Abstract
Dogs are the main reservoir for Leishmania infantum, manifesting from a subclinical to a fatal disease. Limited treatments are available, although new antiparasitics and immunomodulators are pursued. Polyhexamethylene biguanide (PHMB) has a broad antimicrobial spectrum, including antiparasitic activity. Here, we evaluated the potential for Toll-like receptor agonists (TLRa) and PHMB alone, and as polyplex nanoparticles containing PHMB and TLR4 or TLR9 agonists, to selectively kill L. infantum. Susceptibility of L. infantum promastigotes to PHMB, miltefosine, and allopurinol was performed, and the half-maximum inhibitory concentrations (IC50) were determined. Then, DH-82 cells were infected and treated with PHMB alone or combined with TLR4a (MPLA-SM) or TLR9a (CpG ODNs) and allopurinol alone. The IC50 values of L. infantum promastigotes were PHMB (1.495 µM), miltefosine (9.455 µM), and allopurinol (0.124 µM). After infection, treated DH-82 cells displayed a lower percentage (p = 0.0316), intensity (p = 0.0002), and index of infection (p = 0.0022) when compared to non-treated cells. PHMB induced lower percentage of infection alone (p = 0.043), in combination with TLR9a (p = 0.043), and with TLR4a (p = 0.0213). Supernatants were collected and used to measure TNF-α and IL-6 levels. Increased TNF-α was observed after PHMB plus TLR4a, relative to uninfected and infected untreated macrophages (p = 0.043). PHMB combined with TLR4a shows promise as a potential anti-L. infantum drug combination, as well as inducer of proinflammatory response, as demonstrated by decreased infection and increased TNF-α production.
1. Introduction
Leishmaniases are a group of vector-borne infectious diseases in humans and animals caused by different species of Leishmania [1,2]. The genus Leishmania has a digenetic life cycle with two distinct stages recognized. An elongated shape and long flagella characterize the promastigote stage. Promastigotes are further subdivided into procyclic promastigotes that multiply in the gut of blood feeding sandfly phlebotomine and infective metacyclic promastigotes, housed in the mouth and anterior gut of the sand fly vector [3]. Later in the lifecycle, the parasite continues to differentiate in the mammalian host as a non-motile amastigote with rounded or oval shaped cell that lacks flagella. The amastigote stage commonly resides and replicates within the phagolysosome of macrophages [4].
Canine leishmaniosis (CanL) is endemic in many countries of the Mediterranean basin [5]. Domestic dogs are the principal reservoir of Leishmania infantum with a high prevalence of infection [5]. CanL is a zoonotic infectious disease with a wide range of clinical manifestations [6,7]. The development of subclinical or clinical states depends on the potential of the humoral and cell-mediated immune responses to self-eliminate the parasite [8]. The conventional treatment strategies used to control the infection require a long period of sustained treatment, do not guarantee a complete recovery, and do not contain spread [6,9]. Furthermore, most of the current therapeutic strategies can be associated with adverse side effects [10,11,12] and do not reinforce the immune system.
It is important to highlight that the failure of conventional treatments for Leishmania infection is increasing [10,13]. In many cases, drug resistance is responsible for non-successful treatment [13]. However, it is well known that other aspects such as the immunological characteristics of the host, the quality and pharmacokinetics of the drug used, as well as the parasite features or eventual co-infections are also involved [14]. Therefore, identification of new drug strategies and immunomodulators that can help to reduce the length of Leishmania treatments and improve efficacy to combat this zoonotic infection are very important.
Macrophages are targeted host cells for L. infantum infection; even so, they are also capable of antigen-presentation and killing intracellular parasites by reactive oxygen and nitrogen intermediates mechanisms which is an important instrument of response against this infection [15,16,17]. However, Leishmania parasites evade the innate immune system by several mechanisms, such as the ability to survive and replicate within macrophage phagolysosomes by producing compounds such as lipophosphoglycans (LPG). The LPG inhibits phagosome maturation [18] and the interference of signaling pathways [19].
Isolation, culture, and characterization of canine macrophage cell lines derived from malignant histiocytosis have been described [16]. The mechanisms and receptor-ligands implicated in the interaction, attachment, and uptake of Leishmania promastigotes by macrophages have been previously investigated [20]. Therefore, the use of canine macrophage cell lines for functional studies is a very attractive model [21], as it requires a less invasive approach for obtaining the cells comparing to peritoneal or bone marrow-derived macrophages. Such welfare issues are becoming better appreciated when designing experiments in which animals are studied [22].
The effect of stimulation of macrophages receptors such as Toll-like receptors (TLRs) during L. infantum infection has been little studied (see in [18] for a review). TLRs are the crucial regulators of innate immune responses against various pathogens including Leishmania parasites. They are located on the plasma membrane or internal membranes of macrophages, dendritic cells (DCs), natural killer (NK) cells and T and B lymphocytes [23]. TLRs are involved in a variety of phenomena including phagocytosis, maturation, microbicidal activity, and production of cytokines [24]. Once the different TLRs are stimulated by specific ligands, a cascade that induces the production of proinflammatory cytokines such as interleukin (IL)-1α, IL-6, IL-12, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) is activated [25,26]. Several TLRs play important roles in pathogenesis, susceptibility and resistance to control Leishmania infections [27,28]. TLR4 contributes to control the growth of Leishmania in innate and adaptative immune responses. It is also a strong regulator of inducible nitric oxide synthase (iNOS) leading to death of the parasite [29]. TLR9 is required for neutrophil recruitment and DCs activation during Leishmania infection [30]. However, the spectrum and specificity of how Leishmania interaction with TLR agonists (TLRa) induces proinflammatory cytokines is complex and not well established [18] and the mechanisms by which macrophages kill Leishmania in dogs have not fully been elucidated [31].
Polyhexamethylene biguanide (PHMB) is a polymer composed of repeating basic biguanidine units connected by hexamethylene hydrocarbon chains, which provide a cationic and amphipathic structure [32]. PHMB has broad antimicrobial spectrum. It is used to treat certain parasite infections and it is widely used in wound care applications [33]. PHMB’s leishmanicidal mechanisms of action appear to involve disruption of parasite cell membranes, cell entry, and condensation/disruption of parasite chromosomes [34]. Nevertheless, the leishmanicidal effect and mechanisms of PHMB has been investigated only in Leishmania major demonstrating the killing of promastigotes at low concentrations [34]; however, studies related to others Leishmania species are lacking.
In order to elucidate the in vitro antileishmanial properties of PHMB against L. infantum, here we studied the infection profile and proinflammatory cytokines produced by infected DH-82 cells after treatment with PHMB alone or as polyplex nanoparticles containing TLR4 or TLR9 agonists (TLR4a and TLR9a). These studies may underpin the development of new antimicrobial and immunomodulatory strategies for the control of human and animal leishmaniosis.
