Molecular Epidemiology and Genotyping of Infectious Bronchitis Virus and Avian Metapneumovirus in Backyard and Commercial Chickens in Jimma Zone, Southwestern Ethiopia
Abstract
1. Introduction
2. Materials and Methods
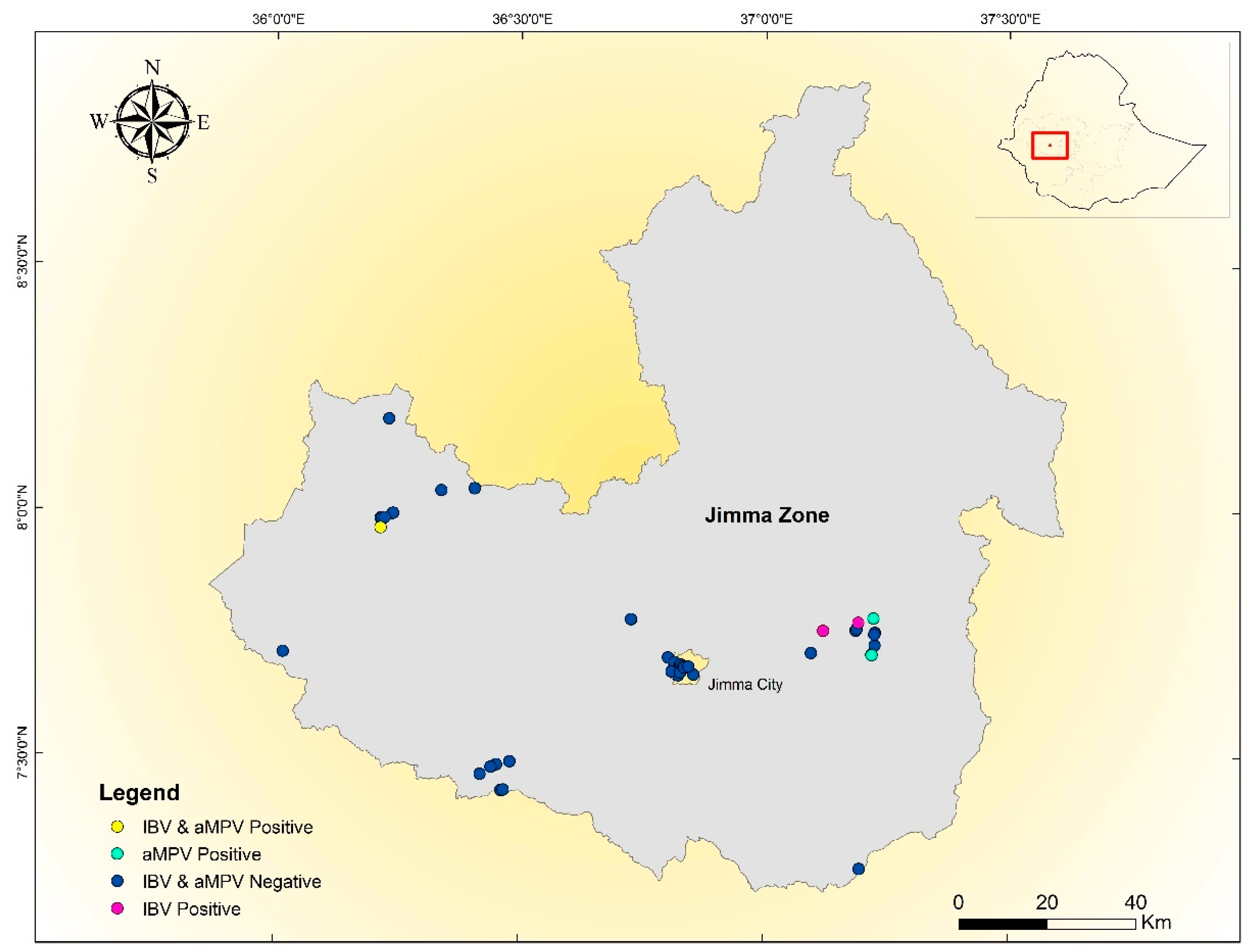
2.1. The Study Area
2.2. Study Design and Sampling Technique
2.3. Data Analysis
2.4. RNA Extraction, RT-PCR, and Sequencing
2.5. Sequence Analysis
2.6. Ethical Approval and Consent
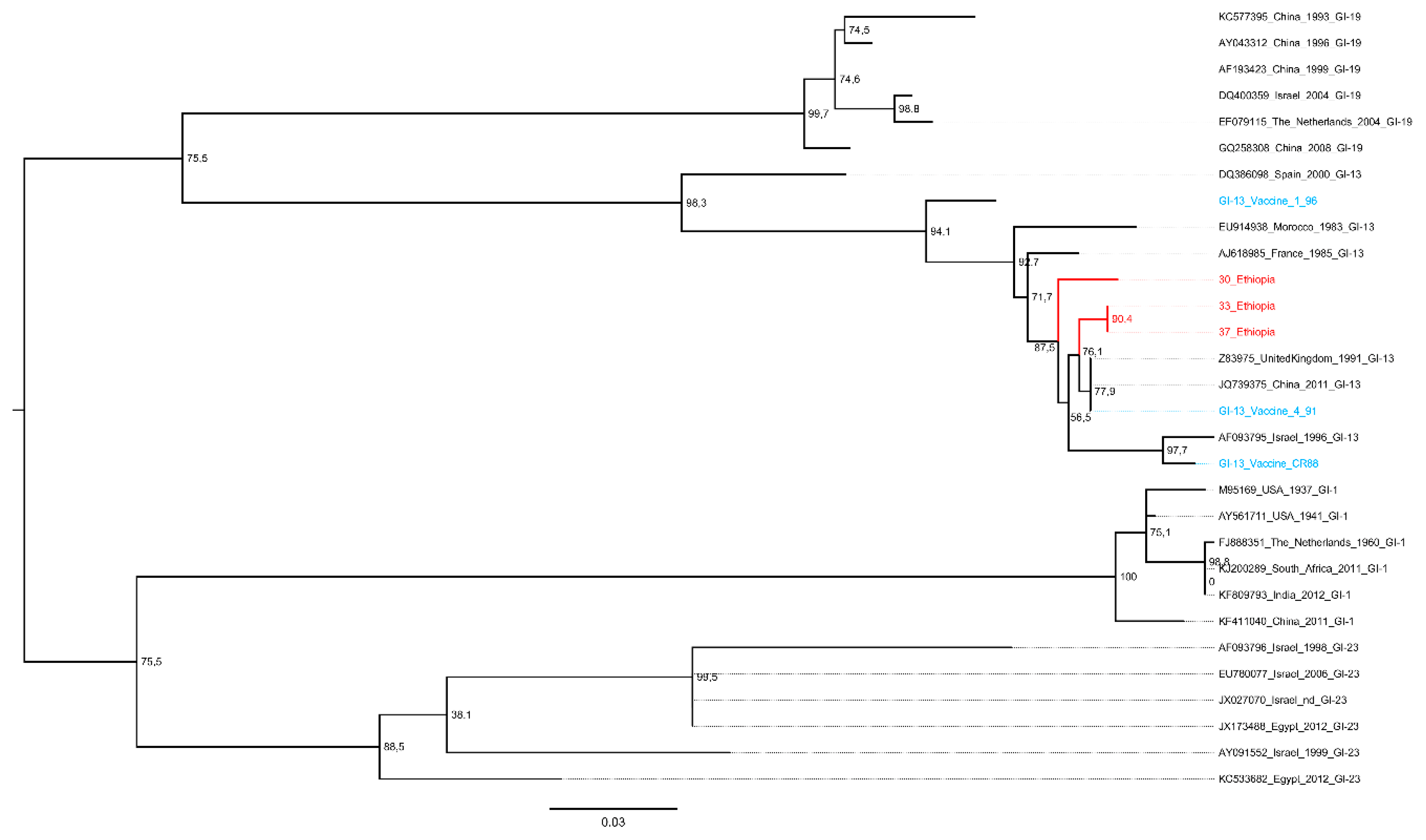
3. Results
Overview of IBV and aMPV Genotypes Detected
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef]
- Dolz, R.; Pujols, J.; Ordóñez, G.; Porta, R.; Majó, N. Molecular epidemiology and evolution of avian infectious bronchitis virus in Spain over a fourteen-year period. Virology 2008, 374, 50–59. [Google Scholar] [CrossRef]
- Raj, G.D.; Jones, R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997, 26, 677–706. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, L.Y.B.; Brandão, P.E.; Chacón, J.L.; Assayag, M.S.; Maiorka, P.C.; Raffi, P.; Saidenberg, A.B.S.; Jones, R.C.; Ferreira, A.J.P. Orchitis in roosters with reduced fertility associated with avian infectious bronchitis virus and avian metapneumovirus infections. Avian Dis. 2007, 51, 900–904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crinion, R.A.P.; Hofstad, M.S. Pathogenicity of Four Serotypes of Avian Infectious Bronchitis Virus for the Oviduct of Young Chickens of Various Ages. Avian Dis. 1972, 16, 351. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W.; de Wit, S.J. Infectious bronchitis. In Diseases of Poultry, 13th ed.; Saif, Y.M., Fadly, A.M., Glisson, J.R., McDougald, L.R., Nolan, L.K., Swayne, D.E., Eds.; Iowa State University Press: Ames, IA, USA, 2013; Volume 13, pp. 139–159. ISBN 9780128008799. [Google Scholar]
- World Bank. World Livestock Disease Atlas. A Quantitative Analysis of Global Animal Health Data (2006–2009); World Bank: Washington, DC, USA, 2011. [Google Scholar]
- González, J.M.; Gomez-Puertas, P.; Cavanagh, D.; Gorbalenya, A.E.; Enjuanes, L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch. Virol. 2003, 148, 2207–2235. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Hall, D.; Handel, A. Molecular evolution and emergence of avian gammacoronaviruses. Infect. Genet. Evol. 2012, 12, 1305–1311. [Google Scholar] [CrossRef]
