Abstract
Diagnosis and treatment of ocular fungal infection in equine seems very challenging for owners and clinicians. The present study aimed to identify and characterize fungal species isolated from the eyes of clinically healthy and diseased equines (N = 100) from Dakahlia Governorate, Egypt. The work also involved morphological and molecular characterization of the major fungal species. In addition, correlations between the occurrence of isolated fungi and some of the potential risk factors were also investigated. Interestingly, the prevalence rate of ocular mycosis in all examined equines in the study was 28% and there were major clinical signs associated with ocular fungal infection. Moreover, the identified fungal species included Aspergillus flavus, A. fumigatus, A. niger, Penicillium spp., Mucor spp., and Alternari spp. with a corresponding prevalence rate of 63.9%, 27.8%, 15.3%, 18.1%, 13.9%, and 4.2%, respectively, in healthy equine eyes, while their prevalence in diseased equine eyes was 57.1%, 32.1%, 21.4%, 7.1%, 3.6%, and 0%. Furthermore, a statistical significant association (p < 0.05) was found between the frequency of isolation of A. fumigatus and Penicillium and several risk factors (breed, sex, and ground type), while the remaining risk factors and occurrence of fungi were not statistically correlated. A subset of the Aspergillus species samples positive by polymerase chain reaction (PCR) were sequenced and their phylogenetic analysis identified three species of Aspergillus. Taken together, our study provides novel data related to the occurrence of ocular mycosis in equine in Egypt. Given the zoonotic potential of some identified fungi, our data may be helpful for implementation of novel diagnostic and therapeutic strategies for combating this sight-threatening infection in equine.
1. Introduction
Ocular diseases and their related complications remain a serious health problem in equines worldwide [1]. These diseases have a great impact on the quality of life and value of affected horses, as well as their athletic and show potential or use for other purposes [1]. In addition, the pain associated with ocular diseases damages the animal welfare and may lead to partial or full blindness, which may result in exclusion of the horse from performance occupations and cause potential risk for the rider [2]. In fact, the ocular surface in horses, mules, and donkeys is naturally inhabited by microorganisms that constitute its normal microflora [3]. Scientists believe such flora play a defensive role against pathogenic microbes by depriving them from nutrients, producing antimicrobial substances, and occupying space on the corneal and conjunctival epithelium [4]. However, under particular disease conditions, the microbes composing the normal flora, including Gram-negative bacteria and fungi, may become opportunistic pathogens, [5,6]. Amongst others, 38% of infectious keratitis cases in equines caused by fungal organisms are believed to be seeded from the environment, thus seasonal variation could affect the equine conjunctival microflora [7,8]. Importantly, these opportunistic pathogens, which include a wide range of fungi and bacteria, can be transmitted to humans and cause potential health risks [9].
There is great variability in pathogenic fungal species and the visual outcome of ocular infection. Fungal infection of the eye is frequently encountered in horses but it is rare in ruminants, dogs, and cats [10]. The ubiquitous presence of fungi on the surface of the conjunctiva and cornea of equines, combined with suspected defects in the equine corneal immune system make corneal infection more likely to occur in equines than in other domestic animal species [10,11]. Furthermore, the progression of keratomycosis is favored by the use of topical corticosteroids and prolonged use of topical antibiotics [12,13]. Equine keratomycosis has been documented in various countries and regions worldwide including the USA [10,11,14,15,16], the United Kingdom [17], Germany [18], Australia [19], and Switzerland [20]. However, it should be stressed that fungal infection of the eye is more common in warm, humid environments such as the southern USA [12]. Fungal keratitis in horses is considered an infrequent problem in temperate regions of the world, where bacterial keratitis is the major problem [21,22]. In Brazil and the USA, fungi were detected most frequently in the eyes of animals that lived in stalls, where molds were more prevalent (91%) than yeasts [21]. This could mean that high humidity, dust and other hygienic problems in the stalls represent major risk factors for the development of keratomycosis in horses confined to stables [7]. Interestingly, the most common isolated fungal organisms from non-diseased eyes include Cladosporium, Alternaria, Fusarium, Aspergillus spp., and Penicillium spp. [7], while the most frequently reported fungal isolates from diseased equine eyes are Aspergillus spp., Fusarium spp., and Candida spp., which vary depending on the location [11,23]. Since there is a lack of information on the available epidemiological data related to the occurrence of keratomoycosis in equines in the Egyptian environment and the potential zoonotic risks of some of the frequently isolated fungal species, the present study was undertaken to investigate and characterize the occurrence of fungal pathogens isolated from equine eyes.
2. Material and Methods
2.1. Ethical Consideration
Ethical approval was obtained from a guidance of Research, Publication and Ethics of the Faculty of Veterinary Medicine, Mansoura University, Egypt, with code number R/31, which complies with all relevant Egyptian legislations in research and publications.
