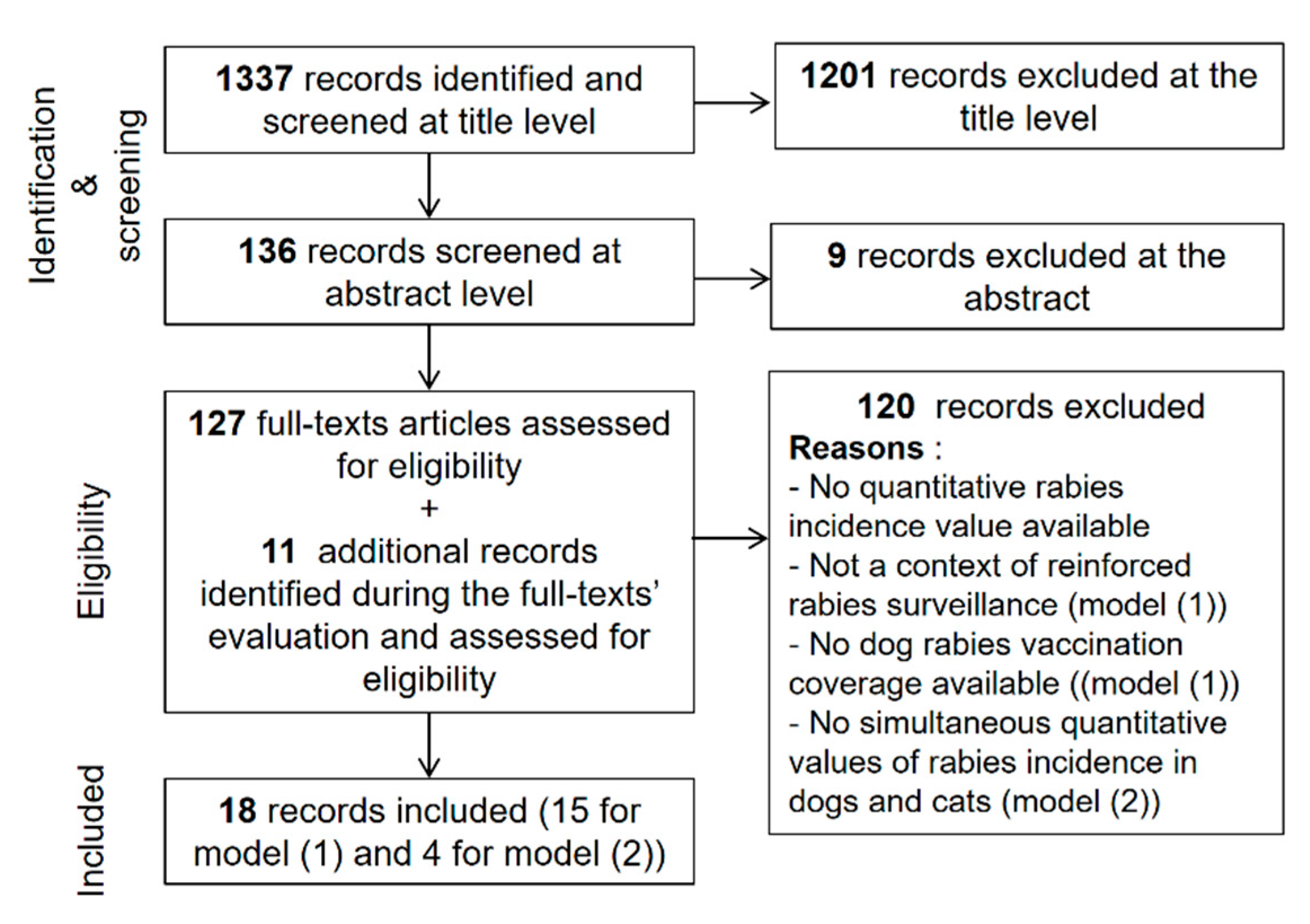
2. Materials and Methods
2.1. Framework Overview and Data Sources
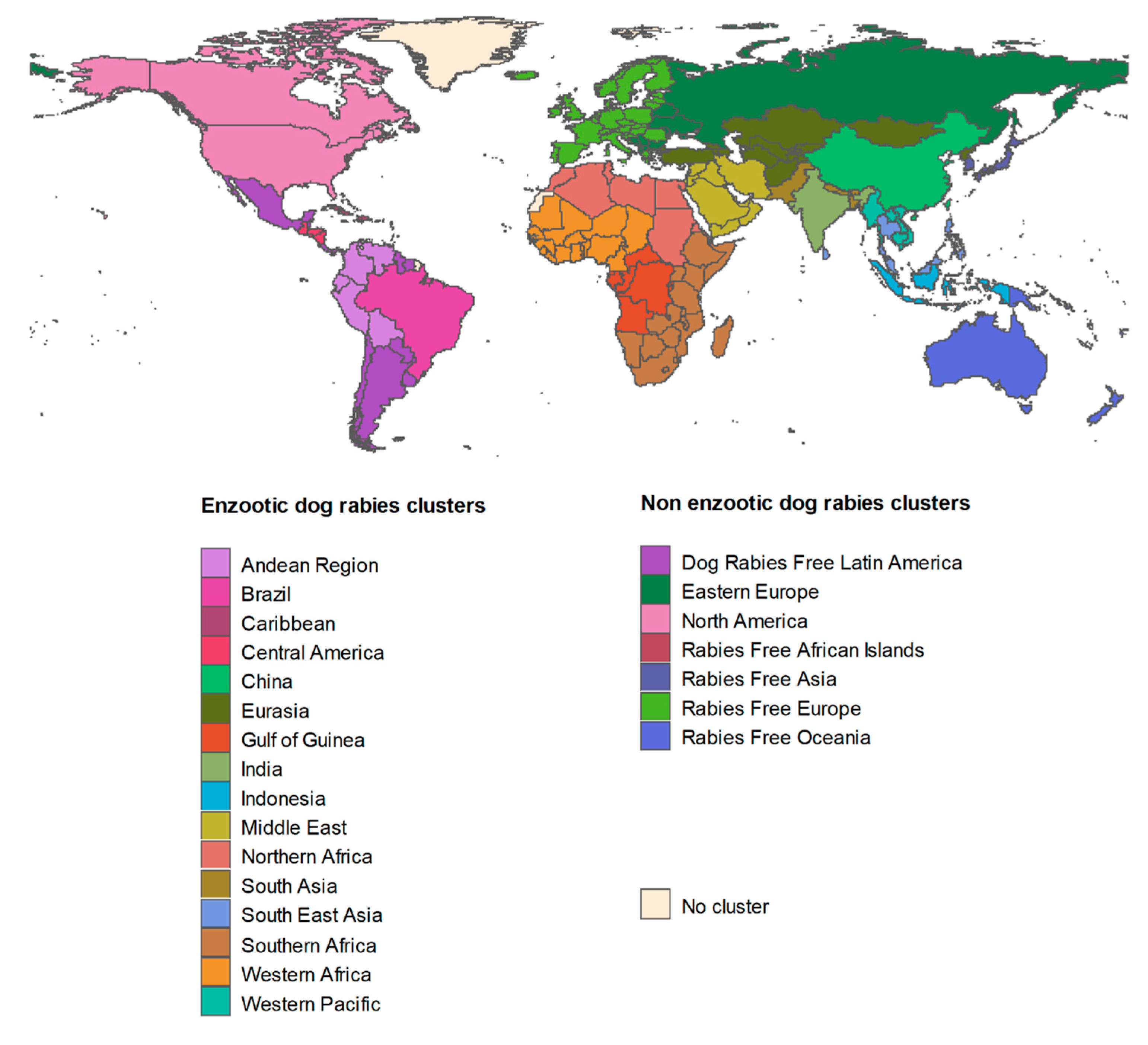
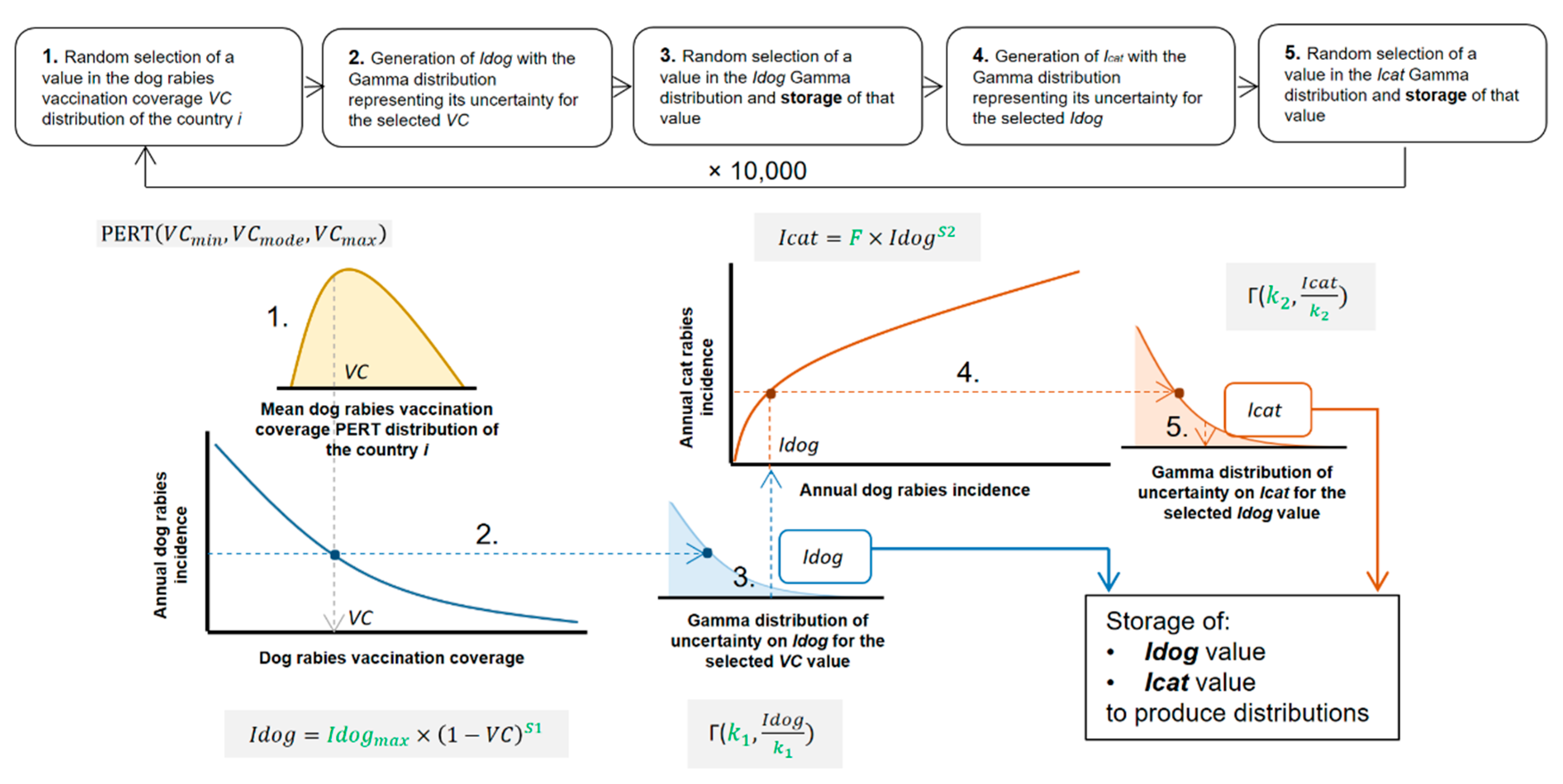
2.2. EDRAs
- Model for dog rabies incidence prediction
- Model for cat rabies incidence prediction
- Prediction of dog and cat rabies incidences
2.3. nEDRAs
3. Results
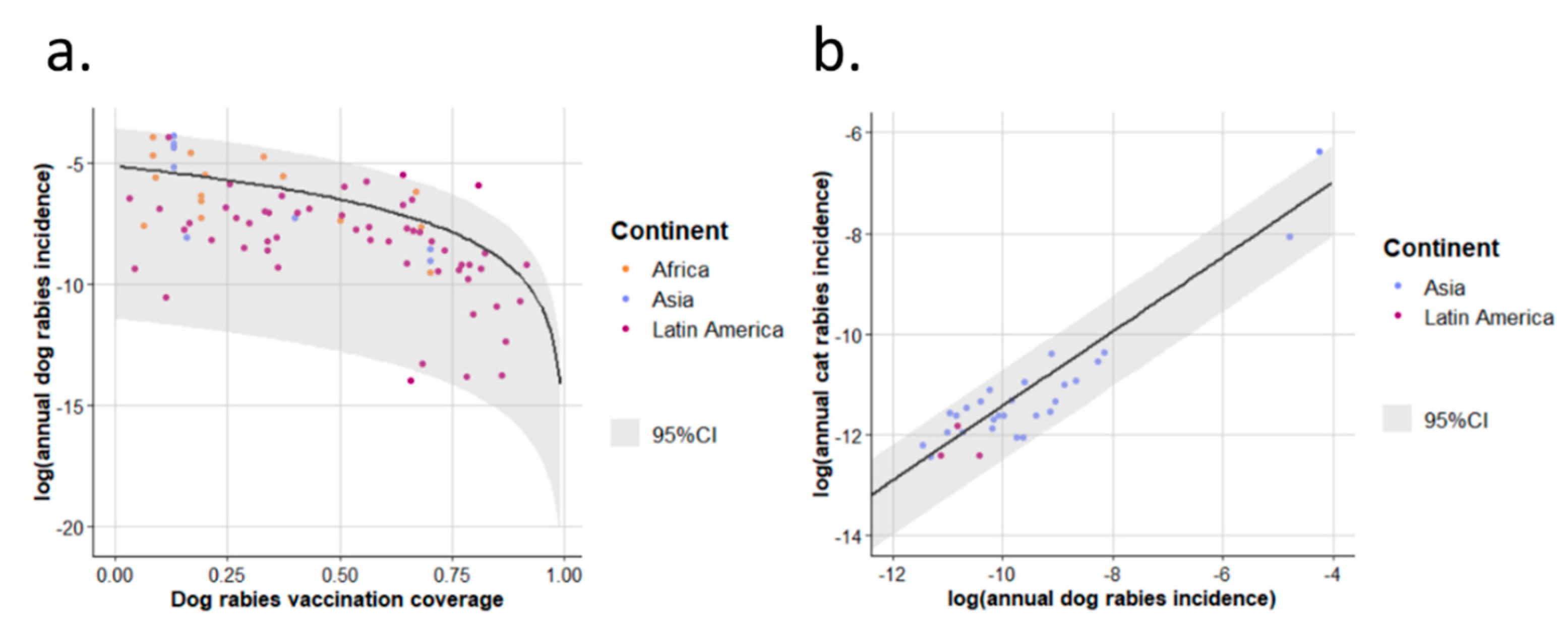
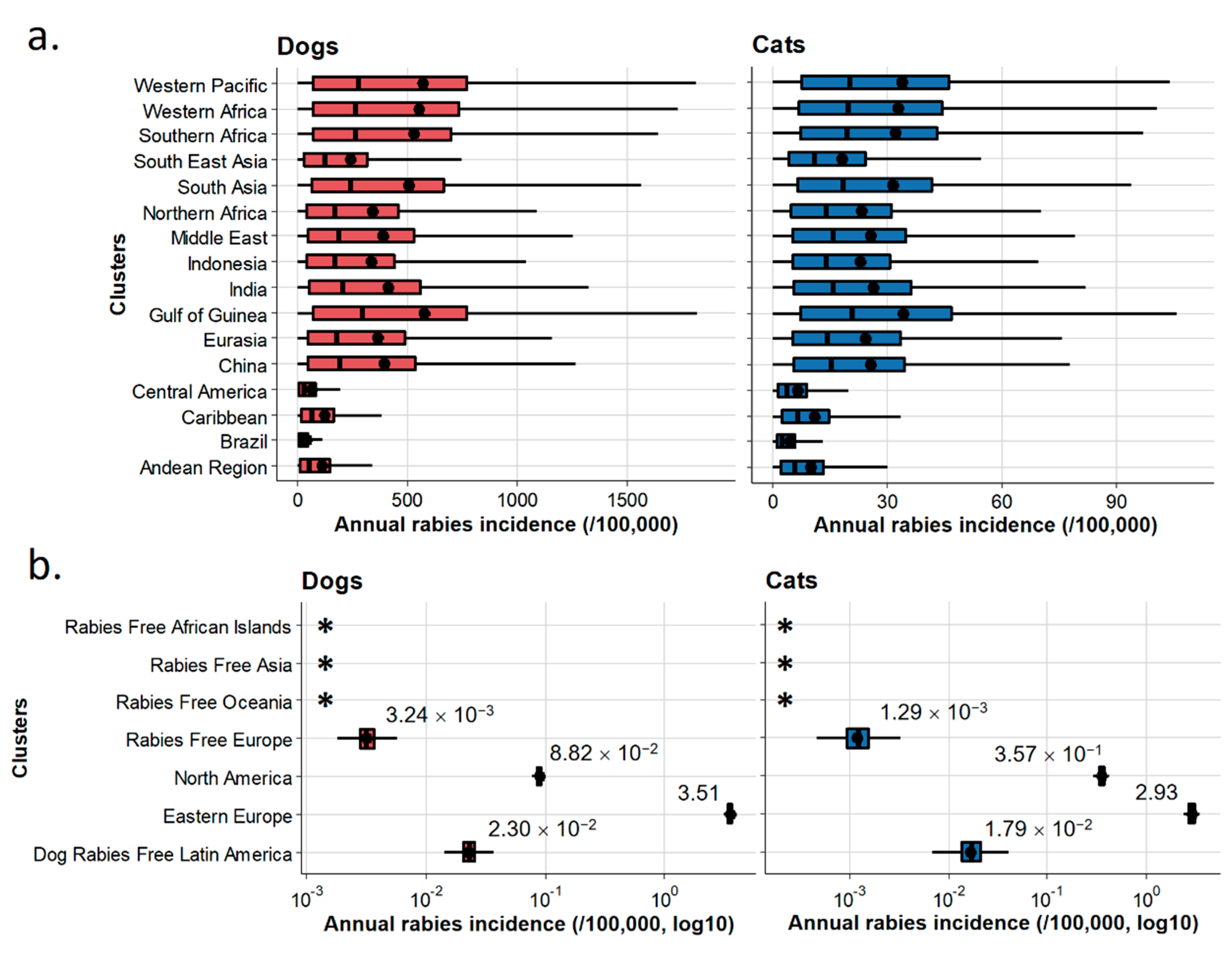
3.1. EDRAs
3.2. nEDRAs
4. Discussion
4.1. Model and Results for EDRAs
4.2. Model and Results for nEDRAs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| City/Area | Country | Continent | Year | Annual Dog Rabies Incidence | Dog Rabies Vaccination Coverage | Reference |
|---|---|---|---|---|---|---|
| Gondar | Ethiopia | Africa | 2009 | 4.12 × 10−3 | 0.2 | [32] |
| Mongar | Bhutan | Asia | 2005 | 1.23 × 10−2 | 0.13 | [33] †, [34] |
| Tashiyangtse | Bhutan | Asia | 2005 | 1.44 × 10−2 | 0.13 | [33] †, [34] |
| Trashigang | Bhutan | Asia | 2005 | 5.75 × 10−3 | 0.13 | [33] †, [34] |
| Serengeti | Tanzania | Africa | 1997 | 3.71 × 10−3 | 0.091 | [35] |
| Serengeti | Tanzania | Africa | 1999 | 4.95 × 10−4 | 0.0639 | [35] |
| Musoma | Tanzania | Africa | 1997 | 1.89 × 10−2 | 0.085 | [35] |
| Musoma | Tanzania | Africa | 1999 | 9.02 × 10−3 | 0.085 | [35] |
| Punjab | India | Asia | 2016 | 3.05 × 10−4 | 0.16 | [36] |
| N’Djamena | Chad | Africa | 2001 | 1.40 × 10−3 | 0.19 | [37] |
| Machakos | Kenya | Africa | 1992 | 8.60 × 10−3 | 0.33 | [22], [38] † |
| Chukha | Bhutan | Asia | 2008 | 2.00 × 10−2 | 0.13 | [33] †, [39] |
| Serengeti | Tanzania | Africa | 2011 | 3.84 × 10−3 | 0.375 | [40], [41] † |
| Pemba | Tanzania | Africa | 2011 | 1.03 × 10−2 | 0.168 | [42] |
| Pemba | Tanzania | Africa | 2016 | 4.88 × 10−4 | 0.682 | [42] |
| N’Djamena | Chad | Africa | 2012 | 7.00 × 10−4 | 0.19 | [37] †, [43] |
| N’Djamena | Chad | Africa | 2014 | 7.30 × 10−5 | 0.7 | [37] †, [43] |
| Unguja | Tanzania | Africa | 2018 | 6.15 × 10−4 | 0.5 | [44] |
| N’Djamena | Chad | Africa | 2016 | 2.00 × 10−3 | 0.67 | [45] |
| N’Djamena | Chad | Africa | 2006 | 1.71 × 10−3 | 0.19 | [46], [47] † |
| Bali Island | Bali | Asia | 2008 | 6.75 × 10−4 | 0.4 | [48] ‡, [49] (making the assumption of linear decrease for dog population between 2008 and 2019) |
| Bali Island | Bali | Asia | 2010 | 1.94 × 10−4 | 0.7 | [48] ‡, [49] (making the assumption of linear decrease for dog population between 2008 and 2019) |
| Bali Island | Bali | Asia | 2011 | 1.16 × 10−4 | 0.7 | [48] ‡, [49] (making the assumption of linear decrease for dog population between 2008 and 2019) |
| Hermosillo | Mexico | South Central America | 1988 | 4.15 × 10−3 | 0.64 | [50] |
| Hermosillo | Mexico | South Central America | 1988 | 3.11 × 10−3 | 0.56 | [50] |
| Hermosillo | Mexico | South Central America | 1988 | 2.65 × 10−3 | 0.81 | [50] |
| Hermosillo | Mexico | South Central America | 1988 | 2.49 × 10−3 | 0.51 | [50] |
| Hermosillo | Mexico | South Central America | 1988 | 1.73 × 10−3 | 0.37 | [50] |
| Hermosillo | Mexico | South Central America | 1988 | 1.48 × 10−3 | 0.66 | [50] |
| Hermosillo | Mexico | South Central America | 1988 | 1.16 × 10−3 | 0.64 | [50] |
| Area | Country | Continent | Year | Annual Cat Rabies Incidence | Annual Dog Rabies Incidence | Reference |
|---|---|---|---|---|---|---|
| Bangkok area | Thailand | Asia | 1987 | 3.17 × 10−5 | 2.85 × 10−4 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1988 | 2.68 × 10−5 | 2.56 × 10−4 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1989 | 1.80 × 10−5 | 1.73 × 10−4 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1990 | 1.67 × 10−5 | 1.39 × 10−4 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1991 | 1.21 × 10−5 | 1.18 × 10−4 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1992 | 9.85 × 10−6 | 1.06 × 10−4 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1993 | 8.99 × 10−6 | 8.27 × 10−5 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1994 | 5.86 × 10−6 | 6.52 × 10−5 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1995 | 5.80 × 10−6 | 5.77 × 10−5 | [51], [52] † |
| Bangkok area | Thailand | Asia | 1996 | 6.98 × 10−6 | 3.68 × 10−5 | [51], [52] † |
| Tashiyangtse | Bhutan | Asia | 2005 | 1.73 × 10−3 | 1.44 × 10−2 | [34] |
| Tashiyangtse | Bhutan | Asia | 2006 | 3.20 × 10−4 | 8.42 × 10−3 | [34] |
| State of Sao Paulo | Brazil | South America | 1993 | 1.64 × 10−6 | 3.80 × 10−6 | [53], [55] † |
| State of Sao Paulo | Brazil | South America | 1994 | 4.10 × 10−6 | 2.96 × 10−5 | [53], [55] † |
| State of Sao Paulo | Brazil | South America | 1995 | 7.38 × 10−6 | 2.00 × 10−5 | [53], [55] † |
| State of Sao Paulo | Brazil | South America | 1996 | 4.10 × 10−6 | 1.46 × 10−5 | [53], [55] † |
| Southern Thailand | Thailand | Asia | 1994 | 3.08 × 10−5 | 1.08 × 10−4 | [52,54] |
| Southern Thailand | Thailand | Asia | 1995 | 1.74 × 10−5 | 6.62 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 1996 | 1.24 × 10−5 | 5.24 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 1997 | 8.94 × 10−6 | 4.23 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 1998 | 8.44 × 10−6 | 3.77 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 1999 | 8.94 × 10−6 | 4.57 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2000 | 1.49 × 10−5 | 3.58 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2001 | 1.19 × 10−5 | 3.01 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2002 | 1.04 × 10−5 | 2.32 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2003 | 6.46 × 10−6 | 2.19 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2004 | 9.44 × 10−6 | 1.73 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2005 | 8.94 × 10−6 | 1.94 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2006 | 6.46 × 10−6 | 1.66 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2007 | 3.97 × 10−6 | 1.20 × 10−5 | [52] †, [54] |