- Laconi, A.; van Beurden, S.J.; Berends, A.J.; Krämer-Kühl, A.; Jansen, C.A.; Spekreijse, D.; Chénard, G.; Philipp, H.C.; Mundt, E.; Rottier, P.J.M.; et al. Deletion of accessory genes 3a, 3b, 5a or 5b from avian coronavirus infectious bronchitis virus induces an attenuated phenotype both in vitro and in vivo. J. Gen. Virol. 2018, 99, 1381–1390. [Google Scholar] [CrossRef]
- Hodgson, T.; Britton, P.; Cavanagh, D. Neither the RNA nor the Proteins of Open Reading Frames 3a and 3b of the Coronavirus Infectious Bronchitis Virus Are Essential for Replication. J. Virol. 2006, 80, 296–305. [Google Scholar] [CrossRef]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Schalk, A.F. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931, 78, 413–423. [Google Scholar]
- Hitchner, S.B.; Winterfield, R.W.; Appleton, G.S. Infectious Bronchitis Virus Types: Incidence in the United States. Avian Dis. 1966, 10, 98. [Google Scholar] [CrossRef]
- Hitchner, S.B. History of Biological Control of Poultry Diseases in the U.S.A. Avian Dis. 2004, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W. Review of Infectious Bronchitis Virus Around the World. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Chen, H.-W. Infectious Bronchitis Virus Variants: Molecular Analysis and Pathogenicity Investigation. Int. J. Mol. Sci. 2017, 18, 2030. [Google Scholar] [CrossRef]
- De Wit, J.J.S.; Cook, J.K.A.; van der Heijden, H.M.J.F. Infectious bronchitis virus variants: A review of the history, current situation and control measures. Avian Pathol. 2011, 40, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Massi, P.; Tucciarone, C.M.; Barbieri, I.; Tosi, G.; Fiorentini, L.; Ciccozzi, M.; Lavazza, A.; Cecchinato, M.; Moreno, A. Think globally, act locally: Phylodynamic reconstruction of infectious bronchitis virus (IBV) QX genotype (GI-19 lineage) reveals different population dynamics and spreading patterns when evaluated on different epidemiological scales. PLoS ONE 2017, 12, e184401. [Google Scholar] [CrossRef]
- Cook, J.K.A.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef]
- Fields, D.B. Arkansas 99, a new infectious bronchitis serotype. Avian Dis. 1973, 17, 659–661. [Google Scholar] [CrossRef]
- Cook, J.K.A.; Orbell, S.J.; Woods, M.A.; Huggins, M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999, 28, 477–485. [Google Scholar] [CrossRef] [PubMed]