2.2. Study Area, Animals, and Medical Records
The study was carried out on a total number of 100 swabs from the eyes of clinically healthy and diseased equines (N = 100) from Dakahlia Governorate, Egypt from October 2018 to May 2019. This included 86 horses (61 clinically healthy and 25 with ophthalmic lesions) at a median age of 11.5 years old (range 0.5–22 years) and median weight of 330 kg (range 100–500 kg). In addition, 14 donkeys (Equus asinus) (11 clinically healthy and three with ophthalmic lesions) at a median age of 6.8 years (range 0.3–13 years) and at a median weight of 220 kg (range 80–350 kg) were also analyzed. The selected horses lived in semi-open stabling in an open yard from 08:00 till 14:00, then they were allowed to enter the closed pens. All horses were fed a concentrated diet and berseem in the winter and a concentrated diet and hay in the summer season. Importantly, horses and equine eyes were examined before application of any topical or systemic treatment. A questionnaire was given to managers at the time of visit, consisting of objective questions to obtain data regarding age, sex, breed, housing, season, hygiene, bedding, any stress factor related to the animal, and antimicrobial drugs used in the treatment of any previous ophthalmic infection. Importantly, the sampling process was carried out by a group of specialists and experts in the field of equine ophthalmology including clinicians, ophthalmologists, veterinary surgeons, and several experts from the Department of Infectious diseases and Internal Medicine and Clinical diagnosis, Faculty of Veterinary Medicine, Mansoura University, Egypt. The experts followed strict regulations during the sampling process to avoid violating any ethical boundaries.
2.3. Clinical Examination and Sample Collection
Data concerned with the case history, clinical findings, and medical record for each horse and donkey were recorded. Ophthalmic history was obtained through a series of questions directed to the owners and farm personnel regarding the observed clinical signs. The clinical examination of the eye was performed without anesthetics for restraint purposes as it was a non-invasive study. The palpebral margins and the conjunctiva were examined for signs of inflammation, and the position of the third eyelid was evaluated. The opening of the palpebral fissure is readily achieved by using the thumb and forefinger from the same hand while approaching from the temporal aspect of the eye. Pupils were dilated with 1% atropine sulfate (atropine eye drops). The anterior chamber and posterior chamber of the eyes were examined with a direct ophthalmoscope. The clinical findings were recorded. Regarding sampling, a conjunctival swab from the inferior conjunctival fornix of both eyes was collected from each animal, without touching the eyelashes or eyelids, using a sterile swab under complete aseptic precautions. All samples were collected before the animals were given neither antibiotics (local or systemic) nor anesthetics as described elsewhere [24]. The collected swabs were directly placed in sterile test tubes containing tryptone soya broth (Oxoid, Oxoid Ltd., Cheshire, UK) as enrichment media and were sent directly to the laboratory of Internal Medicine, Faculty of Veterinary Medicine, Mansoura University, Egypt for further mycological examination.
2.4. Isolation and Identification of Fungal Species
All samples were incubated at 37 °C for 24 h, then a loopful was taken from each sample and streaked on Sabouraud dextrose agar (Oxoid) and incubated at 25 °C for isolation of fungi. Identification of fungal species was done by colony morphology and molecular characterization through sequencing of 18 S ribosomal RNA (rRNAs) gene.
2.5. Fungal DNA Extraction
The genomic DNA was extracted from 5–7 days old fungal cultures grown in malt extract agar media. The mycelial growth from the culture plate was scraped out using 2 mL of sterile distilled water. Two mL of spore suspension was then inoculated in a 100 mL yeast extract with supplements (YES) medium in a universal 250 mL flask and incubated with gentle shaking at 28 °C with 180 rpm for 48 h. Harvesting of the mycelia from the flasks was done by filtration under aseptic condition using a micro-cloth. The mycelia were then washed with sterile distilled water and placed in sterile Petri–dishes, stored at −20 °C overnight and freeze-dried. Later on, the freeze-dried mycelium was ground in a mortar using a sterile pestle and then placed in eppendorf tubes. DNA extraction was conducted using DNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. To ensure the presence of fungal DNA, agarose gel electrophoresis was conducted and the DNA was visualized and imaged using the transilluminator of a gel documentation system (BIO-RAD, Gel Doc 2000, Budapest, Hungary).
2.6. Polymerase Chain Reaction (PCR)
Taq PCR Master mix (Qiagen) was used to amplify or synthesize DNA (genomic or plasmid) fragments that targets the 18 S ribosomal RNA (rRNAs) gene using a thermal cycler machine (Stratagene, San Diego, CA, USA) at the Regional Center for Mycology and Biotechnology Alazhar University, Cairo, Egypt. The primer set used was TCCGTAGGTGAACCTGCGG for the forward primer and TCCTCCGCT TAT TGA TAT GC for the reverse primer, to amplify a 595–600bp fragment [25]. The appropriate PCR conditions included a universal denaturation cycle (5 min at 94 °C), 30 cycles of annealing/extension reactions (20 s at 94 °C, 30 s at an optimum annealing temperature for each amplified fragment and 60 s at 72 °C and cycle of final extension step (5 min at 72 °C) followed by soaking at 4 °C.
2.7. Purification of DNA Fragments from the Gel
DNA fragments (PCR products and linear plasmid) were purified by agarose gel electrophoresis using the appropriate gel concentration stained with ethidium bromide according to the method described elsewhere [26].
2.8. DNA Sequencing, Assembling of Nucleotide Sequences, and Phylogenic Analysis
Some of positive PCR products belonged to Aspergillus fungi were directly purified then sequenced using the Cy5/Cy5.5 Dye Primer Sequencing kit (Visible Genetics Inc., Toronto, ON, Canada) with the Open Gene automated DNA sequencing system [27,28] at the Regional Center For Mycology and Biotechnology, Alazhar University, Cairo, Egypt. The resulting nucleotide sequences were subjected to BLAST algorithms and databases from the National Center for Biotechnology NCBI (http://www.ncbi.nlm.nih.gov/html) to compare the resulting sequence to those available in GenBank to confirm PCR specificity. Molecular evolutionary genetics analysis (MEGA) MEGA X (http://www.megasoftware.net/) software was also used for sequence analysis and alignments [29]. The phylogram is drawn to scale, with branch lengths (next to the branches) in the same units as those of the evolutionary genetic distances (0.05%) [30].