| Southern Thailand | Thailand | Asia | 2008 | 4.97 × 10−6 | 1.05 × 10−5 | [52] †, [54] |
| Country | Cluster | Dogs | Cats | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Interquartile Range | Mean | Median | Interquartile Range | ||||
| 25th Percentile | 75th Percentile | 25th Percentile | 75th Percentile | ||||||
| Algeria | Northern Africa | 3.38 × 10−3 | 1.66 × 10−3 | 4.25 × 10−4 | 4.50 × 10−3 | 2.65 × 10−4 | 1.36 × 10−4 | 4.80 × 10−5 | 9.37 × 10−4 |
| Djibouti | Northern Africa | 5.72 × 10−3 | 2.70 × 10−3 | 7.22 × 10−4 | 7.63 × 10−3 | 3.91 × 10−4 | 1.99 × 10−4 | 7.08 × 10−5 | 1.42 × 10−3 |
| Egypt | Northern Africa | 3.38 × 10−3 | 1.66 × 10−3 | 4.30 × 10−4 | 4.43 × 10−3 | 2.65 × 10−4 | 1.37 × 10−4 | 4.78 × 10−5 | 9.86 × 10−4 |
| Eritrea | Northern Africa | 5.72 × 10−3 | 2.81 × 10−3 | 7.38 × 10−4 | 7.60 × 10−3 | 3.91 × 10−4 | 2.02 × 10−4 | 7.26 × 10−5 | 1.41 × 10−3 |
| Libya | Northern Africa | 3.38 × 10−3 | 1.63 × 10−3 | 4.15 × 10−4 | 4.55 × 10−3 | 2.65 × 10−4 | 1.36 × 10−4 | 4.72 × 10−5 | 9.78 × 10−4 |
| Morocco | Northern Africa | 4.27 × 10−3 | 2.17 × 10−3 | 5.38 × 10−4 | 5.87 × 10−3 | 3.15 × 10−4 | 1.64 × 10−4 | 5.74 × 10−5 | 1.20 × 10−3 |
| Sudan | Northern Africa | 5.71 × 10−3 | 2.81 × 10−3 | 6.96 × 10−4 | 7.50 × 10−3 | 3.90 × 10−4 | 2.05 × 10−4 | 7.04 × 10−5 | 1.40 × 10−3 |
| Tunisia | Northern Africa | 1.58 × 10−3 | 7.76 × 10−4 | 2.06 × 10−4 | 2.13 × 10−3 | 1.51 × 10−4 | 7.82 × 10−5 | 2.83 × 10−5 | 5.33 × 10−4 |
| Angola | Gulf of Guinea | 5.68 × 10−3 | 2.85 × 10−3 | 7.17 × 10−4 | 7.83 × 10−3 | 3.89 × 10−4 | 2.08 × 10−4 | 7.20 × 10−5 | 1.45 × 10−3 |
| Burundi | Gulf of Guinea | 5.68 × 10−3 | 2.77 × 10−3 | 7.19 × 10−4 | 7.40 × 10−3 | 3.89 × 10−4 | 1.99 × 10−4 | 7.13 × 10−5 | 1.43 × 10−3 |
| Central African Republic | Gulf of Guinea | 5.68 × 10−3 | 2.88 × 10−3 | 7.50 × 10−4 | 7.83 × 10−3 | 3.89 × 10−4 | 2.08 × 10−4 | 7.42 × 10−5 | 1.49 × 10−3 |
| Congo | Gulf of Guinea | 5.72 × 10−3 | 2.75 × 10−3 | 6.90 × 10−4 | 7.74 × 10−3 | 3.91 × 10−4 | 2.03 × 10−4 | 6.97 × 10−5 | 1.42 × 10−3 |
| Congo (Dem. Rep.) | Gulf of Guinea | 5.68 × 10−3 | 2.87 × 10−3 | 7.08 × 10−4 | 7.66 × 10−3 | 3.89 × 10−4 | 2.07 × 10−4 | 7.01 × 10−5 | 1.45 × 10−3 |
| Equatorial Guinea | Gulf of Guinea | 5.68 × 10−3 | 2.90 × 10−3 | 7.45 × 10−4 | 7.75 × 10−3 | 3.89 × 10−4 | 2.09 × 10−4 | 7.44 × 10−5 | 1.48 × 10−3 |
| Gabon | Gulf of Guinea | 5.64 × 10−3 | 2.90 × 10−3 | 7.05 × 10−4 | 7.73 × 10−3 | 3.87 × 10−4 | 2.07 × 10−4 | 7.06 × 10−5 | 1.46 × 10−3 |
| Rwanda | Gulf of Guinea | 3.19 × 10−3 | 1.72 × 10−3 | 4.33 × 10−4 | 4.71 × 10−3 | 2.54 × 10−4 | 1.40 × 10−4 | 4.82 × 10−5 | 1.00 × 10−3 |
| Benin | Western Africa | 5.64 × 10−3 | 2.74 × 10−3 | 7.32 × 10−4 | 7.53 × 10−3 | 3.87 × 10−4 | 2.00 × 10−4 | 7.08 × 10−5 | 1.44 × 10−3 |
| Burkina | Western Africa | 5.64 × 10−3 | 2.60 × 10−3 | 6.67 × 10−4 | 7.19 × 10−3 | 3.87 × 10−4 | 1.93 × 10−4 | 6.74 × 10−5 | 1.38 × 10−3 |
| Cameroon | Western Africa | 5.64 × 10−3 | 2.82 × 10−3 | 6.95 × 10−4 | 7.68 × 10−3 | 3.87 × 10−4 | 2.08 × 10−4 | 7.01 × 10−5 | 1.40 × 10−3 |
| Chad | Western Africa | 5.64 × 10−3 | 2.61 × 10−3 | 6.67 × 10−4 | 7.16 × 10−3 | 3.87 × 10−4 | 1.91 × 10−4 | 6.90 × 10−5 | 1.38 × 10−3 |
| Cote d’Ivoire | Western Africa | 5.64 × 10−3 | 2.68 × 10−3 | 7.05 × 10−4 | 7.52 × 10−3 | 3.87 × 10−4 | 1.97 × 10−4 | 7.12 × 10−5 | 1.44 × 10−3 |
| Gambia | Western Africa | 5.64 × 10−3 | 2.79 × 10−3 | 7.02 × 10−4 | 7.46 × 10−3 | 3.87 × 10−4 | 2.03 × 10−4 | 7.09 × 10−5 | 1.38 × 10−3 |
| Ghana | Western Africa | 5.64 × 10−3 | 2.74 × 10−3 | 6.84 × 10−4 | 7.45 × 10−3 | 3.87 × 10−4 | 2.02 × 10−4 | 6.92 × 10−5 | 1.40 × 10−3 |
| Guinea | Western Africa | 5.64 × 10−3 | 2.70 × 10−3 | 6.92 × 10−4 | 7.24 × 10−3 | 3.87 × 10−4 | 1.98 × 10−4 | 6.90 × 10−5 | 1.35 × 10−3 |
| Guinea-Bissau | Western Africa | 5.64 × 10−3 | 2.67 × 10−3 | 7.21 × 10−4 | 7.13 × 10−3 | 3.87 × 10−4 | 1.96 × 10−4 | 7.15 × 10−5 | 1.36 × 10−3 |
| Liberia | Western Africa | 5.64 × 10−3 | 2.59 × 10−3 | 6.55 × 10−4 | 7.31 × 10−3 | 3.87 × 10−4 | 1.97 × 10−4 | 6.66 × 10−5 | 1.39 × 10−3 |
| Mali | Western Africa | 5.64 × 10−3 | 2.77 × 10−3 | 6.95 × 10−4 | 7.31 × 10−3 | 3.87 × 10−4 | 2.02 × 10−4 | 7.22 × 10−5 | 1.36 × 10−3 |
| Mauritania | Western Africa | 5.64 × 10−3 | 2.81 × 10−3 | 7.02 × 10−4 | 7.52 × 10−3 | 3.87 × 10−4 | 2.00 × 10−4 | 6.95 × 10−5 | 1.36 × 10−3 |
| Niger | Western Africa | 5.64 × 10−3 | 2.78 × 10−3 | 6.90 × 10−4 | 7.57 × 10−3 | 3.87 × 10−4 | 2.00 × 10−4 | 6.99 × 10−5 | 1.42 × 10−3 |
| Nigeria | Western Africa | 4.44 × 10−3 | 2.30 × 10−3 | 5.88 × 10−4 | 6.05 × 10−3 | 3.24 × 10−4 | 1.75 × 10−4 | 6.23 × 10−5 | 1.16 × 10−3 |
| Senegal | Western Africa | 5.64 × 10−3 | 2.79 × 10−3 | 6.89 × 10−4 | 7.51 × 10−3 | 3.87 × 10−4 | 2.01 × 10−4 | 7.07 × 10−5 | 1.39 × 10−3 |
| Sierra Leone | Western Africa | 5.64 × 10−3 | 2.74 × 10−3 | 7.25 × 10−4 | 7.25 × 10−3 | 3.87 × 10−4 | 2.00 × 10−4 | 7.06 × 10−5 | 1.37 × 10−3 |
| Togo | Western Africa | 4.85 × 10−3 | 2.43 × 10−3 | 6.07 × 10−4 | 6.42 × 10−3 | 3.46 × 10−4 | 1.84 × 10−4 | 6.25 × 10−5 | 1.25 × 10−3 |
| Botswana | Southern Africa | 5.67 × 10−3 | 2.76 × 10−3 | 6.65 × 10−4 | 7.22 × 10−3 | 3.89 × 10−4 | 2.01 × 10−4 | 6.88 × 10−5 | 1.38 × 10−3 |
| Ethiopia | Southern Africa | 5.67 × 10−3 | 2.70 × 10−3 | 6.90 × 10−4 | 7.08 × 10−3 | 3.89 × 10−4 | 1.94 × 10−4 | 6.85 × 10−5 | 1.42 × 10−3 |
| Kenya | Southern Africa | 5.67 × 10−3 | 2.64 × 10−3 | 7.20 × 10−4 | 7.05 × 10−3 | 3.89 × 10−4 | 1.96 × 10−4 | 7.06 × 10−5 | 1.37 × 10−3 |
| Lesotho | Southern Africa | 5.67 × 10−3 | 2.67 × 10−3 | 6.85 × 10−4 | 7.17 × 10−3 | 3.89 × 10−4 | 1.92 × 10−4 | 6.76 × 10−5 | 1.33 × 10−3 |
| Madagascar | Southern Africa | 5.67 × 10−3 | 2.66 × 10−3 | 6.54 × 10−4 | 7.15 × 10−3 | 3.89 × 10−4 | 1.97 × 10−4 | 6.77 × 10−5 | 1.35 × 10−3 |