- De Wit, J.J.; Cook, J.K.A.; van der Heijden, H.M.J.F. Infectious Bronchitis Virus in Asia, Africa, Australia and Latin America—History, Current Situation and Control Measures. Braz. J. Poult. Sci. 2010, 12, 97–106. [Google Scholar] [CrossRef]
- Ahmed, H.N. Incidence and Treatment of Some Infectious Viral Respiratory Diseases of Poultry in Egypt. Ph.D. Thesis, Cairo University, Cairo, Egypt, 1954. [Google Scholar]
- Hutton, S.; Bettridge, J.; Christley, R.; Habte, T.; Ganapathy, K. Detection of infectious bronchitis virus 793B, avian metapneumovirus, Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Ethiopia. Trop. Anim. Health Prod. 2017, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.; Kassa, T.; Mesfin, S.; Garoma, A.; Koran, T.; Dima, C.; Guyassa, C.; Hailu, H.; Teshale, S. Four Serotypes of Infectious Bronchitis Virus are Widespread in Unvaccinated Backyard Chicken and Commercial Farms in Ethiopia. World J. Vet. Sci. 2019, 1, 1–4. [Google Scholar]
- Tucciarone, C.M.; Franzo, G.; Lupini, C.; Alejo, C.T.; Listorti, V.; Mescolini, G.; Brandão, P.E.; Martini, M.; Catelli, E.; Cecchinato, M. Avian Metapneumovirus circulation in Italian broiler farms. Poult. Sci. 2018, 97, 503–509. [Google Scholar] [CrossRef]
- Jones, R.C. Viral respiratory diseases (ILT, aMPV infections, IB): Are they ever under control? Br. Poult. Sci. 2010, 51, 1–11. [Google Scholar] [CrossRef]
- Georgiades, G.; Iordanidis, P.; Koumbati, M. Cases of swollen head syndrome in broiler chickens in Greece. Avian Dis. 2001, 45, 745–750. [Google Scholar] [CrossRef]
- Sugiyama, M.; Koimaru, H.; Shiba, M.; Ono, E.; Nagata, T.; Ito, T. Drop of egg production in chickens by experimental infection with an avian metapneumovirus strain PLE8T1 derived from swollen head syndrome and the application to evaluate vaccine. J. Vet. Med. Sci. 2006, 68, 783–787. [Google Scholar] [CrossRef][Green Version]
- Easton, A.J.; Domachowske, J.B.; Rosenberg, H.F. Animal Pneumoviruses: Molecular Genetics and Pathogenesis. Clin. Microbiol. Rev. 2004, 17, 390–412. [Google Scholar] [CrossRef]
- Buys, S.B.; du Preez, J.H.; Els, H.J. The Isolation and Attenuation of a Virus Causing Rhinotracheitis in Turkeys in South Africa; Government Printer: Pretoria, South Africa, 1989; Volume 56. [Google Scholar]
- Juhasz, K.; Easton, A.J. Extensive sequence variation in the attachment (G) protein gene of avian pneumovirus: Evidence for two distinct subgroups. J. Gen. Virol. 1994, 75, 2873–2880. [Google Scholar] [CrossRef]