2.9. Statistical Analysis
The statistical analysis was performed on SPSS 21 (IBM, Armonk, New York, NY, USA). Numerical data were expressed as median (range), while categorical data were expressed as number (%). Chi-square tests were conducted to assess the correlation between various risk factors, including age, sex, breed, housing, season, hygiene, ground type, stress and frequency of isolation (percentage) of different fungal species from ocular swabs. The p-value, odds ratio (OR), and 95% confidence interval (CI 95%) were recorded as a multivariable logistic regression model to detect the associated risk factors of infection. For all statistical analysis, variables at p-value < 0.05 were considered to be significant.
3. Results
3.1. Clinical Signs
Out of the 100 animals investigated (86 horses and 14 donkeys), 25 horses and three donkeys (28 in total) developed one or more of the following clinical signs that suggest ophthalmic infection: conjunctival hyperemia, continuous lacrimation, blepharospasm, eye lid edema, mucopurulent ocular discharge, corneal edema, and corneal opacity, examples of which are shown in Figure 1. There were no behavioral or systemic signs of disease in all the studied group. Furthermore, all the parameters of heart rate, respiratory rate, and rectal temperature were within the normal range.
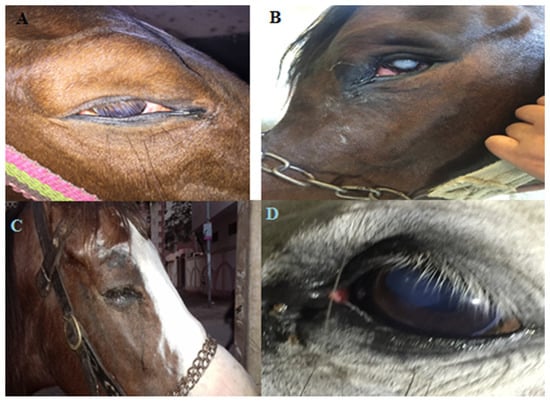
Figure 1.
Clinical signs suggest ophthalmic infection. (A) A horse with ocular infection showing corneal opacity and mucopurulent discharge. (B) A horse’s eye, following exposure to ocular trauma showing corneal opacity. (C) A horse suffering from conjunctivitis showing blepharospasm, eyelid edema and continuous lacrimation. (D) A donkey showing mucopurulent discharge with eyelid edema.
3.2. Morphology of the Isolated Fungal Species
The colonies of the different species of fungi started to be visible after 4–5 days of cultivation on Sabouraud dextrose agar media. The morphology and characters of the different species of fungi are summarized in Table 1.

Table 1.
The morphology and characters of colonies on agar, the colonies of the different species of fungi, and their appearance on Sabouraud dextrose agar media (SDA).
3.3. Molecular Identification, Sequence Analysis, and Phylogeny of Isolated Fungi
Regarding the molecular identification, the amplified DNA fragment size for the targeted genes (18S rRNA) extracted from the colonies revealed an amplicon of approximately 595–600 base pair (bp). The gene sequences and phylogenetic analysis showed that the pair wise comparison illustrates the low distance between the sequences from selected three fungal species of Aspergillus compared with reference sequences from GenBank which is shown in Supplementary Figures S1–S3. In this concern, as shown in Supplementary Figure S1, the percentage identity between the Aspergillus flavus sequence of our study and previous sequences in GeneBank was 100% with A. flavus isolate RF-02 S rRNA gene (accession no. KY933394.1) and A. flavus strain RF-03 S rRNA gene (accession no. MF120213.1), while the identity was 99.83% with A. flavus isolate SF53 S rRNA gene (accession no. MG976497.1) and A. flavus strain CBS 126855 S rRNA gene (accession no. MH864264.1).
Regarding Aspergillus fumigatus, as predicted in Supplementary Figure S2, the percentage of identity between the A. fumigatus sequence obtained in our present work was 99.8% with A. fumigatus strain FJAT-31052 S rRNA (accession no. KU687812.1), while it showed 99.59% identity with the following strains; A. fumigatus isolate EGDA31 S rRNA (accession no. MH591451.1), A. fumigatus isolate 1052 18S S rRNA (accession no. KT826617.1), A. fumigatus strain IHEM 18963 isolate ISHAMITS_ID MITS169 18S rRNA (accession no. KP131566.1), A. fumigatus strain HQ 18S rRNA (Accession no. EU139476.1), and A. fumigatus isolate X 7 18S rRNA (accession no. KJ958365.1). On the other hand, there was 100 percentage identity between the obtained sequences of Aspergillus niger and previous sequences in GeneBank, A. niger strain RAF106 S rRNA (accession no. MN195121.1), A. niger isolate KUASR15 S rRNA (accession no. MN187307.1), A. niger isolate T8 internal transcribed spacer 1 (ITS) (accession no. MN180811.1), A. niger strain 22 ITS1 (accession no. MN133869.1), and A. niger isolate SVUA ITS1 (accession no. MK258199.1).