2. Materials and Methods
2.1. Leishmania infantum Parasite Maintenance
Leishmania infantum (MCAN/ES/92/BCN-83) was used in this experiment. Parasites were cultured in biphasic Novy-MacNeal-Nicolle (NNN) medium and sub-passaged in Schneider’s Drosophila medium (Biowest®, Riverside, MO, USA) supplemented with 20% of fetal bovine serum ((FBS) Biowest®, Riverside, MO, USA), 100 U/mL of penicillin (Life TechnologiesTM, Carlsbad, CA, USA), and 1% of filtrated human urine from a healthy donor.
2.2. Macrophages Maintenance
The canine macrophage-derived cell line DH-82 (ATCC® CRL-10389™, USA), from a dog with malignant histiocytosis, was maintained in Roswell park memorial institute (RPMI) 1640 medium (Biowest®, Riverside, MO, USA) with 10% of inactivated FBS Premium (Biowest®, Riverside, MO, USA) supplemented with 100 U/mL of penicillin, 100 µg/mL streptomycin (Life TechnologiesTM, California, USA), and 2 mM L-glutamine (Biowest®, Riverside, MO, USA) at 37 °C in 5% CO2–95% air.
2.3. Drugs and TLR Agonists
Allopurinol (Sigma-Aldrich, St. Louis, MO, USA) and miltefosine (10 mg/mL, Sigma-Aldrich, MO, USA) were used. Stock final solutions were prepared in ultrapure water for allopurinol (5 mg/mL) and miltefosine (100 µg/mL). TLR4a (monophosphoryl lipid A from Salmonella minnesota; [MPLA-SM], Invivogen, San Diego, CA, USA) and TLR9a (5′tccatgacgttcctgatgct CpG oligodeoxynucleotides (CpG ODNs) Invivogen, San Diego, CA, USA) were tested alone and as a polyplex nanoparticles with PHMB (stock concentration of 1 mg/mL). All drugs and TLRa solutions were stored at 4 °C until used.
2.4. Preparation and Analyses of Polyplex Nanoparticles
The nanoparticles were prepared in aqueous solution by nanoprecipitation within 2 mL polypropylene microfuge tubes [34]. The final ligand and polymer concentrations are listed in Table 1. First, ligand solutions (TLR4 ligand: MPLA-SM in 30% ethanol and TLR9 ligand: CpG ODNs in water) were diluted in water to the 2 x concentrations indicated in Table 1 and to a final volume of 200 μL, followed by addition of 200 μL of PHMB solution; the mixtures were then stirred for 20 min. After preparation, nanoparticle populations were subjected to dynamic light scattering (DLS) measurements to characterize particle populations. Particle sizes and zeta potential of ligand:PHMB polyplexes were measured by using a Zetasizer Nano S-90 (Malvern Panalytical Ltd., Malvern, UK). The formulations were performed in triplicate.

Table 1.
Nanoparticle formulations and particle population profiles.
2.5. Half Inhibitory Concentration (IC50) Promastigote Susceptibility
Promastigote drug susceptibility was determined by using AlamarBlue® cell viability reagent (10% v/v, Life TechnologiesTM, Carlsbad, CA, USA) resazurin reaction. Leishmania infantum promastigotes in the stationary phase of in vitro growth were cultured at a concentration of (1 × 105 parasite/well) in 100 µL each well in flat-bottomed 96-well cell culture microtiter plates (Costar, Corning, New York, NY, USA). Briefly, starting solutions for allopurinol (2.5 µg/mL, Sigma-Aldrich, St. Louis, MO, USA), miltefosine (50 µg/mL, Sigma-Aldrich, St. Louis, MO, USA), and PHMB (10 µg/mL) were subjected to 2-fold serial dilution until a total of 10 dilutions for each drug. Then, 100 µL of each dilution were added to the promastigotes and incubated at 26 °C for 24 h. Finally, 20 μL of AlamarBlue® (10% v/v) was added and the cultures were kept for 24 h for drug susceptibility determination following the manufacturer’s recommendations by reading the absorbance at 540 and 590 nm in a Fluoroskan fluorimeter (ThermoLabsystems, Helsinki, Finland). Relative viability was calculated from the ratio of the optical density (OD) results in parasites exposed to the drugs versus those not exposed. For each drug, three independent and reproducible experiments were performed in triplicate (total 9 wells for each dilution), drug concentrations alone and parasites alone were added as controls to each plate to calculate the inhibitory concentrations that kill 50% of L. infantum promastigotes (IC50).
2.6. Cytotoxicity for DH-82 Cell Line
DH-82 cells in the logarithmic phase of growth were counted in a Neubauer chamber, seeded in a 96-well culture plate (Costar, Corning, NY, USA) at a concentration 1 × 105 cells in 100 µL each well in complete RPMI and incubated in an atmosphere of 5% CO2 at 37 °C. After 24 h, supernatant was gently removed and two washes with 100 µL each of Dulbecco’s phosphate-buffered saline (DPBS) were made, then 100 µL of fresh media was added to the cells. Dilutions of allopurinol, miltefosine, and PHMB were performed as described above and tested on DH-82 cells. After preparation of 2-fold serial dilutions in complete RPMI, 100 µL of each drug was added to the cells and incubated in an atmosphere of 5% CO2 at 37 °C for 24 h. Then, 20 μL of AlamarBlue® (10% v/v) was added and the cultures were kept for 24 h and absorbance was read as described above for promastigotes. For each drug, three independent and reproducible experiments were performed. For each drug, three independent and reproducible experiments were performed in triplicate (total 9 wells for each dilution), drugs concentrations aloneand parasites alone were added as controls to each plate to calculate the inhibitory concentrations that kill 50% of DH-82 cells (IC50).
2.7. Intracellular L. infantum Amastigotes Susceptibility Assay
Macrophages (5 × 104 cells/well) were incubated in 8 wells (Lab-Tek® Chamber Slide™ (Thermo Scientific Nunc®, Waltham, MA, USA)) in 200 µL of complete RPMI medium for 24 h. Then, the medium was removed and DH-82 cells were resuspended with 200 µL of complete RPMI containing promastigotes (stationary phase) at a 10:1 parasite:host cell ratio. The cultures were incubated at 37 °C in 5% CO2–95% ambient air for 3 h and then infected DH-82 cells were washed two times with DPBS (Biowest®, Riverside, MO, USA) prior to addition of the different conditions. Conditions were prepared as follows. (1) PHMB alone (stock concentration of 1 mg/mL), at a maximum dose tolerated by DH-82 cells (6.133 µM). (2) TLR4a alone ([MPLA-SM]: 0.092 mg/mL stock solution, Invivogen®, San Diego, CA, USA) at a final concentration of 1 µg/mL. (3) TLR9a alone ((CpG ODNs) 0.092 mg/mL stock solution, Invivogen®, San Diego, CA, USA) at a final concentration of 1 µg/mL. (4) PHMB associated with TLR4a (0.092 mg/mL stock solution) at a final concentration of 1 µg/mL. (5) PHMB associated with TLR9a (0.092 mg/mL stock solution) at a use concentration of 1 µg/mL. (6) Allopurinol alone (stock concentrations of 50 mg/mL), maximum tolerated dose 0.124 µM for allopurinol. Cytotoxicity of each studied drug for DH-82 cells was previously determined. Also, DH-82 cytotoxicity was determined for TLR agonists alone and for combination with PHMB + TLR agonist compounds (data not shown). All control uninfected conditions were also included. Five independent assays with four replicates per each condition were performed. At 24 h, supernatants were collected and stored at −80 °C and Lab-Tek® Chamber Slide™ were fixed with methanol and Diff-quick stained (QCA, Amposta, Tarragona, Spain).