| Malawi | Southern Africa | 5.67 × 10−3 | 2.66 × 10−3 | 6.59 × 10−4 | 7.27 × 10−3 | 3.89 × 10−4 | 2.00 × 10−4 | 6.75 × 10−5 | 1.34 × 10−3 |
| Mozambique | Southern Africa | 4.67 × 10−3 | 2.24 × 10−3 | 5.78 × 10−4 | 6.15 × 10−3 | 3.36 × 10−4 | 1.71 × 10−4 | 5.96 × 10−5 | 1.25 × 10−3 |
| Namibia | Southern Africa | 5.67 × 10−3 | 2.56 × 10−3 | 6.45 × 10−4 | 7.03 × 10−3 | 3.89 × 10−4 | 1.87 × 10−4 | 6.57 × 10−5 | 1.36 × 10−3 |
| Somalia | Southern Africa | 5.72 × 10−3 | 2.82 × 10−3 | 7.48 × 10−4 | 7.59 × 10−3 | 3.91 × 10−4 | 2.04 × 10−4 | 7.27 × 10−5 | 1.42 × 10−3 |
| South Africa | Southern Africa | 8.27 × 10−4 | 4.09 × 10−4 | 1.06 × 10−4 | 1.12 × 10−3 | 9.37 × 10−5 | 4.93 × 10−5 | 1.68 × 10−5 | 3.51 × 10−4 |
| Swaziland | Southern Africa | 5.67 × 10−3 | 2.70 × 10−3 | 6.50 × 10−4 | 7.09 × 10−3 | 3.89 × 10−4 | 1.96 × 10−4 | 6.76 × 10−5 | 1.35 × 10−3 |
| Uganda | Southern Africa | 4.67 × 10−3 | 2.31 × 10−3 | 6.05 × 10−4 | 6.23 × 10−3 | 3.36 × 10−4 | 1.77 × 10−4 | 6.28 × 10−5 | 1.22 × 10−3 |
| United Republic of Tanzania | Southern Africa | 5.67 × 10−3 | 2.69 × 10−3 | 6.79 × 10−4 | 7.11 × 10−3 | 3.89 × 10−4 | 1.98 × 10−4 | 6.94 × 10−5 | 1.33 × 10−3 |
| Zambia | Southern Africa | 3.71 × 10−3 | 2.00 × 10−3 | 5.15 × 10−4 | 5.42 × 10−3 | 2.84 × 10−4 | 1.59 × 10−4 | 5.63 × 10−5 | 1.08 × 10−3 |
| Zimbabwe | Southern Africa | 4.55 × 10−3 | 2.30 × 10−3 | 5.74 × 10−4 | 6.14 × 10−3 | 3.30 × 10−4 | 1.76 × 10−4 | 6.06 × 10−5 | 1.19 × 10−3 |
| Antigua and Barbuda | Caribbean | 1.20 × 10−3 | 6.04 × 10−4 | 1.53 × 10−4 | 1.61 × 10−3 | 1.24 × 10−4 | 6.59 × 10−5 | 2.29 × 10−5 | 4.45 × 10−4 |
| Cuba | Caribbean | 7.57 × 10−4 | 3.74 × 10−4 | 9.46 × 10−5 | 1.03 × 10−3 | 8.77 × 10−5 | 4.56 × 10−5 | 1.63 × 10−5 | 3.22 × 10−4 |
| Dominican Rep. | Caribbean | 3.13 × 10−3 | 1.56 × 10−3 | 3.96 × 10−4 | 4.23 × 10−3 | 2.50 × 10−4 | 1.33 × 10−4 | 4.56 × 10−5 | 8.98 × 10−4 |
| Haiti | Caribbean | 2.75 × 10−3 | 1.34 × 10−3 | 3.41 × 10−4 | 3.64 × 10−3 | 2.28 × 10−4 | 1.17 × 10−4 | 4.13 × 10−5 | 8.32 × 10−4 |
| El Salvador | Central America | 7.14 × 10−4 | 3.53 × 10−4 | 9.17 × 10−5 | 9.57 × 10−4 | 8.40 × 10−5 | 4.42 × 10−5 | 1.55 × 10−5 | 3.13 × 10−4 |
| Guatemala | Central America | 8.27 × 10−4 | 4.01 × 10−4 | 1.05 × 10−4 | 1.07 × 10−3 | 9.37 × 10−5 | 4.87 × 10−5 | 1.72 × 10−5 | 3.46 × 10−4 |
| Honduras | Central America | 1.93 × 10−4 | 9.00 × 10−5 | 2.24 × 10−5 | 2.53 × 10−4 | 3.20 × 10−5 | 1.60 × 10−5 | 5.49 × 10−6 | 1.20 × 10−4 |
| Nicaragua | Central America | 1.03 × 10−4 | 4.43 × 10−5 | 1.03 × 10−5 | 1.31 × 10−4 | 2.01 × 10−5 | 9.55 × 10−6 | 3.08 × 10−6 | 7.90 × 10−5 |
| Bolivia | Andean Region | 3.10 × 10−4 | 1.45 × 10−4 | 3.81 × 10−5 | 3.96 × 10−4 | 4.53 × 10−5 | 2.27 × 10−5 | 8.12 × 10−6 | 1.66 × 10−4 |
| Colombia | Andean Region | 4.41 × 10−4 | 2.18 × 10−4 | 5.62 × 10−5 | 6.28 × 10−4 | 5.89 × 10−5 | 3.11 × 10−5 | 1.09 × 10−5 | 2.31 × 10−4 |
| Ecuador | Andean Region | 2.88 × 10−4 | 1.77 × 10−4 | 4.48 × 10−5 | 5.01 × 10−4 | 4.30 × 10−5 | 2.61 × 10−5 | 9.18 × 10−6 | 1.93 × 10−4 |
| Peru | Andean Region | 2.65 × 10−3 | 1.16 × 10−3 | 2.95 × 10−4 | 3.24 × 10−3 | 2.21 × 10−4 | 1.05 × 10−4 | 3.70 × 10−5 | 7.78 × 10−4 |
| Venezuela | Andean Region | 3.66 × 10−3 | 1.40 × 10−3 | 3.57 × 10−4 | 3.89 × 10−3 | 2.81 × 10−4 | 1.20 × 10−4 | 4.28 × 10−5 | 9.15 × 10−4 |
| Brazil | Brazil | 5.92 × 10−4 | 2.90 × 10−4 | 7.35 × 10−5 | 7.96 × 10−4 | 7.31 × 10−5 | 3.76 × 10−5 | 1.31 × 10−5 | 2.71 × 10−4 |
| Cambodia | Western Pacific | 5.66 × 10−3 | 2.81 × 10−3 | 7.12 × 10−4 | 7.66 × 10−3 | 3.88 × 10−4 | 2.03 × 10−4 | 7.10 × 10−5 | 1.44 × 10−3 |
| Laos | Western Pacific | 5.58 × 10−3 | 2.77 × 10−3 | 6.78 × 10−4 | 7.40 × 10−3 | 3.84 × 10−4 | 2.03 × 10−4 | 6.98 × 10−5 | 1.43 × 10−3 |
| Myanmar | Western Pacific | 5.66 × 10−3 | 2.90 × 10−3 | 7.19 × 10−4 | 7.67 × 10−3 | 3.88 × 10−4 | 2.08 × 10−4 | 7.33 × 10−5 | 1.43 × 10−3 |
| Vietnam | Western Pacific | 3.27 × 10−3 | 1.50 × 10−3 | 3.89 × 10−4 | 4.21 × 10−3 | 2.59 × 10−4 | 1.29 × 10−4 | 4.49 × 10−5 | 9.45 × 10−4 |
| Bangladesh | South Asia | 5.18 × 10−3 | 2.45 × 10−3 | 6.39 × 10−4 | 6.65 × 10−3 | 3.63 × 10−4 | 1.87 × 10−4 | 6.61 × 10−5 | 1.30 × 10−3 |
| Bhutan | South Asia | 4.67 × 10−3 | 2.25 × 10−3 | 6.11 × 10−4 | 6.21 × 10−3 | 3.36 × 10−4 | 1.74 × 10−4 | 6.12 × 10−5 | 1.21 × 10−3 |
| Nepal | South Asia | 5.71 × 10−3 | 2.84 × 10−3 | 7.36 × 10−4 | 7.62 × 10−3 | 3.91 × 10−4 | 2.05 × 10−4 | 7.20 × 10−5 | 1.44 × 10−3 |
| Pakistan | South Asia | 5.18 × 10−3 | 2.45 × 10−3 | 5.85 × 10−4 | 6.86 × 10−3 | 3.63 × 10−4 | 1.81 × 10−4 | 6.16 × 10−5 | 1.30 × 10−3 |
| Malaysia | South East Asia | 2.38 × 10−3 | 1.16 × 10−3 | 3.06 × 10−4 | 3.24 × 10−3 | 2.05 × 10−4 | 1.06 × 10−4 | 3.65 × 10−5 | 7.62 × 10−4 |
| Philippines | South East Asia | 4.67 × 10−3 | 2.37 × 10−3 | 5.89 × 10−4 | 6.37 × 10−3 | 3.36 × 10−4 | 1.80 × 10−4 | 6.25 × 10−5 | 1.22 × 10−3 |
| Sri Lanka | South East Asia | 1.53 × 10−3 | 7.58 × 10−4 | 1.93 × 10−4 | 2.03 × 10−3 | 1.47 × 10−4 | 7.66 × 10−5 | 2.70 × 10−5 | 5.59 × 10−4 |
| Thailand | South East Asia | 1.52 × 10−3 | 7.47 × 10−4 | 1.87 × 10−4 | 2.04 × 10−3 | 1.47 × 10−4 | 7.73 × 10−5 | 2.65 × 10−5 | 5.49 × 10−4 |
| India | India | 4.18 × 10−3 | 2.02 × 10−3 | 4.89 × 10−4 | 5.52 × 10−3 | 3.10 × 10−4 | 1.58 × 10−4 | 5.41 × 10−5 | 1.12 × 10−3 |
| China | China | 4.25 × 10−3 | 1.86 × 10−3 | 4.76 × 10−4 | 5.10 × 10−3 | 3.14 × 10−4 | 1.51 × 10−4 | 5.30 × 10−5 | 1.06 × 10−3 |
| Indonesia | Indonesia | 3.38 × 10−3 | 1.65 × 10−3 | 4.20 × 10−4 | 4.52 × 10−3 | 2.65 × 10−4 | 1.36 × 10−4 | 4.72 × 10−5 | 9.80 × 10−4 |
| Bahrain | Middle East | 3.85 × 10−3 | 1.86 × 10−3 | 4.83 × 10−4 | 5.22 × 10−3 | 2.92 × 10−4 | 1.55 × 10−4 | 5.36 × 10−5 | 1.08 × 10−3 |
| Iran | Middle East | 3.88 × 10−3 | 1.94 × 10−3 | 4.87 × 10−4 | 5.33 × 10−3 | 2.93 × 10−4 | 1.55 × 10−4 | 5.23 × 10−5 | 1.03 × 10−3 |