3.4. Frequency of Isolated Fungi between Healthy and Diseased Equines’ Eyes and Associated Risk Factors
As depicted in Table 2, Aspergillus flavus was isolated from 63.9% of healthy eyes and 57.1% of diseased eyes while Aspergillus fumigatus was isolated from 27.8% of healthy eyes and from 32.1% of diseased eyes. In addition, Aspergillus niger was isolated from 11 (15.3%) healthy animals and six (21.4%) animals with diseased eyes, while Mucor species was isolated from 13 (18.1%) healthy eyes and two (7.1%) diseased eyes. Meanwhile, Penicillium species were isolated from 10 (13.9%) healthy eyes and one (3.6%) diseased eye while Alternaria species was isolated from three (4.2%) healthy eyes but was not isolated from any diseased eyes. Collectively, Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Mucor species, Penicillium species and Alternaria species were the most commonly isolated fungal species from healthy and diseased equine eyes; however, there was no statistical significant difference in the frequency of isolated fungi between healthy and diseased equine eyes.

Table 2.
The frequency of isolated fungal species between healthy and diseased equine eyes.
Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8 show the correlation between the variable studied risk factors and the frequency of isolated species of fungi in healthy and diseases eyes of examined animals. As mentioned above, the studied risk factors included age, sex, breed, housing, season, hygiene, ground type, and other stress factors. Among others, there was a significant association noted between the frequency of the isolation of Aspergillus fumigatus from eyes and both the breed and ground type (both p-value = 0.025; OR = 0.337; 99% CI: 0.260–0.437). In this concern, the frequency of isolation of Aspergillus fumigatus was considerably higher in Arabian horses than other breeds and donkeys, where this fungus was isolated from 16 (55.2%) Arabian horses versus 13 (44.8%) horses from the other breeds and donkeys (Table 4). In addition, Aspergillus fumigatus was isolated at significantly higher levels from animals housed on concrete flooring (55.2%) compared with mud flooring (44.8%) (Table 4). Also, there was a significant association between Penicillium spp. isolation and the following risk factors, sex (p = 0.021) and housing (p = 0.024) (Table 7). With respective to the sex of the animal, Penicillium was only isolated in females (100%) (Table 7). Meanwhile for housing, the percentage of isolated Penicillium was higher in mixed housing (90.9%) in comparison with indoor systems (9.1%) (Table 7). On the other hand, there were no statistical significance association noted between Aspergillus flavus, Aspergillus niger, Mucor spp., Alternaria spp. and all the mentioned risk factors (Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8).

Table 3.
Risk factors of Aspergillus flavus.

Table 4.
Risk factors of Aspergillus fumigatus.

Table 5.
Risk factors of Aspergillus niger.

Table 6.
Risk factors of Mucor.

Table 7.
Risk factors of Penicillium.

Table 8.
Risk factors of Alternaria.
4. Discussion
The corneal epithelium and tear film of the eyes represent a major barrier for protection of equine eyes from many contaminates and from invasion by pathogens that include bacteria and fungi [31]. The physiology of the equines eye may contribute in potentiating their susceptibility to infection [31,32]. For example, the large size of the eye, the prominence and unprotected position of the eye, and the tear film instability of equine eyes expose them to environmental factors such as trauma of the cornea with vegetative materials, organic matter, head collar, and contaminated soils [15,31,32]. These factors, which either alone or in association with other factors, represent leading causes of keratomycosis as they destruct the main barriers, favoring pathogen entry [33]. Among other pathogens, fungal infection represents one of sight-threatening disease in different animals species, but equines seem most frequently affected due to the lateral location of their eyes [34,35]. Previous studies have shown that fungi were isolated from normal, healthy eyes of cows (100%), horses (12%), dogs (22%), cats (40%), and sheep (86%) [8,36]. The conjunctiva of theses animal species were colonized by a subset of fungal species including Aspergillus spp. in 12% of cows, 56% of horses, and 8% of the cats, but not detected in dogs. Penicillium spp. and Cladosporium spp. were isolated ubiquitously from these species [8]. Moreover, five species of fungi were isolated from normal sheep conjunctiva, including Mucor spp., Aspergillus spp., Penicillium spp., Alternaria spp., and Cladosporium spp. [36].The present study provides novel data related to the occurrence of different species of fungi isolated from healthy and diseased eyes of equines in Dakahlia governorate, Egypt. To achieve this objective, the work involved morphological identification and molecular characterization of the major isolated fungi combined with an assessment of some possible risk factors that might influence the epidemiological pattern of the disease. This seems to be the first study involving isolation and identification of different mycosis from the eyes of equines in Egypt.
In accordance with its pathogenesis, keratomycosis is considered a subset of eye infection which is associated with primary disruption of the corneal epithelium and responsible for binding of environmental organisms to the corneal stroma [32,37]. Upon their binding, the fungal hyphae release proteases that inhibit the angiogenesis in susceptible cornea, corneal melting and anterior uveitis [37]. The hallmark of mycotic keratitis in horses is the induction of rapidly and progressive ulceration and perforation on the cornea [38]. More importantly, some of these major leading ubiquitous organisms causing keratomycosis may be of potential zoonotic concern, e.g., Aspergillus spp., and therefore may be occupation risks for agricultural workers and farmers [39]. Clearly, early identification of the causative fungi is considered a cornerstone for designing control strategies. At the same time, the identification of the causative agent represent a major challenge for proper therapeutic intervention and consequently for salvage of the vision [32]. The prevalence of keratomycosis or ocular mycosis in equines has been documented in some previous studies worldwide where the diagnosis in these studies was carried out using either one or often a combination of the following methods: cytology, culture, histopathology, and molecular identification [11,14,23,40,41,42,43,44]. In accordance with the reported clinical signs, the majority of infected horses and donkeys experienced one or more of the various clinical signs that include conjunctival hyperemia, continuous lacrimation, blepharospasm, eye lid edema, and mucopurulent ocular discharge. Taken into account, these clinical signs are not specific for certain species of fungi but similar clinical signs were recorded in previous reports with equine ulcerative fungal keratitis [45,46].