2.8. Percentage of L. infantum-Infected Macrophages, Intensity of Infection and Infection Index
The percentage of infected cells (i.e., number of infected cells per 100 macrophages) and the intensity of infection (i.e., number of intracellular amastigotes per number of infected macrophages) were determined by light microscopy under oil (×1000) by counting at least 300 cells per well. Additionally, the infection index (II) was obtained as previously reported multiplying the percentage of infected macrophages and the number of internalized amastigotes per infected macrophage [35].
2.9. Sandwich ELISA for the Determination of TNF-α and IL-6
Cytokine analysis of TNF-α and IL-6 were performed according to manufacturer’s instructions, (DuoSet® ELISA by Development System R&D TM, Abingdon, UK) using a 96-well cell plate flat bottom (Costar ® Corning, USA). Standard curve for TNF-α started with 1000 pg/mL and twofold dilutions were made until 15.63 pg/mL concentration. Standard curve for IL-6 started with 4000 pg/mL and twofold dilutions were made until 62.5 pg/mL concentration. Optical density was measured with an ELISA reader (Anthos 2020, UK) at wavelength of 450 nm. The standard curve for each cytokine was calculated using a computer generated four parameter logistic curve-fit with program myassays (http://www.myassays.com/). Plate was repeated when R2-value of standard curve was below 0.98.
2.10. Statistical Analysis
Data were analyzed with one-way ANOVA and post hoc Dunnett’s multiple comparisons test to compare every treatment mean with the control uninfected macrophages mean. Then, a nonparametric Wilcoxon signed rank test was used to compare among related several treatments.
The statistical analysis in the case of Wilcoxon signed rank test was performed using the SPSS 17.0 for Windows software (SPSS Inc., Chicago, IL, USA), ANOVA and post hoc Dunnett’s test were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Differences were considered significant with a 5% significance level (p < 0.05). All graphs were performed using excel GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Polyplex Nanoparticles
To complex TLR ligand into polyplex nanoparticles, we used nanoprecipitation with the cationic polymer PHMB. Aqueous solutions of two TLR ligands were separately prepared and complex with an aqueous solution of polymer and polyplex nanoparticle populations were profiled by DLS. Values for size and polydispersity are indicated in Table 1. Based on the particle population results, low dispersity formulations for TLR4 and TLR9 ligands were selected for cell studies.
3.2. Promastigotes and Macrophage Drugs Susceptibility
Drug susceptibility of L. infantum promastigotes and DH-82 canine macrophages were performed in separate experiments. The IC50 values for allopurinol, miltefosine, and PHMB were calculated and then summarized in Table 2.

Table 2.
IC50 values for all drugs studied for L. infantum promastigotes and cytotoxicity for DH-82 macrophages.
3.3. Percentages of Infected Macrophages
To determine the infectivity degree, the mean ± standard deviation (mean ± sd) of the percentage of infected macrophages was calculated and plotted in Figure 1. Infected macrophages without any treatment displayed a range of infection within 30% to 53% with a mean ± sd of 38.5 ± 8.0%. As expected, infected macrophages alone showed the higher percentage of infection when compared to all treatment conditions studied (ANOVA: p = 0.0316). Infected macrophages that were un-treated showed a higher level of infection when compared with infected cells treated with TLR4a alone (Dunnett’s test: p = 0.0154), TLR9a alone (Dunnett’s test: p = 0.0269), PHMB plus TLR4a (Dunnett’s test: p = 0.0213), and PHMB plus TLR9a (Dunnett’s test: p = 0.0121).
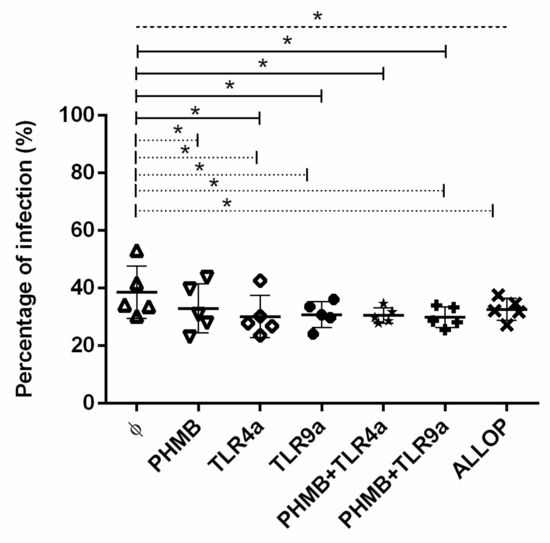
Figure 1.
Percentage of in vitro L. infantum infected macrophages after 24 h treatment with the different conditions: Medium alone (Ø), Polyhexamethylene biguanide alone (PHMB), MPLA Toll-like receptor agonist 4 (TLR4a), PHMB plus TLR4a (PHMB + TLR4a), CpG ODN Toll-like receptor agonist 9 (TLR9a), PHMB plus TLR9a (PHMB + TLR9a), and allopurinol (ALLOP). Significant results are described as * 0.05 > p > 0.01. Lines in graphic represent ANOVA (dashed lines), Dunnett’s test (solid lines), and Wilcoxon signed-rank test (dotted lines).
Significantly higher percentage of infection was detected when untreated infected macrophages were compared to infected cells treated with PHMB alone (% mean ± sd of 34.7 ± 6), TLR4a alone (% mean ± sd of 30.4 ± 6.4), TLR9a alone (% mean ± sd of 32.7 ± 2.1), PHMB plus TLR9a (% mean ± sd of 30.8 ± 3.2), and allopurinol (% mean ± sd of 30.9 ± 5) alone (Wilcoxon signed-rank test: p = 0.043). A tendency towards higher percentage of infection was noticed when infected macrophages without treatment were compared with macrophages treated with PHMB plus TLR4a (Wilcoxon signed-rank test: p = 0.08). Additionally, no differences were detected when PHMB alone was compared with the rest of the conditions studied. Moreover, no differences were found when comparing TLR4a (% mean ± sd of 30.4 ± 6.4) or TLR9a (% mean ± sd of 32.7 ± 2.5) alone with PHMB plus TLR4a (% mean ± sd of 28.4 ± 2.5) or PHMB plus TLR9a (% mean ± sd of 30.8 ± 3.2) (Figure 1).