- Seal, B.S. Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains. Virus Res. 1998, 58, 45–52. [Google Scholar] [CrossRef]
- Lee, E.h.; Song, M.S.; Shin, J.Y.; Lee, Y.M.; Kim, C.J.; Lee, Y.S.; Kim, H.; Choi, Y.K. Genetic characterization of avian metapneumovirus subtype C isolated from pheasants in a live bird market. Virus Res. 2007, 128, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bayon-Auboyer, M.H.; Arnauld, C.; Toquin, D.; Eterradossi, N. Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subgroup. J. Gen. Virol. 2000, 81, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Owoade, A.A.; Ducatez, M.F.; Muller, C.P. Seroprevalence of Avian Influenza Virus, Infectious Bronchitis Virus, Reovirus, Avian Pneumovirus, Infectious Laryngotracheitis Virus, and Avian Leukosis Virus in Nigerian Poultry. Avian Dis. 2006, 50, 222–227. [Google Scholar] [CrossRef]
- Worlund, D.D.; Taylor, G. Estimation of Disease Incidence in Fish Populations. Can. J. Fish. Aquat. Sci. 1983, 40, 2194–2197. [Google Scholar] [CrossRef]
- Cavanagh, D.; Mawditt, K.; Britton, P.; Naylor, C.J. Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions. Avian Pathol. 1999, 28, 593–605. [Google Scholar] [CrossRef]
- Cecchinato, M.; Lupini, C.; Munoz Pogoreltseva, O.S.; Listorti, V.; Mondin, A.; Drigo, M.; Catelli, E. Development of a real-time RT-PCR assay for the simultaneous identification, quantitation and differentiation of avian metapneumovirus subtypes A and B. Avian Pathol. 2013, 42, 283–289. [Google Scholar] [CrossRef]
- Standley, K. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability (outlines version 7). Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Wilson, R.T. Poultry production and performance in the Federal Democratic Republic of Ethiopia. Worlds. Poult. Sci. J. 2010, 66, 441–453. [Google Scholar] [CrossRef]
- Solomon, D. Ethiopia: Poultry Sector Country Review; FAO: Rome, Italy, 2008. [Google Scholar]
- Aklilu, H.A.; Almekinders, C.J.M.; Udo, H.M.J.; Van Der Zijpp, A.J. Village poultry consumption and marketing in relation to gender, religious festivals and market access. Trop. Anim. Health Prod. 2007, 39, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Ebsa, Y.A.; Harpal, S.; Negia, G.G. Challenges and Chicken Production Status of Poultry Producers in Bishoftu, Ethiopia. Available online: https://www.sciencedirect.com/science/article/pii/S0032579119457490 (accessed on 15 June 2020).