In our present results, the prevalence of kertaomycosis was 28% in relation to the total number of the examined ophthalmic cases. Interestingly, this prevalence is higher than that reported in several previous studies [17,40]. In a previous study carried out by McLaughlin et al. (1992) [47], the reported prevalence of keratomycosis was 10%. Furthermore, other studies have reported prevalence rates of 8.62% and 7%, respectively, which are lower than our current findings [40,48]. This may relate to the fact that a higher number of equines were examined in our study versus the majority of the previous reports worldwide [17,40]. Alternatively, the difference in the prevalence of fungal infection compared to our study might be attributed to the hypothesis that the seasonal variation and temperature conditions, housing and management conditions might influence the epidemiological pattern of keratomycosis [35,49]. Along the same line, equine fungal keratitis is usually recorded in warm and humid climate regions, which suggest that seasonal variation is an important factor influencing the occurrence of the disease [31,50,51]. However, there is raised awareness of its prevalence in subtropical areas and during summer and autumn months with sufficient temperature and precipitation [12,43,50,52]. Clearly, the difference in geographic location might favor the existence of certain species of fungi in specific regions than others [17,21,44]. Our present results showed isolation of six major species of fungi from healthy and diseased equine eyes. These fungal species are Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Penicillium spp., and Mucor spp. Furthermore, a sixth species, Alternari spp., was only isolated from the healthy eyes of examined animals. Given the little difference on the frequency and the prevalence of the isolated fungi, Aspergillus flavus, Aspergillus fumigatus, and Aspergillus niger were the most common etiological agents have been isolated from eyes of healthy and diseased equine, which is consistent with several previous studies [35,40,41,43]. Similar studies revealed that Aspergillus and Fusarium counts for one-third of all major causes of traumatic keratitis [35,40,49], while some other fungal genera could be isolated such as Candida, Penicillium, and Alternaria [11,14,23,42]. In the same line, several previous works concluded that mixed infections of Aspergillus and Pseudomonas spp. in horses represent the major isolated pathogens in severe corneal diseases [3,16].
Analysis of the data presented here revealed that there are several predisposing factors such as; breed, age, sex, species, season, and stabling that might influence the epidemiological pattern of keratomycosis [14]. However, it should be borne in mind that the small number of clinical cases in our present study makes it difficult to study the correlation of predisposing (risk) factors to the occurrence of the disease, and therefore, it might influence the significance of the results of the statistical analysis difference or not as documented elsewhere. As shown in Table 2, the percentage of isolation from fungi was higher in healthy equines than diseased equines, which is consistent with several previous studies [35,53]. The occurrence of Aspergillus fumigatus was higher in Arabian horses (55.2%) in comparison with other horse breeds and donkeys (44.8%) that implicate the breed as a possible predisposing factor for fungal infection of equine eyes (Table 2). Similar results were reported in previous studies which found that the occurrence of keratomycosis affected some horse breeds more than others [12]. Furthermore, the highest percentage of isolation of Aspergillus fumigatus was recorded in animals stabled with concrete flooring (55.2%) compared with those stabled with mud flooring (44.8%) (Table 4). On the other hand, there was a significant association between the frequency of the isolation of Penicillium and both the sex of the animal and housing factors. In this concern, there was correlation between the positivity to Penicillium from eyes of infected equids and the sex; where more female equids were infected than male equids. As depicted in Table 7, for housing, the isolation percentage of Penicillium from animals kept indoors or outdoors was 9.1% versus 90.9%, respectively. This finding is in line with previous studies from USA and Brazil that reported a higher occurrence of fungi isolated from the eyes of stabled equids compared to those horses living outside [7,21,54]. These data reflects that the high humidity, dust, and other hygienic problems in the stables represent an important risk factor for keratomycosis in equids confined to stables [7]. However, the majority of outdoors living donkeys in rural areas were found to harbor fungi in their eyes [55]. It should be stressed that it is very hard to compare the prevalence of ocular fungal infection of horses and donkeys living outside versus stabled ones because of their peculiar kind of husbandry, especially those from rural areas and kept outdoors all day. In Tuscany, stabled horses with increased levels of humidity and dust were more frequently positive to kertaomycosis than those living outside [56], leading to a higher risk of ocular contamination by fungi [21,56].
Meanwhile, for the other risk factors and the remaining fungal species, despite the existence of differences between the frequency of isolation of fungi and risk factors, no significant correlation was reported, which is consistent with several previous studies [14,57,58]. Furthermore, there was no correlation and no statistical significant difference between the other studied possible risk factors and the frequency of the isolation of the other isolated fungal species, which is consistent with the hypothesis that keratomycosis can affect any horse and has no breed, age, or sex predisposition, but geographic location and housing and management practices might influence the epidemiology of the disease [14,57,58]. The role of molecular methods in detection of keratomycososis in equine has been documented in several previous studies [59]. In our present results, the amplified DNA fragment size target 18S rRNA gene from the colonies revealed an amplicon of 594–600 bp in size, which is similar to that was reported elsewhere [60]. It is worth mentioning that our present work investigated the molecular identity of the major isolated species of fungi that were Aspergillus flavus, Aspergillus fumigatus, and Aspergillus niger. In addition, the data were then confirmed illustrated through a phylogenetic analysis for three isolated of three Aspergillus species and this might reveal the wide spread of these genotypes to Egypt.