3.4. Intensity of Infection
The intensities of infection were analyzed, and the result of all experiments is displayed in Figure 2. Statistically significant differences were observed when infected macrophages alone were compared to the rest of the treatment conditions (ANOVA: p = 0.0002). Particularly, infected macrophages treated with TLR4a alone (Dunnett’s test: p = 0.0040), TLR9a alone (Dunnett’s test: p = 0.0018), PHMB plus TLR4a (Dunnett’s test: p = 0.0011) and PHMB plus TLR9a (Dunnett’s test: p = 0.0138) showed lower intensity of infection than infected non-treated cells. A lower number of amastigotes were observed in macrophages treated with TLR4a (mean ± sd of 1.56 ± 0.0) and TLR9a (mean ± sd of 1.53 ± 0.0), PHMB plus TLR4a (mean ± sd of 1.50 ± 0.0), and PHMB plus TLR9a (mean ± sd of 1.57 ± 0.0) when compared to infected macrophages without treatment (Wilcoxon signed-rank test: p = 0.043). Additionally, macrophages treated with TLR4a or TLR9a alone showed lower intensity of infection than macrophages treated with allopurinol (Wilcoxon signed-rank test: p = 0.043) (Figure 2). In addition, a lower significantly intensity of infection was observed on cells treated with PHMB plus TLR4a when compared to PHMB plus TLR9a treatment (Wilcoxon signed-rank test: p = 0.043) (Figure 2).
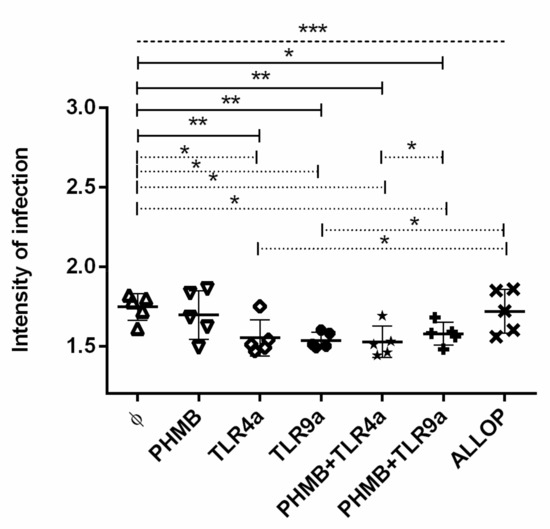
Figure 2.
Intensity of in vitro L. infantum macrophage infection after 24 h treatment with the different conditions: Medium alone (Ø), Polyhexamethylene biguanide alone (PHMB), MPLA Toll-like receptor agonist 4 (TLR4a), PHMB plus TLR4a (PHMB + TLR4a), CpG ODN Toll-like receptor agonist 9 (TLR9a), PHMB plus TLR9a (PHMB + TLR9a), and allopurinol (ALLOP). Significant results are described as * 0.05 > p > 0.01, ** 0.01 > p > 0.001, *** 0.001 > p > 0.0001. Lines in graphic represent ANOVA (dashed lines), Dunnett´s test (solid lines), and Wilcoxon signed-rank test (dotted lines).
Finally, a tendency towards higher intensity of infection was noticed when infected macrophages without treatment were compared with macrophages treated with PHMB alone (Wilcoxon signed-rank test: p = 0.08) as well as when infected macrophages treated with PHMB plus TLR4a were compared to infected macrophages only treated with TLR4a alone (Wilcoxon signed-rank test: p = 0.08).
3.5. Infectivity Index
At 24 h post-infection, we compared the infectivity index of the macrophages under different conditions (Figure 3). As previously observed, a higher infection index was achieved in non-treated macrophages (mean ± sd of 66.4 ± 16.1) when compared to the rest of the treatments (ANOVA: p = 0.0022). Differences were found with TLR4a alone (Dunnett’s test: p = 0.0048), TLR9a alone (Dunnett’s test: p = 0.0129), PHMB plus TLR4a (Dunnett’s test: p = 0.0004) and PHMB plus TLR9a (Dunnett’s test: p = 0.0058), and allopurinol (Dunnett’s test: p = 0.0479) when compared with un-treated macrophages. Furthermore, lower but not significantly differences (Dunnett’s test: p = 0.2103) were found when PHMB alone (mean ± sd of 56.3 ± 13.0) was compared with macrophages without treatment.
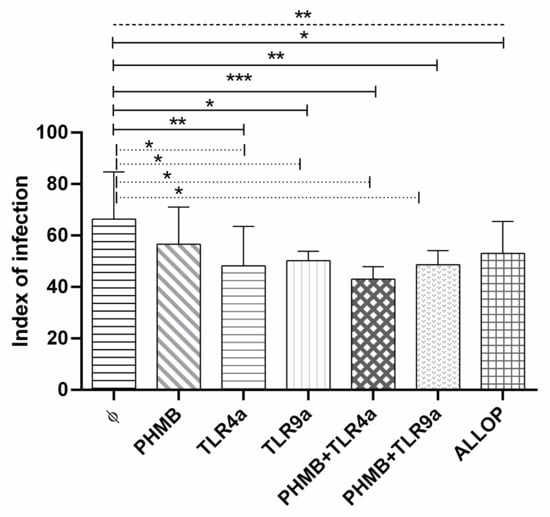
Figure 3.
Infection index of in vitro L. infantum macrophage infection after 24 h treatment with the different conditions: Medium alone (Ø), Polyhexamethylene biguanide alone (PHMB), MPLA Toll-like receptor agonist 4 (TLR4a), PHMB plus TLR4a (PHMB + TLR4a), CpG ODN Toll-like receptor agonist 9 (TLR9a), PHMB plus TLR9a (PHMB + TLR9a), and allopurinol (ALLOP). Significant results are described as * 0.05 > p > 0.01, ** 0.01 > p > 0.001, *** 0.001 > p > 0.0001. Lines in graphic represent ANOVA (dashed lines), Dunnett´s test (solid lines), and Wilcoxon signed-rank test (dotted lines).
A lower infection index was found in the infected macrophages treated with PHMB plus TLR4a (mean ± sd of 42.9 ± 4.2, Wilcoxon signed-rank test: p = 0.043), and PHMB plus TLR9a (mean ± sd of 48.5 ± 4.9, Wilcoxon signed-rank test: p = 0.042) and also in TLRa treatments alone, TLR4a (mean ± sd of 48.2 ± 13.4, Wilcoxon signed-rank test: p = 0.041) and TLR9a (mean ± sd of 50.1 ± 3.3, Wilcoxon signed-rank test: p = 0.042) when compared with non-treated infected macrophages (Figure 3).