- Mazengia, H. Review on major viral diseases of chickens reported in Ethiopia. J. Infect. Dis. Immun. 2012, 4, 1–9. [Google Scholar] [CrossRef]
- Al-Shekaili, T.; Baylis, M.; Ganapathy, K. Molecular detection of infectious bronchitis and avian metapneumoviruses in Oman backyard poultry. Res. Vet. Sci. 2015, 99, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Seger, W.; GhalyanchiLangeroudi, A.; Karimi, V.; Madadgar, O.; Marandi, M.V.; Hashemzadeh, M. Genotyping of infectious bronchitis viruses from broiler farms in Iraq during 2014–2015. Arch. Virol. 2016, 161, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Shokri, S.; Karimi, V.; Langeroudi, A.G.; Marandi, M.V.; Hashamzadeh, M.; Zabihipetroudi, T.; Najafi, H.; Tehrani, F. Seroprevalence and Genotyping of Avian Infectious Bronchitis Virus Detected from Iranian Unvaccinated Backyard Chickens. Available online: https://pubmed.ncbi.nlm.nih.gov/29922421/?from_single_result=Seroprevalence+and+genotyping+of+avian+infectious+bronchitis+virus+detected+from+Iranian+unvaccinated+backyard+chickens (accessed on 15 June 2020).
- Boroomand, Z.; Jafari, R.A.; Mayahi, M. Detection of Newcastle disease, H9N2 avian influenza, and infectious bronchitis viruses in respiratory diseases in backyard chickens in Ahvaz, Iran, in 2014–2015. Arch. Razi Inst. 2018, 73, 19–25. [Google Scholar] [CrossRef]
- Kouakou, A.V.; Kouakou, V.; Kouakou, C.; Godji, P.; Kouassi, A.L.; Krou, H.A.; Langeois, Q.; Webby, R.J.; Ducatez, M.F.; Couacy-Hymann, E. Prevalence of Newcastle disease virus and infectious bronchitis virus in avian influenza negative birds from live bird markets and backyard and commercial farms in Ivory-Coast. Res. Vet. Sci. 2015, 102, 83–88. [Google Scholar] [CrossRef]
- Garba, J.; Nwankwo, I.; Manu, I.J.; Faleke, O.O. Detection of Avian Influenza, Newcastle Disease and Infectious Bronchitis Viruses in Domestic and Captive Migratory Wild Birds Using Nested Polymerase, Yobe State Nigeria. J. Vet. Adv. 2012, 2, 481–487. [Google Scholar]
- Jones, R.C. Europe: History, Current Situation and Control Measures for Infectious Bronchitis. Braz. J. Poult. Sci. 2010, 12, 125–128. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Wit, S. Infectious Bronchitis. In Diseases of Poultry; Wiley: Hoboken, NJ, USA, 2020; Volume 12, pp. 167–188. [Google Scholar]
- Franzo, G.; Naylor, C.J.; Lupini, C.; Drigo, M.; Catelli, E.; Listorti, V.; Pesente, P.; Giovanardi, D.; Morandini, E.; Cecchinato, M. Continued use of IBV 793B vaccine needs reassessment after its withdrawal led to the genotype’s disappearance. Vaccine 2014, 32, 6765–6767. [Google Scholar] [CrossRef]
- Legnardi, M.; Franzo, G.; Koutoulis, K.C.; Wiśniewski, M.; Catelli, E.; Tucciarone, C.M.; Cecchinato, M. Vaccine or field strains: The jigsaw pattern of infectious bronchitis virus molecular epidemiology in Poland. Poult. Sci. 2019, 98, 6388–6392. [Google Scholar] [CrossRef]
- Karabozhilova, I.; Wieland, B.; Alonso, S.; Salonen, L.; Häsler, B. Backyard chicken keeping in the Greater London Urban Area: Welfare status, biosecurity and disease control issues. Br. Poult. Sci. 2012, 53, 421–430. [Google Scholar] [CrossRef]
- Pohjola, L.; Tammiranta, N.; Ek-Kommonen, C.; Soveri, T.; Hänninen, M.L.; Fredriksson Ahomaa, M.; Huovilainen, A. A survey for selected avian viral pathogens in backyard chicken farms in Finland. Avian Pathol. 2017, 46, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Avery, A. Red Meat and Poultry Production and Consumption in Ethiopia and Distribution in Addis Ababa. Borlaug-Ruan World Food Prize Intern, International Livestock Research Institute, Addis Ababa, Ethiopia. Available online: https://www.worldfoodprize.org/documents/filelibrary/images/youth_programs/2004_interns/AveryAbbey_ABB75F25DEDEC.pdf (accessed on 24 November 2020).