5. Conclusions
Given the findings, our study reveals high prevalence of fungal infection in the investigated equine eyes from Dakahlia governorate, Egypt and provides novel information that should turn our attention toward the importance of early recognition of the disease agent for the prompt intervention and successful management of the case. Our study also proposes further future research to explore more about fungal mycotic infection in different animal species together with investigation of the occurrence of ocular mycosis in people who are in contact with these infected animals. Other alternative prophylactic strategies, good management practices, and periodical surveillance regimes may also be helpful to reduce the risk of infection.
Supplementary Materials
The following are available online at https://www.mdpi.com/2306-7381/7/3/130/s1.
Author Contributions
A.T., H.M.M.I., M.A.Y., and S.E.-K. designed the study protocol and conception of the research idea. H.K.E., A.T., A.A., H.E., E.K.E., A.M.R., A.E., and H.M.M.I. participated in the design of the methodology and sampling; and performed the laboratory work. A.T., H.M.M.I., A.M.R., H.E.-S., A.E., and E.K.E. performed data analysis and interpretation. A.T., H.K.E., A.A., H.M.M.I., M.A.Y., and S.E.-K. participated in data analysis. A.T., E.K.E., H.K.E., and H.E.-S. wrote and prepared the manuscript for publication and revision. H.K.E., H.M.M.I., M.A.Y., and S.E. contributed their scientific advice. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors also thank the veterinarians and farm owners for their support and help in providing data and samples collection throughout the study. The authors would also like to thank Kirsty Jensen from the University of Edinburgh for improving the English style of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Consideration
Ethical approval was obtained from a guidance of Research, Publication and Ethics of the Faculty of Veterinary Medicine, Mansoura University, Egypt, which complies with all relevant Egyptian legislations.
References
- Gelatt, K.N.; Gilger, B.C.; Kern, T.J. Veterinary Ophthalmology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Paschalis-Trela, K.; Cywinska, A. The prevalence of ocular diseases in polish Arabian horses. BMC Vet. Res. 2017, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Johns, I.C.; Baxter, K.; Booler, H.; Hicks, C.; Menzies-Gow, N. Conjunctival bacterial and fungal flora in healthy horses in the UK. Vet. Ophthalmol. 2011, 14, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.R.; Chengappa, M.; Roberts, A.W.; Claus, G.W.; Rikihisa, Y. Essentials of Veterinary Microbiology; Smithsonian Libraries: Washington, DC, USA, 1995; 394p. [Google Scholar]
- Kodikara, D.S.; De Silva, N.; Makuloluwa, C.A.; De Silva, N.; Gunatilake, M. Bacterial and fungal pathogens isolated from corneal ulcerations in domesticated elephants (Elephas maximus maximus) in Sri Lanka. Vet. Ophthalmol. 1999, 2, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Petersen-Jones, S. Quantification of conjunctival sac bacteria in normal dogs and those suffering from keratoconjunctivitis sicca. Vet. Comp. Ophthalmol. 1997, 7, 29–35. [Google Scholar]
- Moore, C.P.; Heller, N.; Majors, L.J.; Whitley, R.D.; Burgess, E.C.; Weber, J. Prevalence of ocular microorganisms in hospitalized and stabled horses. Am. J. Vet. Res. 1988, 49, 773–777. [Google Scholar]
- Samuelson, D.A.; Andresen, T.L.; Gwin, R.M. Conjunctival fungal flora in horses, cattle, dogs, and cats. J. Am. Vet. Med. Assoc. 1984, 184, 1240–1242. [Google Scholar]
- Seyedmousavi, S.; Bosco, S.M.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56, 165–187. [Google Scholar] [CrossRef]
- Moore, C.P.; Collins, B.K.; Fales, W.H. Antibacterial susceptibility patterns for microbial isolates associated with infectious keratitis in horses: 63 cases (1986-1994). J. Am. Vet. Med. Assoc. 1995, 207, 928–933. [Google Scholar]
- Andrew, S.E.; Brooks, D.E.; Smith, P.J.; Gelatt, K.N.; Chmielewski, N.T.; Whittaker, C.J. Equine ulcerative keratomycosis: Visual outcome and ocular survival in 39 cases (1987-1996). Equine Vet. J. 1998, 30, 109–116. [Google Scholar] [CrossRef]
- Reed, Z.; Thomasy, S.M.; Good, K.L.; Maggs, D.J.; Magdesian, K.G.; Pusterla, N.; Hollingsworth, S.R. Equine keratomycoses in California from 1987 to 2010 (47 cases). Equine Vet. J. 2013, 45, 361–366. [Google Scholar] [CrossRef]
- Beech, J.; Sweeney, C.R.; Irby, N. Keratomycoses in 11 horses. Equine Vet. J. 1983, 15, 39–44. [Google Scholar] [CrossRef]