3.6. TNF-α and IL-6 Concentrations on Supernatant of DH-82 Treated Cells
Production of proinflammatory TNF-α and IL-6 cytokines of infected and uninfected cells treated with the different conditions is plotted in Figure 4a,b. TNF-α production in uninfected macrophages without treatment was significantly lower than the rest of the treatments with the exception of PHMB alone, TLR9a, PHMB + TLR9a, and allopurinol (ANOVA: p < 0.0001). Uninfected macrophages controls secreted lower TNF-α than uninfected cells treated with TLR4a alone (Dunnett’s test: p = 0.0017) and with PHMB in combination with TLR4a (Dunnett’s test: p = 0.0134); and when compared to infected cells treated with PHMB complex with TLR4a (Dunnett’s test: p = 0.0014) (Figure 4a).
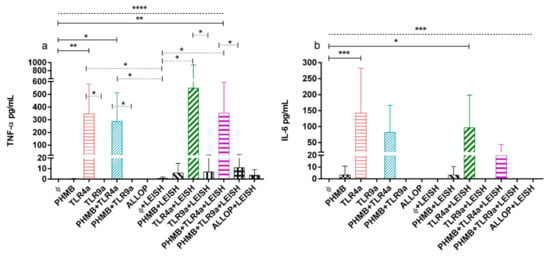
Figure 4.
(a) TNF-α and (b) IL-6 concentrations from uninfected and infected macrophages treated with the following conditions. Medium alone (Ø), Polyhexamethylene biguanide alone (PHMB), MPLA Toll-like receptor agonist 4 (TLR4a), PHMB plus TLR4a (PHMB + TLR4a), CpG ODN Toll-like receptor agonist 9 (TLR9a), PHMB plus TLR9a (PHMB + TLR9a), and allopurinol (ALLOP). All the conditions indicated with LEISH are L. infantum infected replicates. Significant results are described as * 0.05 > p > 0.01, ** 0.01 > p > 0.001, *** 0.001 > p > 0.0001, **** 0.0001 > p. Lines in graphic represent ANOVA (dashed lines), Dunnett´s test (solid lines), and Wilcoxon signed-rank test (dotted lines).
In addition, TNF-α concentrations of non-treated and infected macrophages were significantly lower than uninfected cells treated with TLR4a alone or in combination with PHMB (Wilcoxon signed-rank test: p = 0.043). Moreover, infected macrophages alone secreted lower TNF-α concentrations when compared to infected cells treated with TLR4a alone or in combination with PHMB (Wilcoxon signed-rank test: p = 0.043). Furthermore, infected macrophages without treatment produced lower TNF-α concentrations than infected cells treated with TLR4a alone or associated to PHMB (Wilcoxon signed-rank test: p = 0.043). In addition, infected and uninfected macrophages treated with TLR4a were significantly more potent on production of TNF-α than TLR9a, either associated or not with PHMB (Wilcoxon signed-rank test: p = 0.043) (Figure 4a).
Uninfected macrophages without treatment produced lower significant levels of IL-6 when compared to the rest of the conditions (ANOVA: p < 0.0001), particularly when compared to uninfected macrophages treated with TLR4a alone (Dunnett’s test: p = 0.0002) and infected macrophages treated with TLR4a alone (Dunnett’s test: p = 0.0228) (Figure 4b).
At 24 h, a trend of higher IL-6 concentration was detected after TLR4a treatment in complex or not with PHMB, on either infected or uninfected cells. In addition, only a trend of higher concentration of IL-6 was observed on infected and uninfected cells treated with TLR4a when compared with TLR9a, in complex or not with PHMB.
4. Discussion
To the best of our knowledge, this study is the first to demonstrate the susceptibility of the L. infantum promastigote and amastigote forms to PHMB. In addition, the susceptibility of the L. infantum strain to conventional drugs, such as miltefosine and allopurinol was corroborated [36]. Furthermore, the study evaluated the use of TLR ligands as polyplex nanoparticles with PHMB, observing a potent antiparasitic and proinflammatory effect in combination with TLR4a.
The susceptibility of promastigotes to conventional antileishmanial drugs was determined. As expected, the IC50 value obtained for miltefosine (9.455 µM) was in the range determined by others [36]. Allopurinol IC50 value (1.495 µM) was also in the range registered by one group [36], but also lower than other studies [37,38,39]. Several studies recommended to avoid the use of miltefosine as monotherapy, and have been encouraged to advocate for its use in combined therapies in order to evade drug resistance [6,36,40]. Additionally, a previous in vitro study involving different strains of Leishmania parasite remarked how easily Leishmania parasite adapts to allopurinol [36]. The genetic characteristics of miltefosine resistance in human patients with leishmaniosis have been described [41,42]. Unfortunately, less conclusive studies are available related to miltefosine resistance in CanL [36,43]. Regarding allopurinol, resistance has been largely investigated in CanL [37,38,39].
Here, we studied the antileishmanial effect of PHMB, a compound not investigated as a treatment for L. infantum. However, a previous study demonstrated its properties against L. major [34]. In addition, the same study determined an IC50 value PHMB concentration of 0.41 µM for L. major promastigotes [34], which was lower but similar to that observed in our study for L. infantum promastigotes (1.495 µM). In this case, diverse drug sensitivities encountered with each Leishmania species might be the cause of such minor difference observed when IC50 values were compared.
The mechanism(s) of action of PHMB remain unclear. However, a previous study demonstrated that this polymer preferentially disrupts microbial membranes, specifically interacting with phospholipid acids [44]. Recently, it was indicated that Acanthamoeba castellanii trophozoites and cysts exposed to PHMB presented cell shrinkage and membrane blebbing, which is a hallmark for apoptosis [45]. Firdessa et al. [34] observed that L. major incubated with PHMB displayed chromosome disruption, consistent with chromosome effects observed in bacteria [32], indicating that multiple mechanisms are likely involved. It is likely that similar mechanisms of action of PHMB are valid for L. infantum investigated in our study.
PHMB displayed low cell toxicity in DH-82 canine macrophages (IC50 = 6.13 µM) which was at a similar IC50 range when compared to other cell lines studied before, such as bone marrow-derived macrophage (BMDM) from BALBc mice (IC50 = 4 µM) and 293T kidney epithelial cells(IC50 = 26 µM) [34]. In accordance with a previous study, the conventional drugs miltefosine and allopurinol were also minimally cytotoxic [46] with higher IC50 concentrations than the limit of detection of the experiments here performed (Table 2). However, a recent study concluded that cytotoxicity of antileishmanial drugs depends on the type of macrophage source under investigation [47].