- Hinsemu, F. Review on Challenges and Opportunities of Poultry Breeds. J. Dairy Vet. Sci. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Andreopoulou, M.; Franzo, G.; Tucciarone, C.M.; Prentza, Z.; Koutoulis, K.C.; Cecchinato, M.; Chaligianni, I. Molecular epidemiology of infectious bronchitis virus and avian metapneumovirus in Greece. Poult. Sci. 2019, 98, 5374–5384. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Tucciarone, C.M.; Enache, M.; Bejan, V.; Ramon, G.; Koutoulis, K.C.; Cecchinato, M. First Report of Avian Metapneumovirus Subtype B Field Strain in a Romanian Broiler Flock During an Outbreak of Respiratory Disease. Avian Dis. 2017, 61, 250. [Google Scholar] [CrossRef]
| Place | No. of Pooled Samples | Positive (n) Farms | |
|---|---|---|---|
| IBV | aMPV | ||
| Intensive | |||
| Jimma city and surroundings | 16 | 2 | |
| Goma | 4 | ||
| Shebe | 5 | ||
| Backyard | |||
| Setema | 4 | ||
| Sigimo | 3 | 1 | 2 |
| Dedo | 4 | ||
| Omonada | 4 | ||
| Asendabo | 5 | 2 | |
| Dedo, Kersa | 5 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tegegne, D.; Deneke, Y.; Sori, T.; Abdurahaman, M.; Kebede, N.; Cecchinato, M.; Franzo, G. Molecular Epidemiology and Genotyping of Infectious Bronchitis Virus and Avian Metapneumovirus in Backyard and Commercial Chickens in Jimma Zone, Southwestern Ethiopia. Vet. Sci. 2020, 7, 187. https://doi.org/10.3390/vetsci7040187
Tegegne D, Deneke Y, Sori T, Abdurahaman M, Kebede N, Cecchinato M, Franzo G. Molecular Epidemiology and Genotyping of Infectious Bronchitis Virus and Avian Metapneumovirus in Backyard and Commercial Chickens in Jimma Zone, Southwestern Ethiopia. Veterinary Sciences. 2020; 7(4):187. https://doi.org/10.3390/vetsci7040187
Chicago/Turabian StyleTegegne, Dechassa, Yosef Deneke, Takele Sori, Mukarim Abdurahaman, Nigatu Kebede, Mattia Cecchinato, and Giovanni Franzo. 2020. "Molecular Epidemiology and Genotyping of Infectious Bronchitis Virus and Avian Metapneumovirus in Backyard and Commercial Chickens in Jimma Zone, Southwestern Ethiopia" Veterinary Sciences 7, no. 4: 187. https://doi.org/10.3390/vetsci7040187
APA StyleTegegne, D., Deneke, Y., Sori, T., Abdurahaman, M., Kebede, N., Cecchinato, M., & Franzo, G. (2020). Molecular Epidemiology and Genotyping of Infectious Bronchitis Virus and Avian Metapneumovirus in Backyard and Commercial Chickens in Jimma Zone, Southwestern Ethiopia. Veterinary Sciences, 7(4), 187. https://doi.org/10.3390/vetsci7040187