| Iraq | Middle East | 3.85 × 10−3 | 1.86 × 10−3 | 4.70 × 10−4 | 5.07 × 10−3 | 2.92 × 10−4 | 1.49 × 10−4 | 5.19 × 10−5 | 1.03 × 10−3 |
| Israel | Middle East | 8.20 × 10−4 | 4.02 × 10−4 | 1.02 × 10−4 | 1.11 × 10−3 | 9.30 × 10−5 | 4.75 × 10−5 | 1.69 × 10−5 | 3.48 × 10−4 |
| Jordan | Middle East | 3.85 × 10−3 | 1.93 × 10−3 | 4.80 × 10−4 | 5.22 × 10−3 | 2.92 × 10−4 | 1.51 × 10−4 | 5.32 × 10−5 | 1.10 × 10−3 |
| Kuwait | Middle East | 3.85 × 10−3 | 1.94 × 10−3 | 4.79 × 10−4 | 5.13 × 10−3 | 2.92 × 10−4 | 1.51 × 10−4 | 5.31 × 10−5 | 1.04 × 10−3 |
| Lebanon | Middle East | 3.85 × 10−3 | 1.85 × 10−3 | 4.65 × 10−4 | 5.20 × 10−3 | 2.92 × 10−4 | 1.50 × 10−4 | 5.24 × 10−5 | 1.07 × 10−3 |
| Oman | Middle East | 4.18 × 10−3 | 2.12 × 10−3 | 5.25 × 10−4 | 5.61 × 10−3 | 3.10 × 10−4 | 1.61 × 10−4 | 5.64 × 10−5 | 1.13 × 10−3 |
| Qatar | Middle East | 3.85 × 10−3 | 1.94 × 10−3 | 4.78 × 10−4 | 5.25 × 10−3 | 2.92 × 10−4 | 1.56 × 10−4 | 5.35 × 10−5 | 1.06 × 10−3 |
| Saudi Arabia | Middle East | 3.85 × 10−3 | 1.86 × 10−3 | 4.81 × 10−4 | 5.04 × 10−3 | 2.92 × 10−4 | 1.50 × 10−4 | 5.34 × 10−5 | 1.10 × 10−3 |
| Syria | Middle East | 3.85 × 10−3 | 1.87 × 10−3 | 4.77 × 10−4 | 5.33 × 10−3 | 2.92 × 10−4 | 1.51 × 10−4 | 5.26 × 10−5 | 1.08 × 10−3 |
| United Arab Emirates | Middle East | 3.85 × 10−3 | 1.93 × 10−3 | 5.12 × 10−4 | 5.16 × 10−3 | 2.92 × 10−4 | 1.54 × 10−4 | 5.50 × 10−5 | 1.09 × 10−3 |
| Yemen | Middle East | 3.85 × 10−3 | 1.94 × 10−3 | 4.73 × 10−4 | 5.16 × 10−3 | 2.92 × 10−4 | 1.55 × 10−4 | 5.21 × 10−5 | 1.08 × 10−3 |
| Turkey | Eurasia | 3.62 × 10−3 | 1.78 × 10−3 | 4.38 × 10−4 | 4.69 × 10−3 | 2.79 × 10−4 | 1.44 × 10−4 | 4.94 × 10−5 | 1.02 × 10−3 |
| Armenia | Eurasia | 3.62 × 10−3 | 1.84 × 10−3 | 4.44 × 10−4 | 4.87 × 10−3 | 2.79 × 10−4 | 1.50 × 10−4 | 4.98 × 10−5 | 1.03 × 10−3 |
| Azerbaijan | Eurasia | 3.62 × 10−3 | 1.71 × 10−3 | 4.33 × 10−4 | 4.83 × 10−3 | 2.79 × 10−4 | 1.41 × 10−4 | 4.84 × 10−5 | 1.02 × 10−3 |
| Georgia | Eurasia | 3.62 × 10−3 | 1.76 × 10−3 | 4.60 × 10−4 | 4.87 × 10−3 | 2.79 × 10−4 | 1.45 × 10−4 | 5.05 × 10−5 | 1.03 × 10−3 |
| Afghanistan | Eurasia | 5.62 × 10−3 | 2.76 × 10−3 | 7.40 × 10−4 | 7.50 × 10−3 | 3.86 × 10−4 | 2.04 × 10−4 | 7.34 × 10−5 | 1.43 × 10−3 |
| Korea (north) | Eurasia | 3.62 × 10−3 | 1.85 × 10−3 | 4.52 × 10−4 | 4.88 × 10−3 | 2.79 × 10−4 | 1.48 × 10−4 | 5.13 × 10−5 | 1.02 × 10−3 |
| Kazakhstan | Eurasia | 3.62 × 10−3 | 1.75 × 10−3 | 4.47 × 10−4 | 4.76 × 10−3 | 2.79 × 10−4 | 1.44 × 10−4 | 5.15 × 10−5 | 1.02 × 10−3 |
| Kyrgyzstan | Eurasia | 3.62 × 10−3 | 1.77 × 10−3 | 4.50 × 10−4 | 4.83 × 10−3 | 2.79 × 10−4 | 1.44 × 10−4 | 5.02 × 10−5 | 1.04 × 10−3 |
| Mongolia | Eurasia | 4.18 × 10−3 | 2.14 × 10−3 | 5.60 × 10−4 | 5.74 × 10−3 | 3.10 × 10−4 | 1.70 × 10−4 | 5.84 × 10−5 | 1.14 × 10−3 |
| Tajikistan | Eurasia | 4.20 × 10−3 | 2.10 × 10−3 | 5.43 × 10−4 | 5.70 × 10−3 | 3.11 × 10−4 | 1.65 × 10−4 | 5.78 × 10−5 | 1.17 × 10−3 |
| Turkmenistan | Eurasia | 3.62 × 10−3 | 1.76 × 10−3 | 4.57 × 10−4 | 4.90 × 10−3 | 2.79 × 10−4 | 1.46 × 10−4 | 5.10 × 10−5 | 1.02 × 10−3 |
| Uzbekistan | Eurasia | 2.10 × 10−3 | 1.05 × 10−3 | 2.58 × 10−4 | 2.87 × 10−3 | 1.86 × 10−4 | 9.93 × 10−5 | 3.38 × 10−5 | 7.26 × 10−4 |
| Country | Cluster | Dogs | Cats | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Interquartile Range | Mean | Median | Interquartile Range | ||||
| 25th Percentile | 75th Percentile | 25th Percentile | 75th Percentile | ||||||
| Argentina | Dog Rabies Free Latin America | 4.78 × 10−7 | 4.70 × 10−7 | 4.00 × 10−7 | 5.46 × 10−7 | 7.92 × 10−7 | 7.58 × 10−7 | 5.90 × 10−7 | 1.42 × 10−6 |
| Chile | Dog Rabies Free Latin America | 4.20 × 10−8 | 2.89 × 10−8 | 1.21 × 10−8 | 5.79 × 10−8 | 0 | 0 | 0 | 0 |
| Paraguay | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Uruguay | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Costa Rica | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Panama | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bahamas | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Barbados | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dominica | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grenada | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Jamaica | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| St. Kitts & Nevis | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| St. Lucia | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| St. Vincent & the Grenadines | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trinidad & Tobago | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Belize | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Guyana | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Suriname | Dog Rabies Free Latin America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mexico | Dog Rabies Free Latin America | 2.19 × 10−7 | 2.15 × 10−7 | 1.87 × 10−7 | 2.47 × 10−7 | 2.86 × 10−8 | 1.95 × 10−8 | 7.92 × 10−9 | 1.08 × 10−7 |
| Canada | North America | 8.12 × 10−7 | 8.03 × 10−7 | 7.21 × 10−7 | 8.93 × 10−7 | 3.39 × 10−7 | 3.31 × 10−7 | 2.74 × 10−7 | 5.41 × 10−7 |
| United States | North America | 8.89 × 10−7 | 8.88 × 10−7 | 8.57 × 10−7 | 9.20 × 10−7 | 3.94 × 10−6 | 3.93 × 10−6 | 3.71 × 10−6 | 4.44 × 10−6 |