Our principal goal was to probe the in vitro antileishmanial properties of PHMB alone and in polyplex nanoparticles with TLR4a or TLR9a. As discussed in the introduction, TLRs have key roles in the induction of immunological responses to infection, so combinations of antileishmanial drugs and TLR agonists as adjuvant might have a synergic effect. For this purpose, we measured the infection rate and proinflammatory cytokines production after different combination treatments using an amastigote-infected macrophage system. The amastigote-infected macrophage system has been widely demonstrated to be correlated with the clinical response to Leishmania infection when testing drug performance [46,47,48,49,50,51]. The leishmanicidal effect of PHMB on L. infantum was demonstrated in our study by evaluating the response to several treatments. When compared to untreated cells, we detected a lower percentage of infection in cells exposed to PHMB and less, but not statistically significant intensity of infection. In agreement with results from L. major in vitro study, we described an intracellular action of PHMB against the L. infantum amastigote forms [34]. As expected, percentage, intensity, and index of infection were significantly higher in the cells not receiving any treatment when compared with cells stimulated with TLR4a or TLR9a alone or in complexes with PHMB.
The contribution of TLR4 during Leishmania infection and treatment has been extensively studied, since it is considered to be important for efficient parasite control during innate immune responses [29,52,53,54,55,56]. A previous study performed on whole blood of dogs stimulated with L. infantum antigen and different TLRa, demonstrated that TLR4a induced a potent pro-inflammatory cytokine stimulation [53]. Additionally, secretion of proinflammatory cytokines such as TNF-α [54] as well as its genetic expression [57] due to TLR4 activation, has been demonstrated using peripheral blood mononuclear cells (PBMC) from patients with cutaneous leishmaniosis due to L. braziliensis infection. In accordance with those results, we show that stimulation with TLR4a alone (or associated with PHMB) induced a higher production of TNF-α when compared to the rest of the treatments including TLR9a alone or associated to PHMB. However, a trend of higher production of IL-6 was observed after stimulation with PHMB alone or associated with TLR4a.
The critical role of TLR9 during activation of the protective innate immune response has been demonstrated using an in vitro and in vivo mouse model of L. infantum infection, in which TLR9 was associated with neutrophil recruitment together with DC activation to combat parasite invasion [30]. We showed that PHMB association with TLR9a managed to reduce the percentage of infection, the index of infection and intensity of infection, when compared to un-treated infected macrophages. In accordance, the potential immunostimulatory activity of TLR9a such as CpG ODN to control intracellular parasites as Leishmania has been already demonstrated [34,58]. Moreover, PHMB can strongly interact with CpG ODN (TLR9a) as previously described [34], and with MPLA-SM (TLR4a) as demonstrated in our work. The immunomodulatory effect of TLRa for treatment and/or prevention of leishmaniosis is a current topic of investigation. As observed in our study, Firdessa et al. [34] concluded that the antileishmanial effect of PHMB might be facilitated due to its ability to deliver immunomodulators as TLRs agonist. Further studies are needed to assess the possible clinical use of PHMB in polyplex nanoparticles with TLR4a or TLR9a or both in human and animal leishmaniosis.
5. Conclusions
We demonstrated, for the first time, the antileishmanial properties of PHMB against L. infantum parasites. In addition, our results support a crucial role for TLR4a in providing protection against L. infantum by enhancing parasite elimination and pro-inflammatory cytokine production. Moreover, PHMB combined with TLR4a shows promise as a potential anti-L. infantum drug combination, as well as inducer of proinflammatory response, as demonstrated by decreased infection and increased TNF-α production. Further investigations regarding the use of immunomodulators such as TLRa associated with new antileishmanial compounds such as PHMB, introduces interesting alternatives for the treatment of a wide-spread neglected zoonotic disease using combined antimicrobial and immunomodulatory strategies.
Author Contributions
Conceptualization, L.S.-G.; Methodology, L.S.-G. and P.M.-O.; Validation, P.M.-O. and L.G.; Investigation, L.S.-G. and P.M.-O.; Formal analysis and data curation, L.S.-G., P.M.-O., and M.B. interpretation; Writing, L.S.-G. and P.M.-O.; Review and editing, L.S.-G., P.M.-O., M.B., L.G.; Visualization and supervision, L.S.-G.; Resources, L.S.-G. and L.G.; Funding acquisition, L.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Spanish ministry grant, Ministerio de Economía y competitividad and Fondo Europeo de Desarrollo Regional (FEDER, EU) (AGL2015-68477).
Acknowledgments
The authors thank Klaudia Kloc-Muniak for her technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gramiccia, M.; Gradoni, L. The current status of zoonotic leishmaniases and approaches to disease control. Int. J. Parasitol. 2005, 35, 1169–1180. [Google Scholar] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar]
- Ready, P.D. Biology of Phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [PubMed]
- Olivier, M.; Gregory, D.J.; Forget, G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: A signaling point of view. Clin. Microbiol. Rev. 2005, 18, 293–305. [Google Scholar] [PubMed]
- Baneth, G.; Koutinas, A.F.; Solano-Gallego, L.; Bourdeau, P.; Ferrer, L. Canine leishmaniosis–new concepts and insights on an expanding zoonosis: Part one. Trends Parasitol. 2008, 24, 324–330. [Google Scholar] [PubMed]
- Solano-Gallego, L.; Koutinas, A.F.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar]
- Solano-Gallego, L.; Cardoso, L.; Pennisi, M.G.; Petersen, C.; Bourdeau, P.; Oliva, G.; Miró, G.; Ferrer, L.; Baneth, G. Diagnostic challenges in the era of canine Leishmania infantum vaccines. Trends Parasitol. 2017, 33, 706–717. [Google Scholar]