- Gaarder, J.E.; Rebhun, W.C.; Ball, M.A.; Patten, V.; Shin, S.; Erb, H. Clinical appearances, healing patterns, risk factors, and outcomes of horses with fungal keratitis: 53 cases (1978-1996). J. Am. Vet. Med. Assoc. 1998, 213, 105–112. [Google Scholar] [PubMed]
- Brooks, D.E.; Andrew, S.E.; Dillavou, C.L.; Ellis, G.; Kubilis, P.S. Antimicrobial susceptibility patterns of fungi isolated from horses with ulcerative keratomycosis. Am. J. Vet. Res. 1998, 59, 138–142. [Google Scholar] [PubMed]
- Moore, C.P.; Fales, W.H.; Whittington, P.; Bauer, L. Bacterial and fungal isolates from Equidae with ulcerative keratitis. J. Am. Vet. Med. Assoc. 1983, 182, 600–603. [Google Scholar] [PubMed]
- Sansom, J.; Featherstone, H.; Barnett, K.C. Keratomycosis in six horses in the United Kingdom. Vet. Rec. 2005, 156, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Marolt, J.; Naglić, T.; Hajsig, D. Aspergillus oryzae as a cause of keratomycosis in the horse. Tierarztliche Praxis 1984, 12, 489–492. [Google Scholar]
- Chopin, J.; Sigler, L.; Connole, M.; O’Boyle, D.; Mackay, B.; Goldstein, L. Keratomycosis in a Percheron cross horse caused by Cladorrhinum bulbillosum. J. Med. Vet. Mycol. 1997, 35, 53–55. [Google Scholar] [CrossRef]
- Richter, M.; Hauser, B.; Kaps, S.; Spiess, B.M. Keratitis due to Histoplasma spp. in a horse. Vet. Ophthalmol. 2003, 6, 99–103. [Google Scholar] [CrossRef]
- Rosa, M.; Cardozo, L.M.; da Silva Pereira, J.; Brooks, D.E.; Martins, A.L.; Florido, P.S.; Stussi, J.S. Fungal flora of normal eyes of healthy horses from the State of Rio de Janeiro, Brazil. Vet. Ophthalmol 2003, 6, 51–55. [Google Scholar] [CrossRef]
- Whitley, R.D.; Moore, C.P. Microbiology of the equine eye in health and disease. Vet. Cinics N. Am. 1984, 6, 451–466. [Google Scholar] [CrossRef]
- Ledbetter, E.C.; Patten, V.H.; Scarlett, J.M.; Vermeylen, F.M. In vitro susceptibility patterns of fungi associated with keratomycosis in horses of the northeastern United States: 68 cases (1987-2006). J. Am. Vet. Med. Assoc. 2007, 231, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.d.A.; Santana, A.F.; Almeida, A.C.d.V.R.; Sousa, R.F.; Perecmanis, S.; Galera, P.D. Bacterial culture and antibiotic sensitivity from the ocular conjunctiva of horses. Ciência Rural 2017, 47. [Google Scholar] [CrossRef]
- Mirhendi, H.; Diba, K.; Kordbacheh, P.; Jalalizand, N.; Makimura, K. Identification of pathogenic Aspergillus species by a PCR-restriction enzyme method. J. Med. Microbiol. 2007, 56, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sriramajayam, K.; Luo, D.; Liao, J. A Quick, Cost-Free Method of Purification of DNA Fragments from Agarose Gel. J. Cancer 2012, 3, 93–95. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Tabor, S.; Richardson, C.C. A single residue in DNA polymerases of the Escherichia coli DNA polymerase I family is critical for distinguishing between deoxy- and dideoxyribonucleotides. Proc. Natl. Acad. Sci. USA 1995, 92, 6339–6343. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Brooks, D.E.; Matthews, A. Equine Ophtalmology; Saunders: Philadelphia, PA, USA, 2005; pp. 323–339. [Google Scholar]
- Galera, P.D.; Brooks, D.E. Optimal management of equine keratomycosis. Vet. Med. (Auckl) 2012, 3, 7–17. [Google Scholar] [CrossRef]
- Bharathi, M.J.; Ramakrishnan, R.; Vasu, S.; Meenakshi, R.; Palaniappan, R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J. Ophthalmol. 2003, 51, 315–321. [Google Scholar]
- Mann, S.S.; Singh, J.; Kalra, D.; Parihar, J.; Gupta, N.; Kumar, P. Medical and Surgical Management of Keratomycosis. Med. J. Armed Forces India 2008, 64, 40–42. [Google Scholar] [CrossRef]
- Sherman, A.B.; Clode, A.B.; Gilger, B.C. Impact of fungal species cultured on outcome in horses with fungal keratitis. Vet. Ophthalmol. 2017, 20, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Barsotti, G.; Attili, A.R.; Mugnaini, L.; Cuteri, V.; Preziuso, S.; Corazza, M.; Preziuso, G.; Sgorbini, M. Conjunctival bacterial and fungal flora in clinically normal sheep. Vet. Rec. Open. 2014, 1, e000017. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.L.; Kinikar, A.G.; Bhalerao, D.S.; Roushani, S. A Case of Keratomycosis Caused by Fusarium Solani at Rural Tertiary Care Center. J. Clin. Diagn. Res. 2017, 11, DD01–DD03. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Krishnan, T.; Rajaraman, R.; Patel, S.; Shah, R.; Srinivasan, M.; Das, M.; Ray, K.J.; Oldenburg, C.E.; McLeod, S.D.; et al. Predictors of Corneal Perforation or Need for Therapeutic Keratoplasty in Severe Fungal Keratitis: A Secondary Analysis of the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol. 2017, 135, 987–991. [Google Scholar] [CrossRef]