| Japan | Rabies Free Asia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maldives | Rabies Free Asia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Korea (south) | Rabies Free Asia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Singapore | Rabies Free Asia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brunei | Rabies Free Asia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| East Timor | Rabies Free Asia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Australia | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cook islands | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fiji | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kiribati | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Marshall Islands | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Micronesia | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nauru | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Zealand | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Niue | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Palau | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Papua New Guinea | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Samoa | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Solomon Islands | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tonga | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tuvalu | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vanuatu | Rabies Free Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Andorra | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Austria | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Belgium | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Croatia | Rabies Free Europe | 3.67 × 10−7 | 2.43 × 10−7 | 1.01 × 10−7 | 5.03 × 10−7 | 0 | 0 | 0 | 0 |
| Cyprus | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Czech Republic | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Denmark | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Estonia | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| France | Rabies Free Europe | 2.23 × 10−8 | 1.50 × 10−8 | 6.23 × 10−9 | 3.01 × 10−8 | 2.19 × 10−8 | 1.43 × 10−8 | 5.94 × 10−9 | 8.68 × 10−8 |
| Germany | Rabies Free Europe | 1.76 × 10−8 | 1.18 × 10−8 | 4.79 × 10−9 | 2.37 × 10−8 | 0 | 0 | 0 | 0 |
| Greece | Rabies Free Europe | 4.17 × 10−7 | 3.62 × 10−7 | 2.29 × 10−7 | 5.45 × 10−7 | 1.36 × 10−7 | 8.97 × 10−8 | 3.83 × 10−8 | 5.45 × 10−7 |
| Hungary | Rabies Free Europe | 1.52 × 10−7 | 1.01 × 10−7 | 4.15 × 10−8 | 2.08 × 10−7 | 0 | 0 | 0 | 0 |
| Iceland | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ireland | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Italy | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Latvia | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuania | Rabies Free Europe | 5.36 × 10−7 | 3.52 × 10−7 | 1.46 × 10−7 | 7.25 × 10−7 | 0 | 0 | 0 | 0 |
| Luxembourg | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monaco | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norway | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Poland | Rabies Free Europe | 1.59 × 10−7 | 1.30 × 10−7 | 7.16 × 10−8 | 2.12 × 10−7 | 7.77 × 10−8 | 5.09 × 10−8 | 2.06 × 10−8 | 3.04 × 10−7 |
| Portugal | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Romania | Rabies Free Europe | 6.14 × 10−7 | 5.39 × 10−7 | 3.68 × 10−7 | 7.85 × 10−7 | 3.01 × 10−7 | 2.40 × 10−7 | 1.33 × 10−7 | 9.27 × 10−7 |
| San Marino | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slovakia | Rabies Free Europe | 5.46 × 10−7 | 4.43 × 10−7 | 2.49 × 10−7 | 7.29 × 10−7 | 0 | 0 | 0 | 0 |
| Slovenia | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spain | Rabies Free Europe | 3.19 × 10−8 | 2.16 × 10−8 | 8.82 × 10−9 | 4.36 × 10−8 | 0 | 0 | 0 | 0 |
| Sweden | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Switzerland | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| United Kingdom | Rabies Free Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Albania | Eastern Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Belarus | Eastern Europe | 3.50 × 10−5 | 3.48 × 10−5 | 3.30 × 10−5 | 3.68 × 10−5 | 2.33 × 10−5 | 2.31 × 10−5 | 2.12 × 10−5 | 2.88 × 10−5 |
| Bosnia and Herzegovina | Eastern Europe | 8.98 × 10−7 | 7.50 × 10−7 | 4.38 × 10−7 | 1.20 × 10−6 | 0 | 0 | 0 | 0 |
| Bulgaria | Eastern Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moldova | Eastern Europe | 6.69 × 10−5 | 6.67 × 10−5 | 6.26 × 10−5 | 7.08 × 10−5 | 2.83 × 10−5 | 2.80 × 10−5 | 2.53 × 10−5 | 3.66 × 10−5 |
| Serbia | Eastern Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Macedonia | Eastern Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ukraine | Eastern Europe | 6.34 × 10−5 | 6.34 × 10−5 | 6.11 × 10−5 | 6.56 × 10−5 | 7.14 × 10−5 | 7.14 × 10−5 | 6.59 × 10−5 | 8.25 × 10−5 |
| Russian Federation | Eastern Europe | 3.12 × 10−5 | 3.12 × 10−5 | 3.01 × 10−5 | 3.22 × 10−5 | 2.15 × 10−5 | 2.15 × 10−5 | 1.98 × 10−5 | 2.49 × 10−5 |
| Montenegro | Eastern Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cape Verde | Rabies Free African Islands | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sao Tome and Principe | Rabies Free African Islands | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comoros | Rabies Free African Islands | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mauritius | Rabies Free African Islands | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Seychelles | Rabies Free African Islands | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Ribadeau-Dumas, F.; Cliquet, F.; Gautret, P.; Robardet, E.; Le Pen, C.; Bourhy, H. Travel-Associated Rabies in Pets and Residual Rabies Risk, Western Europe. Emerg. Infect. Dis. 2016, 22, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.C.; Stull, J.W.; Weese, J.S. Impact of Dog Transport on High-Risk Infectious Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Polak, K. Dog Transport and Infectious Disease Risk: An International Perspective. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Stull, J.W.; Anderson, M.E.C.; Weese, J.S. The Dynamic Nature of Canine and Feline Infectious Disease Risks in the Twenty-first Century. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Amanatin, A.; Sudarnika, E.; Lukman, D.W.; Wibawan, I.W.T. Risk assessment on rabies entry through hunting dog movement with semi-quantitative approach to Sumatera Island, Indonesia. J. Adv. Vet. Anim. Res. 2019, 6, 148–157. [Google Scholar] [CrossRef]