- Koutinas, A.F.; Koutinas, C.K. Pathologic mechanisms underlying the clinical findings in canine leishmaniosis due to Leishmania infantum/chagasi. Vet. Pathol. 2014, 51, 527–538. [Google Scholar]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar]
- Ikeda-Garcia, F.A.; Lopes, R.S.; Marques, F.J.; de Lima, V.M.F.; Morinishi, C.K.; Bonello, F.L.; Zanette, M.F.; Perri, S.H.V.; Feitosa, M.M. Clinical and parasitological evaluation of dogs naturally infected by Leishmania (Leishmania) chagasi submitted to treatment with meglumine antimoniate. Vet. Parasitol. 2007, 143, 254–259. [Google Scholar]
- Miró, G.; Oliva, G.; Cruz, I.; Cañavate, C.; Mortarino, M.; Vischer, C.; Bianciardi, P. Multicentric, controlled clinical study to evaluate effectiveness and safety of miltefosine and allopurinol for canine leishmaniosis. Vet. Dermatol. 2009, 20, 397–404. [Google Scholar] [PubMed]
- Koutinas, A.F.; Saridomichelakis, M.N.; Mylonakis, M.E.; Leontides, L.; Polizopoulou, Z.; Billinis, C.; Argyriadis, D.; Diakou, N.; Papadopoulos, O. A randomised, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniosis. Vet. Parasitol. 2001, 98, 247–261. [Google Scholar] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, 1–24. [Google Scholar]
- Vanaerschot, M.; Dumetz, F.; Roy, S.; Ponte-Sucre, A.; Arevalo, J.; Dujardin, J.C. Treatment failure in leishmaniasis: Drug-resistance or another (epi-) phenotype? Expert Rev. Anti. Infect. Ther. 2014, 12, 937–946. [Google Scholar] [PubMed]
- Murray, H.W.; Nathan, C.F. Macrophage microbicidal mechanisms in vivo: Reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 1999, 189, 741–746. [Google Scholar]
- Pinelli, E.; Gebhard, D.; Mommaas, A.M.; Van Hoeij, M.; Langermans, J.A.M.; Ruitenberg, E.J.; Rutten, V.P.M.G. Infection of a canine macrophage cell line with Leishmania infantum: Determination of nitric oxide production and anti-leishmanial activity. Vet. Parasitol. 2000, 92, 181–189. [Google Scholar]
- Holzmuller, P.; Hide, M.; Sereno, D.; Lemesre, J.L. Leishmania infantum amastigotes resistant to nitric oxide cytotoxicity: Impact on in vitro parasite developmental cycle and metabolic enzyme activities. Infect. Genet. Evol. 2006, 6, 187–197. [Google Scholar]
- Tuon, F.F.; Amato, V.S.; Bacha, H.A.; AlMusawi, T.; Duarte, M.I.; Neto, V.A. Toll-like receptors and leishmaniasis. Infect. Immun. 2008, 76, 866–872. [Google Scholar]
- Sacks, D.; Sher, A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002, 3, 1041–1047. [Google Scholar]
- Mosser, D.M.; Rosenthal, L.A. Leishmania-macrophage interactions: Multiple receptors, multiple ligands and diverse cellular responses. Semin. Cell Biol. 1993, 4, 315–322. [Google Scholar]
- Saldarriaga, O.A.; Velásquez, J.I.; Ossa, J.E.; Rugeles, M.T. Standardization of bovine macrophage monolayers and isolation and culture of Trypanosomes. Memórias Inst. Oswaldo Cruz 2003, 98, 269–271. [Google Scholar]
- Graham, M.L.; Prescott, M.J. The multifactorial role of the 3Rs in shifting the harm-bene fi t analysis in animal models of disease. Eur. J. Pharmacol. 2015, 759, 19–29. [Google Scholar] [PubMed]
- Carpenter, S.; O’Neill, L.A.J. How important are Toll-like receptors for antimicrobial responses? Cell. Microbiol. 2007, 9, 1891–1901. [Google Scholar] [PubMed]
- Janssens, S.; Beyaert, R. Role of Toll-Like receptors in pathogen recognition. Clin. Microbiol. Rev. 2003, 16, 637–646. [Google Scholar] [PubMed]
- Zeytun, A.; Chaudhary, A.; Pardington, P.; Cary, R.B.; Gupta, G. Induction of cytokines and chemokines by Toll-like receptor signaling: Strategies for control of inflammation. Crit. Rev. Immunol. 2010, 30, 53–67. [Google Scholar] [PubMed]
- Akira, S.; Sato, S. Toll-like receptors and their signaling mechanisms. Scand. J. Infect. Dis. 2003, 35, 555–562. [Google Scholar] [PubMed]
- de Veer, M.J.; Curtis, J.M.; Baldwin, T.M.; DiDonato, J.A.; Sexton, A.; McConville, M.J.; Handman, E.; Schofield, L. MyD88 is essential for clearance of Leishmania major: Possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 2003, 33, 2822–2831. [Google Scholar]
- Flandin, J.F.; Chano, F.; Descoteaux, A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-γ-primed macrophages. Eur. J. Immunol. 2006, 36, 411–420. [Google Scholar]
- Kropf, P.; Freudenberg, M.A.; Modolell, M.; Price, H.P.; Herath, S.; Antoniazi, S.; Galanos, C.; Smith, D.F.; Müller, I. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 2004, 72, 1920–1928. [Google Scholar]
- Sacramento, L.; Trevelin, S.C.; Nascimento, M.S.; Lima-Jùnior, D.S.; Costa, D.L.; Almeida, R.P.; Cunha, F.Q.; Silva, J.S.; Carregaro, V. Toll-like receptor 9 signaling in dendritic cells regulates neutrophil recruitment to inflammatory foci following Leishmania infantum infection. Infect. Immun. 2015, 83, 4604–4616. [Google Scholar]
- Viana, K.F.; Aguiar-Soares, R.D.O.; Roatt, B.M.; Resende, L.A.; Silveira-Lemos, D.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Moura, S.L.; Zanini, M.S.; Araújo, M.S.S.; et al. Analysis using canine peripheral blood for establishing in vitro conditions for monocyte differentiation into macrophages for Leishmania chagasi infection and T-cell subset purification. Vet. Parasitol. 2013, 198, 62–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chindera, K.; Mahato, M.; Kumar Sharma, A.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef] [PubMed]
- Worsley, A.; Vassileva, K.; Tsui, J.; Song, W.; Good, L. Polyhexamethylene biguanide: Polyurethane blend nanofibrous membranes for wound infection control. Polymers 2019, 11, 915. [Google Scholar]
- Firdessa, R.; Good, L.; Amstalden, M.C.; Chindera, K.; Kamaruzzaman, N.F.; Schultheis, M.; Röger, B.; Hecht, N.; Oelschlaeger, T.A.; Meinel, L.; et al. Pathogen- and host-directed antileishmanial effects mediated by polyhexanide (PHMB). PLoS Negl. Trop. Dis. 2015, 9, 1–22. [Google Scholar] [CrossRef]
- Vanaerschot, M.; Maes, I.; Ouakad, M.; Adaui, V.; Maes, L.; de Doncker, S.; Rijal, S.; Chappuis, F.; Dujardin, J.C.; Decuypere, S. Linking in vitro and in vivo survival of clinical Leishmania donovani strains. PLoS ONE 2010, 5, e12211. [Google Scholar] [CrossRef]
- Maia, C.; Nunes, M.; Marques, M.; Henriques, S.; Rolão, N.; Campino, L. In vitro drug susceptibility of Leishmania infantum isolated from humans and dogs. Exp. Parasitol. 2013, 135, 36–41. [Google Scholar]