- Ahmad, I.; Owais, M.; Shahid, M.; Aqil, F. Combating Fungal Infections: Problems and Remedy; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Galán, A.; Martín-Suárez, E.; Gallardo, J.; Molleda, J. Clinical findings and progression of 10 cases of equine ulcerative keratomycosis (2004–2007). Equine Vet. Educ. 2009, 21, 236–242. [Google Scholar] [CrossRef]
- Blomme, E.; Piero, F.D.; Perle, K.L.; Wilkins, P.A. Aspergillosis in horses: A review. Equine Vet. Educ. 1998, 10, 86–93. [Google Scholar] [CrossRef]
- Seyedmousavi, S. Aspergillosis in Humans and Animals. In Recent Trends in Human and Animal Mycology; Singh, K., Srivastava, N., Eds.; Springer: Singapore, 2019; pp. 81–98. [Google Scholar]
- Wada, S.; Hobo, S.; Ode, H.; Niwa, H.; Moriyama, H. Equine keratomycosis in Japan. Vet. Ophthalmol. 2013, 16, 1–9. [Google Scholar] [CrossRef]
- Scotty, N.C. Equine keratomycosis. Clin. Tech. Equine Pract. 2005, 4, 29–36. [Google Scholar] [CrossRef]
- Teferi, K.; Adem, A. Common Ophthalmic Problems in Equines. Glob. Vet. 2019, 21, 246–254. [Google Scholar]
- Andrew, S.; Willis, M. Disease of the cornea and sclera. In Equine Ophthalmology, 1st ed.; Gilger, B., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2005; pp. 157–251. [Google Scholar]
- McLaughlin, S.A.; Gilger, B.C.; Whitley, R.D. Infectious keratitisin horses: Evaluation and management. Compend. Contin. Educ. Vet. 1992, 14, 372–379. [Google Scholar]
- Grahn, B.; Wolfer, J.; Keller, C.; Wilcock, B. Equinekeratomycosis: Clinical and laboratory findings in 23 cases. Prog. Vet. Comp. Ophthalmol. 1993, 3, 1–7. [Google Scholar]
- Mustikka, M.P.; Gronthal, T.S.C.; Pietila, E.M. Equine infectious keratitis in Finland: Associated microbial isolates and susceptibility profiles. Vet. Ophthalmol. 2020, 23, 148–159. [Google Scholar] [CrossRef] [PubMed]
- De Linde Henriksen, M.; Andersen, P.H.; Thomsen, P.D.; Plummer, C.E.; Mangan, B.; Heegaard, S.; Toft, N.; Brooks, D.E. Equine deep stromal abscesses (51 cases-2004–2009)—Part 1: The clinical aspects with attention to the duration of the corneal disease, treatment history, clinical appearance, and microbiology results. Vet. Ophthalmol. 2014, 17 (Suppl. 1), 6–13. [Google Scholar] [CrossRef] [PubMed]
- Proietto, L.R.; Plummer, C.E.; Maxwell, K.M.; Lamb, K.E.; Brooks, D.E. A retrospective analysis of environmental risk factors for the diagnosis of deep stromal abscess in 390 horses in North Central Florida from 1991 to 2013. Vet. Ophthalmol. 2016, 19, 291–296. [Google Scholar] [CrossRef]
- De Linde Henriksen, M.; Brooks, D.E. Standing ophthalmic surgeries in horses. Vet. Clin. N. Am. Equine Pract. 2014, 30, 91–110. [Google Scholar] [CrossRef]
- Aho, R.; Tala, M.; Kivalo, M. Mycotic keratitis in a horse caused by Aspergillus fumigatus. The first reported case in Finland. Acta Vet. Scand. 1991, 32, 373–376. [Google Scholar]
- Whitley, R.; Burgess, E.C.; Moore, C. Microbial isolates of the normal equine eye. Equine Vet. J. 1983, 15, 138–140. [Google Scholar] [CrossRef]
- Nikaido, H. Crossing the envelope: How cephalosporins reach their targets. Clin. Microbiol. Infect. 2000, 6 (Suppl. 3), 22–26. [Google Scholar] [CrossRef][Green Version]
- Barsotti, G.; Sgorbini, M.; Nardoni, S.; Corazza, M.; Mancianti, F. Occurrence of fungi from conjunctiva of healthy horses in Tuscany, Italy. Vet. Res. Commun. 2006, 30, 903–906. [Google Scholar] [CrossRef]
- Robinson, E.; Sprayberry, K. Preface. In Robinson’s Current Therapy in Equine Medicine, 7th ed.; Sprayberry, K.A., Robinson, N.E., Eds.; W.B. Saunders: St. Louis, MO, USA, 2015; Section XIV. [Google Scholar]
- Sellon, D.C.; Long, M.T. E Equine Infectious Diseases, 2nd ed.; Saunders: St. Louis, MO, USA, 2014. [Google Scholar]
- Gajjar, D.U.; Pal, A.K.; Ghodadra, B.K.; Vasavada, A.R. Microscopic evaluation, molecular identification, antifungal susceptibility, and clinical outcomes in fusarium, Aspergillus and, dematiaceous keratitis. BioMed Res. Int. 2013, 2013, 605308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banos, S.; Lentendu, G.; Kopf, A.; Wubet, T.; Glockner, F.O.; Reich, M. A comprehensive fungi-specific 18S rRNA gene sequence primer toolkit suited for diverse research issues and sequencing platforms. BMC Microbiol. 2018, 18, 190. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).