- Brookes, V.J.; Keponge-Yombo, A.; Thomson, D.; Ward, M.P. Risk assessment of the entry of canine-rabies into Papua New Guinea via sea and land routes. Prev. Vet. Med. 2017, 145, 49–66. [Google Scholar] [CrossRef]
- Goddard, A.D.; Donaldson, N.M.; Horton, D.L.; Kosmider, R.; Kelly, L.A.; Sayers, A.R.; Breed, A.C.; Freuling, C.M.; Müller, T.; Shaw, S.E.; et al. A quantitative release assessment for the noncommercial movement of companion animals: Risk of rabies reintroduction to the United Kingdom. Risk Anal. Off. Publ. Soc. Risk Anal. 2012, 32, 1769–1783. [Google Scholar] [CrossRef]
- Have, P.; Alban, L.; Berndtsson, L.T.; Cliquet, F.; Hostnik, P.; Rodeia, S.C.; Sanaa, M. Risk of rabies introduction by non-commercial movement of pets. Dev. Biol. 2008, 131, 177–185. [Google Scholar]
- Hudson, E.G.; Brookes, V.J.; Ward, M.P. Assessing the Risk of a Canine Rabies Incursion in Northern Australia. Front. Vet. Sci. 2017, 4, 141. [Google Scholar] [CrossRef]
- Jones, R.D.; Kelly, L.; Fooks, A.R.; Wooldridge, M. Quantitative risk assessment of rabies entering Great Britain from North America via cats and dogs. Risk Anal. Off. Publ. Soc. Risk Anal. 2005, 25, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Kamakawa, H.; Koiwai, M.; Satomura, S.; Eto, M.; Sugiura, K. Quantitative assessment of the risk of rabies entering Japan through the importation of dogs and cats from the USA. Epidemiol. Infect. 2009, 137, 1149–1154. [Google Scholar] [CrossRef]
- Kwan, N.C.L.; Sugiura, K.; Hosoi, Y.; Yamada, A.; Snary, E.L. Quantitative risk assessment of the introduction of rabies into Japan through the importation of dogs and cats worldwide. Epidemiol. Infect. 2017, 145, 1168–1182. [Google Scholar] [CrossRef]
- Kwan, N.C.L.; Ogawa, H.; Yamada, A.; Sugiura, K. Quantitative risk assessment of the introduction of rabies into Japan through the illegal landing of dogs from Russian fishing boats in the ports of Hokkaido, Japan. Prev. Vet. Med. 2016, 128, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Napp, S.; Casas, M.; Moset, S.; Paramio, J.L.; Casal, J. Quantitative risk assessment model of canine rabies introduction: Application to the risk to the European Union from Morocco. Epidemiol. Infect. 2010, 138, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Ramnial, V.; Kosmider, R.; Aylan, O.; Freuling, C.; Müller, T.; Fooks, A.R. Quantitative risk assessment to compare the risk of rabies entering the UK from Turkey via quarantine, the Pet Travel Scheme and the EU Pet Movement Policy. Epidemiol. Infect. 2010, 138, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.Y.; Wu, P.I.; Yang, P.C.; Tsai, Y.L.; Chang, C.C. A quantitative risk assessment model to evaluate effective border control measures for rabies prevention. Vet. Res. 2010, 41, 11. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons: Chichester, UK, 2018; ISBN 978-1-118-28028-7. [Google Scholar]
- Vose, D. Risk Analysis: A Quantitative Guide; John Wiley & Sons: Chichester, UK, 2008; ISBN 978-0-470-51284-5. [Google Scholar]
- Great Britain Advisory Group on Quarantine. Quarantine and Rabies: A Reappraisal: Report by the Advisory Group on Quarantine to the Right Honourable Nick Brown MP, Minister of Agriculture, Fisheries and Food: Summary of recommendations; Ministry of Agriculture, Fisheries and Food: London, UK, 1998. [Google Scholar]
- Nel, L.H. Discrepancies in data reporting for rabies, Africa. Emerg. Infect. Dis. 2013, 19, 529–533. [Google Scholar] [CrossRef]
- Kitala, P.M.; McDermott, J.J.; Kyule, M.N.; Gathuma, J.M. Community-based active surveillance for rabies in Machakos District, Kenya. Prev. Vet. Med. 2000, 44, 73–85. [Google Scholar] [CrossRef]
- Fuentes, B.; Panunzio, A.; Larreal, Y.; Leal, J.; Villarroel, F.; Parra, I.; Velasco, D.; Prieto, Y. Presence of urban rabies in Zulia State, Venezuela. Years 1996–2006. Investig. Clin. 2008, 49, 487–498. [Google Scholar]
- Lembo, T.; Hampson, K.; Haydon, D.T.; Craft, M.; Dobson, A.; Dushoff, J.; Ernest, E.; Hoare, R.; Kaare, M.; Mlengeya, T.; et al. Exploring reservoir dynamics: A case study of rabies in the Serengeti ecosystem. J. Appl. Ecol. 2008, 45, 1246–1257. [Google Scholar] [CrossRef]
- Tenzin; Dhand, N.K.; Ward, M.P. Patterns of rabies occurrence in Bhutan between 1996 and 2009. Zoonoses Public Health 2011, 58, 463–471. [Google Scholar] [CrossRef]
- Anonymous. Rabies confirmed in an imported kitten in France. Vet. Rec. 2013, 173, 435. [Google Scholar] [CrossRef]
- Cleaveland, S.; Hampson, K. Rabies elimination research: Juxtaposing optimism, pragmatism and realism. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef]
- Fehlner-Gardiner, C. Rabies control in North America—Past, present and future. Rev. Sci. Tech. Int. Off. Epizoot. 2018, 37, 421–437. [Google Scholar] [CrossRef]
- Hercules, Y.; Bryant, N.J.; Wallace, R.M.; Nelson, R.; Palumbo, G.; Williams, J.N.; Ocana, J.M.; Shapiro, S.; Leavitt, H.; Slavinsk, S.; et al. Rabies in a Dog Imported from Egypt—Connecticut, 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1388–1391. [Google Scholar] [CrossRef]
- Singh, A.J.; Chipman, R.B.; de Fijter, S.; Gary, R.; Haskell, M.G.; Kirby, J.; Yu, L.; Condori, R.E.; Orciari, L.; Wallace, R. Translocation of a Stray Cat Infected with Rabies from North Carolina to a Terrestrial Rabies-Free County in Ohio, 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1174–1177. [Google Scholar] [CrossRef]
- Velasco-Villa, A.; Mauldin, M.R.; Shi, M.; Escobar, L.E.; Gallardo-Romero, N.F.; Damon, I.; Olson, V.A.; Streicker, D.G.; Emerson, G. The history of rabies in the Western Hemisphere. Antivir. Res. 2017, 146, 221–232. [Google Scholar] [CrossRef]