- Yasur-Landau, D.; Jaffe, C.L.; Doron-Faigenboim, A.; David, L.; Baneth, G. Induction of allopurinol resistance in Leishmania infantum isolated from dogs. PLoS Negl. Trop. Dis. 2017, 11, e0005910. [Google Scholar] [CrossRef] [PubMed]
- Yasur-Landau, D.; Jaffe, C.L.; David, L.; Baneth, G. Allopurinol resistance in Leishmania infantum from dogs with disease relapse. PLoS Negl. Trop. Dis. 2016, 10, 1–13. [Google Scholar] [CrossRef]
- Yasur-Landau, D.; Jaffe, C.L.; David, L.; Doron-Faigenboim, A.; Baneth, G. Resistance of Leishmania infantum to allopurinol is associated with chromosome and gene copy number variations including decrease in the S-adenosylmethionine synthetase (METK) gene copy number. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 403–410. [Google Scholar]
- Campino, L.; Maia, C. The role of reservoirs: Canine leishmaniasis. In Drug Resistance in Leishmania Parasites–Consequences, Molecular Mechanism and Possible Treatments; Ponte-Sucre, A., Padrón-Nieves, M., Eds.; Springer: Vienna, Austria, 2018; pp. 45–64. [Google Scholar]
- Cojean, S.; Houzé, S.; Haouchine, D.; Huteau, F.; Sylvie Lariven, V.H.; Michard, F.; Bories, C.; Pratlong, F.; Le Bras, J.; Loiseau, P.M.; et al. Leishmania resistance to miltefosine associated with genetic marker. Emerg. Infect. Dis. 2012, 18, 704–706. [Google Scholar]
- Mondelaers, A.; Sanchez-Cañete, M.P.; Hendrickx, S.; Eberhardt, E.; Garcia-Hernandez, R.; Lachaud, L.; Cotton, J.; Sanders, M.; Cuypers, B.; Imamura, H.; et al. Genomic and molecular characterization of miltefosine resistance in Leishmania infantum strains with either natural or acquired resistance through experimental selection of intracellular amastigotes. PLoS ONE 2016, 11, e0154101. [Google Scholar] [CrossRef] [PubMed]
- Gómez Pérez, V.; García-Hernandez, R.; Corpas-López, V.; Tomás, A.M.; Martín-Sanchez, J.; Castanys, S.; Gamarro, F. Decreased antimony uptake and overexpression of genes of thiol metabolism are associated with drug resistance in a canine isolate of Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tazuke, S.; Watanabe, M. Interaction of biologically active molecules with phospholipid membranes. Biochim. Biophys. Acta Biomembr. 1983, 735, 380–386. [Google Scholar] [CrossRef]
- Moon, E.; Choi, H.; Kong, H.; Quan, F. Experimental parasitology polyhexamethylene biguanide and chloroquine induce programmed cell death in Acanthamoeba castellanii. Exp. Parasitol. 2018, 191, 31–35. [Google Scholar] [PubMed]
- Maia, C.; Rolão, N.; Nunes, M.; Gonçalves, L.; Campino, L. Infectivity of five different types of macrophages by Leishmania infantum. Acta Trop. 2007, 103, 150–155. [Google Scholar] [CrossRef]
- Terreros, M.J.S.; de Luna, L.A.V.; Giorgio, S. Evaluation of antileishmanial drugs activities in an ex vivo model of leishmaniasis. Parasitol. Int. 2019, 71, 163–166. [Google Scholar] [CrossRef]
- da Luz, R.I.; Vermeersch, M.; Dujardin, J.C.; Cos, P.; Maes, L. In vitro sensitivity testing of Leishmania clinical field isolates: Preconditioning of promastigotes enhances infectivity for macrophage host cells. Antimicrob. Agents Chemother. 2009, 53, 5197–5203. [Google Scholar]
- Vermeersch, M.; da Luz, R.I.; Tote, K.; Timmermans, J.P.; Cos, P.; Maes, L. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: Practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef]
- Rezaei Riabi, T.; Sharifi, I.; Miramin Mohammadi, A.; Khamesipour, A.; Hakimi Parizi, M. Evaluation of a possible synergistic effect of meglumine antimoniate with paromomycin, miltefosine or allopurinol on in vitro susceptibility of Leishmania tropica resistant isolate. Iran. J. Parasitol. 2013, 8, 396–401. [Google Scholar]
- Das, S.; Rani, M.; Pandey, K.; Sahoo, G.C.; Rabidas, V.N.; Singh, D.; Das, P. Combination of paromomycin and miltefosine promotes TLR4-dependent induction of antileishmanial immune response in vitro. J. Antimicrob. Chemother. 2012, 67, 2373–2378. [Google Scholar] [CrossRef]
- Martínez-Orellana, P.; Montserrat-Sangrà, S.; Quirola-Amores, P.; González, N.; Solano-Gallego, L. Cytokine effect of TLR3, TLR4, and TLR7 agonists alone or associated with Leishmania infantum antigen on blood from dogs. Biomed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galdino, H.; Saar Gomes, R.; dos Santos, J.C.; Pessoni, L.L.; Maldaner, A.E.; Marques, S.M.; Gomes, C.M.; Dorta, M.L.; de Oliveira, M.A.P.; Joosten, L.A.B.; et al. Leishmania (Viannia) braziliensis amastigotes induces the expression of TNFα and IL-10 by human peripheral blood mononuclear cells in vitro in a TLR4-dependent manner. Cytokine 2016, 88, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Hosein, S.; Rodríguez-Cortés, A.; Blake, D.P.; Allenspach, K.; Alberola, J.; Solano-Gallego, L. Transcription of toll-like receptors 2, 3, 4 and 9, FoxP3 and Th17 cytokines in a susceptible experimental model of canine Leishmania infantum infection. PLoS ONE 2015, 10, e0140325. [Google Scholar] [CrossRef] [PubMed]
- Turchetti, A.P.; da Costa, L.F.; de Romão, E.L.; Fujiwara, R.T.; da Paixão, T.A.; Santos, R.L. Transcription of innate immunity genes and cytokine secretion by canine macrophages resistant or susceptible to intracellular survival of Leishmania infantum. Vet. Immunol. Immunopathol. 2015, 163, 67–76. [Google Scholar] [CrossRef]
- Quixabeira, V.B.L.; de Pereira, L.I.A.; Veras, P.R.V.; da Costa, A.C.V.; Fonseca, L.G.; Galdino, H., Jr.; da Silva, I.A., Jr.; Morato, C.I.; Pinto, S.A.; Pereira, A.J.C.S.; et al. Alterations in monocyte subsets and cytokine production after TLR activation in american cutaneous leishmaniasis. Parasite Immunol. 2019, 41, e12623. [Google Scholar] [CrossRef]
- Ramírez-Pineda, J.R.; Fröhlich, A.; Berberich, C.; Moll, H. Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J. Immunol. 2004, 172, 6281–6289. [Google Scholar] [CrossRef]
- Roatt, B.M.; de Aguiar-Soares, R.D.O.; Coura-Vital, W.; Ker, H.G.; das Moreira, N.D.; Vitoriano-Souza, J.; Giunchetti, R.C.; Carneiro, C.M.; Reis, A.B. Immunotherapy and immunochemotherapy in visceral leishmaniasis: Promising treatments for this neglected disease. Front. Immunol. 2014, 5, 1–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).