- Jemberu, W.T.; Molla, W.; Almaw, G.; Alemu, S. Incidence of rabies in humans and domestic animals and people’s awareness in North Gondar Zone, Ethiopia. PLoS Negl. Trop. Dis. 2013, 7, e2216. [Google Scholar] [CrossRef]
- Department of Livestock. Ministry of Agriculture and Forests, Royal Government of Bhutan. Rabies Prevention and Control Project for Bhutan. Available online: https://www.ncah.gov.bt/Downloads/File_102.pdf (accessed on 13 December 2019).
- Tenzin, T.; Dhand, N.K.; Dorjee, J.; Ward, M.P. Re-emergence of rabies in dogs and other domestic animals in eastern Bhutan, 2005–2007. Epidemiol. Infect. 2011, 139, 220–225. [Google Scholar] [CrossRef]
- Cleaveland, S.; Kaare, M.; Tiringa, P.; Mlengeya, T.; Barrat, J. A dog rabies vaccination campaign in rural Africa: Impact on the incidence of dog rabies and human dog-bite injuries. Vaccine 2003, 21, 1965–1973. [Google Scholar] [CrossRef]
- Gill, G.S.; Singh, B.B.; Dhand, N.K.; Aulakh, R.S.; Sandhu, B.S.; Ward, M.P.; Brookes, V.J. Estimation of the incidence of animal rabies in Punjab, India. PLoS ONE 2019, 14, e0222198. [Google Scholar] [CrossRef] [PubMed]
- Kayali, U.; Mindekem, R.; Yémadji, N.; Oussiguéré, A.; Naïssengar, S.; Ndoutamia, A.G.; Zinsstag, J. Incidence of canine rabies in N’Djaména, Chad. Prev. Vet. Med. 2003, 61, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kitala, P.M.; McDermott, J.J.; Kyule, M.N.; Cathuma, J.M. Features of dog ecology relevant to rabies spread in Machakos District, Kenya. Onderstepoort J. Vet. Res. 1993, 60, 445–449. [Google Scholar]
- Sharma, B.; Dhand, N.K.; Timsina, N.; Ward, M.P. Reemergence of Rabies in Chhukha District, Bhutan, 2008. Emerg. Infect. Dis. 2010, 16, 1925–1930. [Google Scholar] [CrossRef]
- Hampson, K.; Abela-Ridder, B.; Brunker, K.; Bucheli, S.T.M.; Carvalho, M.; Caldas, E.; Changalucha, J.; Cleaveland, S.; Dushoff, J.; Gutierrez, V.; et al. Surveillance to Establish Elimination of Transmission and Freedom from Dog-mediated Rabies. bioRxiv 2016, 096883. [Google Scholar] [CrossRef]
- Sambo, M.; Johnson, P.C.D.; Hotopp, K.; Changalucha, J.; Cleaveland, S.; Kazwala, R.; Lembo, T.; Lugelo, A.; Lushasi, K.; Maziku, M.; et al. Comparing Methods of Assessing Dog Rabies Vaccination Coverage in Rural and Urban Communities in Tanzania. Front. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Lushasi, K.S.; Cleaveland, S.; Changalucha, J.J.; Haydon, D.; Kazwala, R.; Lembo, T.; Masoud, M.; Maziku, M.; Mchau, G.; Mtema, Z.; et al. Progress towards rabies elimination from Pemba Island, Southern Tanzania. Online J. Public Health Inform. 2017, 9. [Google Scholar] [CrossRef]
- Léchenne, M.; Oussiguere, A.; Naissengar, K.; Mindekem, R.; Mosimann, L.; Rives, G.; Hattendorf, J.; Moto, D.D.; Alfaroukh, I.O.; Zinsstag, J.; et al. Operational performance and analysis of two rabies vaccination campaigns in N’Djamena, Chad. Vaccine 2016, 34, 571–577. [Google Scholar] [CrossRef]
- Coetzer, A.; Scott, T.P.; Noor, K.; Gwenhure, L.F.; Nel, L.H. A Novel Integrated and Labile eHealth System for Monitoring Dog Rabies Vaccination Campaigns. Vaccines 2019, 7, 108. [Google Scholar] [CrossRef]
- Laager, M.; Mbilo, C.; Madaye, E.A.; Naminou, A.; Léchenne, M.; Tschopp, A.; Naïssengar, S.K.; Smieszek, T.; Zinsstag, J.; Chitnis, N. The importance of dog population contact network structures in rabies transmission. PLoS Negl. Trop. Dis. 2018, 12, e0006680. [Google Scholar] [CrossRef] [PubMed]
- Dürr, S.; Naïssengar, S.; Mindekem, R.; Diguimbye, C.; Niezgoda, M.; Kuzmin, I.; Rupprecht, C.E.; Zinsstag, J. Rabies Diagnosis for Developing Countries. PLoS Negl. Trop. Dis. 2008, 2, e206. [Google Scholar] [CrossRef] [PubMed]
- Mindekem, R.; Kayali, U.; Yemadji, N.; Ndoutamia, A.G.; Zinsstag, J. [Impact of canine demography on rabies transmission in N’djamena, Chad]. Med. Trop. Rev. Corps Sante Colon. 2005, 65, 53–58. [Google Scholar]
- Corrieri, L.; Adda, M.; Miklósi, Á.; Kubinyi, E. Companion and free-ranging Bali dogs: Environmental links with personality traits in an endemic dog population of South East Asia. PLoS ONE 2018, 13, e0197354. [Google Scholar] [CrossRef] [PubMed]
- Putra, A.A.G.; Hampson, K.; Girardi, J.; Hiby, E.; Knobel, D.; Mardiana, I.W.; Townsend, S.; Scott-Orr, H. Response to a rabies epidemic, Bali, Indonesia, 2008–2011. Emerg. Infect. Dis. 2013, 19, 648–651. [Google Scholar] [CrossRef]
- Eng, T.R.; Fishbein, D.B.; Talamante, H.E.; Hall, D.B.; Chavez, G.F.; Dobbins, J.G.; Muro, F.J.; Bustos, J.L.; de los Angeles Ricardy, M.; Munguia, A. Urban epizootic of rabies in Mexico: Epidemiology and impact of animal bite injuries. Bull. World Health Organ. 1993, 71, 615–624. [Google Scholar]
- Mitmoonpitak, C.; Tepsumethanon, V.; Wilde, H. Rabies in Thailand. Epidemiol. Infect. 1998, 120, 165–169. [Google Scholar] [CrossRef]
- Toukhsati, S.R.; Phillips, C.J.C.; Podberscek, A.L.; Coleman, G.J. Companion Animals in Thailand: Human Factors that Predict Sterilization of Cats and Dogs. Soc. Anim. 2015, 23, 569–593. [Google Scholar] [CrossRef]
- Queiroz, L.H.; de Carvalho, C.; Buso, D.S.; de Lucca Ferrari, C.I.; Pedro, W.A. Perfil epidemiológico da raiva na região Noroeste do Estado de São Paulo no período de 1993 a 2007. Rev. Soc. Bras. Med. Trop. 2009, 42, 9–14. [Google Scholar] [CrossRef][Green Version]
- Thiptara, A.; Atwill, E.R.; Kongkaew, W.; Chomel, B.B. Epidemiologic Trends of Rabies in Domestic Animals in Southern Thailand, 1994–2008. Am. J. Trop. Med. Hyg. 2011, 85, 138–145. [Google Scholar] [CrossRef]
- Alves, M.C.G.P.; de Matos, M.R.; de Lourdes Reichmann, M.; Dominguez, M.H. Estimation of the dog and cat population in the State of São Paulo. Rev. Saúde Pública 2005, 39, 891–897. [Google Scholar] [CrossRef]
- The World Bank. Population, Total|Data. Available online: https://data.worldbank.org/indicator/SP.POP.TOTL?end=2019&start=1960 (accessed on 15 December 2019).
- Bolker, B. bbmle: Tools for General Maximum Likelihood Estimation; R Package Version 1.0.22. 2019. Available online: https://cran.r-project.org/package=bbmle (accessed on 15 December 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Pouillot, R.; Delignette-Muller, M.L. Evaluating variability and uncertainty separately in microbial quantitative risk assessment using two R packages. Int. J. Food Microbiol. 2010, 142, 330–340. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. Rabies in Canada. Available online: https://www.inspection.gc.ca/animals/terrestrial-animals/diseases/reportable/rabies/rabies-in-canada/eng/ (accessed on 12 December 2019).
- CDC. Rabies Surveillance in the United States. Available online: https://www.cdc.gov/rabies/resources/publications/index.html (accessed on 12 December 2019).
- AVMA. U.S. Pet Ownership Statistics. Available online: https://www.avma.org/resources-tools/reports-statistics/us-pet-ownership-statistics (accessed on 15 December 2019).
- ICSA. Latest Canadian Pet Population Figures Released. Available online: https://www.cahi-icsa.ca/press-releases/latest-canadian-pet-population-figures-released (accessed on 15 December 2019).
- OIE. World Animal Health Information Database (WAHID). Available online: https://www.oie.int/wahis_2/public/wahid.php (accessed on 12 December 2019).
- Rabies-Bulletin-Europe. Rabies Information System of the WHO Collaboration Centre for Rabies Surveillance and Research. Available online: https://www.who-rabies-bulletin.org/site-page/queries (accessed on 12 December 2019).
- Carvelli, A.; Iacoponi, F.; Scaramozzino, P. A Cross-Sectional Survey to Estimate the Cat Population and Ownership Profiles in a Semirural Area of Central Italy. BioMed Res. Int. 2016, 2016, 3796872. [Google Scholar] [CrossRef]
- Carvelli, A.; Scaramozzino, P.; Iacoponi, F.; Condoleo, R.; Della Marta, U. Size, demography, ownership profiles, and identification rate of the owned dog population in central Italy. PLoS ONE 2020, 15, e0240551. [Google Scholar] [CrossRef]
- FEDIAF. European Statistics. Available online: http://www.fediaf.org/who-we-are/european-statistics.html (accessed on 15 December 2019).
- PANAFTOSA—PAHO/WHO. Regional Information System for Epidemiological Surveillance of Rabies (SIRVERA). Available online: http://sirvera.panaftosa.org.br/ (accessed on 15 January 2020).
- Fishbein, D.B.; Frontini, M.G.; Dobbins, J.G.; Flores Collins, E.; Quiroz Huerta, G.; Gamez Rodriguez, J.J.; Woo-Ming, B.; Garza Ramos, J.; Belotto, A.J.; Balderas Torres, J.M. Prevention of canine rabies in rural Mexico: An epidemiologic study of vaccination campaigns. Am. J. Trop. Med. Hyg. 1992, 47, 317–327. [Google Scholar] [CrossRef]
- Flores-Ibarra, M.; Estrella-Valenzuela, G. Canine ecology and socioeconomic factors associated with dogs unvaccinated against rabies in a Mexican city across the US-Mexico border. Prev. Vet. Med. 2004, 62, 79–87. [Google Scholar] [CrossRef]
- Suzuki, K.; González, E.T.; Ascarrunz, G.; Loza, A.; Pérez, M.; Ruiz, G.; Rojas, L.; Mancilla, K.; Pereira, J.A.C.; Guzman, J.A.; et al. Antibody response to an anti-rabies vaccine in a dog population under field conditions in Bolivia. Zoonoses Public Health 2008, 55, 414–420. [Google Scholar] [CrossRef]
- Dias, R.A.; Garcia, R.; da Silva, D.F.; Amaku, M.; Ferreira Neto, J.S.; Ferreira, F. [Estimate of the owned canine and feline populations in urban area in Brazil]. Rev. Saude Publica 2004, 38, 565–570. [Google Scholar] [CrossRef]
- Ibarra, L.; Morales, M.A.; Acuña, P. Aspectos demográficos de la población de perros y gatos en la ciudad de Santiago, Chile. Av. Cienc. Vet. 2003, 18, 13–20. [Google Scholar] [CrossRef]
- Freire de Carvalho, M.; Vigilato, M.A.N.; Pompei, J.A.; Rocha, F.; Vokaty, A.; Molina-Flores, B.; Cosivi, O.; Del Rio Vilas, V.J. Rabies in the Americas: 1998–2014. PLoS Negl. Trop. Dis. 2018, 12, e0006271. [Google Scholar] [CrossRef]
- Cleaveland, S.; Dye, C. Maintenance of a microparasite infecting several host species: Rabies in the Serengeti. Parasitology 1995, 111, S33–S47. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Dushoff, J.; Bingham, J.; Brückner, G.; Ali, Y.H.; Dobson, A. Synchronous cycles of domestic dog rabies in sub-Saharan Africa and the impact of control efforts. Proc. Natl. Acad. Sci. USA 2007, 104, 7717–7722. [Google Scholar] [CrossRef] [PubMed]
- Morters, M.K.; Restif, O.; Hampson, K.; Cleaveland, S.; Wood, J.L.N.; Conlan, A.J.K. Evidence-based control of canine rabies: A critical review of population density reduction. J. Anim. Ecol. 2013, 82, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Dushoff, J.; Cleaveland, S.; Haydon, D.T.; Kaare, M.; Packer, C.; Dobson, A. Transmission Dynamics and Prospects for the Elimination of Canine Rabies. PLOS Biol. 2009, 7, e1000053. [Google Scholar] [CrossRef]
- Ma, X.; Monroe, B.P.; Cleaton, J.M.; Orciari, L.A.; Yager, P.; Li, Y.; Kirby, J.D.; Blanton, J.D.; Petersen, B.W.; Wallace, R.M. Rabies surveillance in the United States during 2016. J. Am. Vet. Med. Assoc. 2018, 252, 945–957. [Google Scholar] [CrossRef]
- Dalla Villa, P.; Kahn, S.; Stuardo, L.; Iannetti, L.; Di Nardo, A.; Serpell, J.A. Free-roaming dog control among OIE-member countries. Prev. Vet. Med. 2010, 97, 58–63. [Google Scholar] [CrossRef]


| Cluster | Nature of the Residual Rabies Risk | Species | Number of Rabies Cases | Human:Animal Ratios | References |
|---|---|---|---|---|---|
| North America | Wildlife and importations | Dog | 439 | Uniform (4.26; 4.52) | [61,62] †, [63,64] ‡ |
| Cat | 1552 | Uniform (4.46; 5.6) | |||
| Rabies Free Asia | Importations | Dog | 0 | ND | [65] † |
| Cat | 0 | ND | |||
| Rabies Free Oceania | Importations | Dog | 0 | ND | [65] † |
| Cat | 0 | ND | |||
| Rabies Free Europe | Importations (and Wildlife) | Dog | 23 | PERT (4.86; 8.18; 16.2) | [66] †, [67,68,69] ‡ |
| Cat | 8 | PERT (4.31; 7.47; 17.87) | |||
| Eastern Europe | Wildlife and importations | Dog | 5346 | Uniform (8.26; 9.49) | [66] †, [69] ‡ |
| Cat | 5215 | Uniform (6.42; 8.87) | |||
| Dog Rabies Free Latin America | Wildlife and importations | Dog | 63 | PERT (3.40; 4.64; 6.40) | [70] †, [71,72,73,74,75] ‡ |
| Cat | 11 | PERT (14.7; 20.56; 30.57) § | |||
| Rabies Free African Islands | Importations | Dog | 0 | ND | [65] † |
| Cat | 0 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crozet, G.; Rivière, J.; Canini, L.; Cliquet, F.; Robardet, E.; Dufour, B. Evaluation of the Worldwide Occurrence of Rabies in Dogs and Cats Using a Simple and Homogenous Framework for Quantitative Risk Assessments of Rabies Reintroduction in Disease-Free Areas through Pet Movements. Vet. Sci. 2020, 7, 207. https://doi.org/10.3390/vetsci7040207
Crozet G, Rivière J, Canini L, Cliquet F, Robardet E, Dufour B. Evaluation of the Worldwide Occurrence of Rabies in Dogs and Cats Using a Simple and Homogenous Framework for Quantitative Risk Assessments of Rabies Reintroduction in Disease-Free Areas through Pet Movements. Veterinary Sciences. 2020; 7(4):207. https://doi.org/10.3390/vetsci7040207
Chicago/Turabian StyleCrozet, Guillaume, Julie Rivière, Laetitia Canini, Florence Cliquet, Emmanuelle Robardet, and Barbara Dufour. 2020. "Evaluation of the Worldwide Occurrence of Rabies in Dogs and Cats Using a Simple and Homogenous Framework for Quantitative Risk Assessments of Rabies Reintroduction in Disease-Free Areas through Pet Movements" Veterinary Sciences 7, no. 4: 207. https://doi.org/10.3390/vetsci7040207
APA StyleCrozet, G., Rivière, J., Canini, L., Cliquet, F., Robardet, E., & Dufour, B. (2020). Evaluation of the Worldwide Occurrence of Rabies in Dogs and Cats Using a Simple and Homogenous Framework for Quantitative Risk Assessments of Rabies Reintroduction in Disease-Free Areas through Pet Movements. Veterinary Sciences, 7(4), 207. https://doi.org/10.3390/vetsci7040207





