Zoonotic Pathogens of Dromedary Camels in Kenya: A Systematised Review
Abstract
1. Introduction
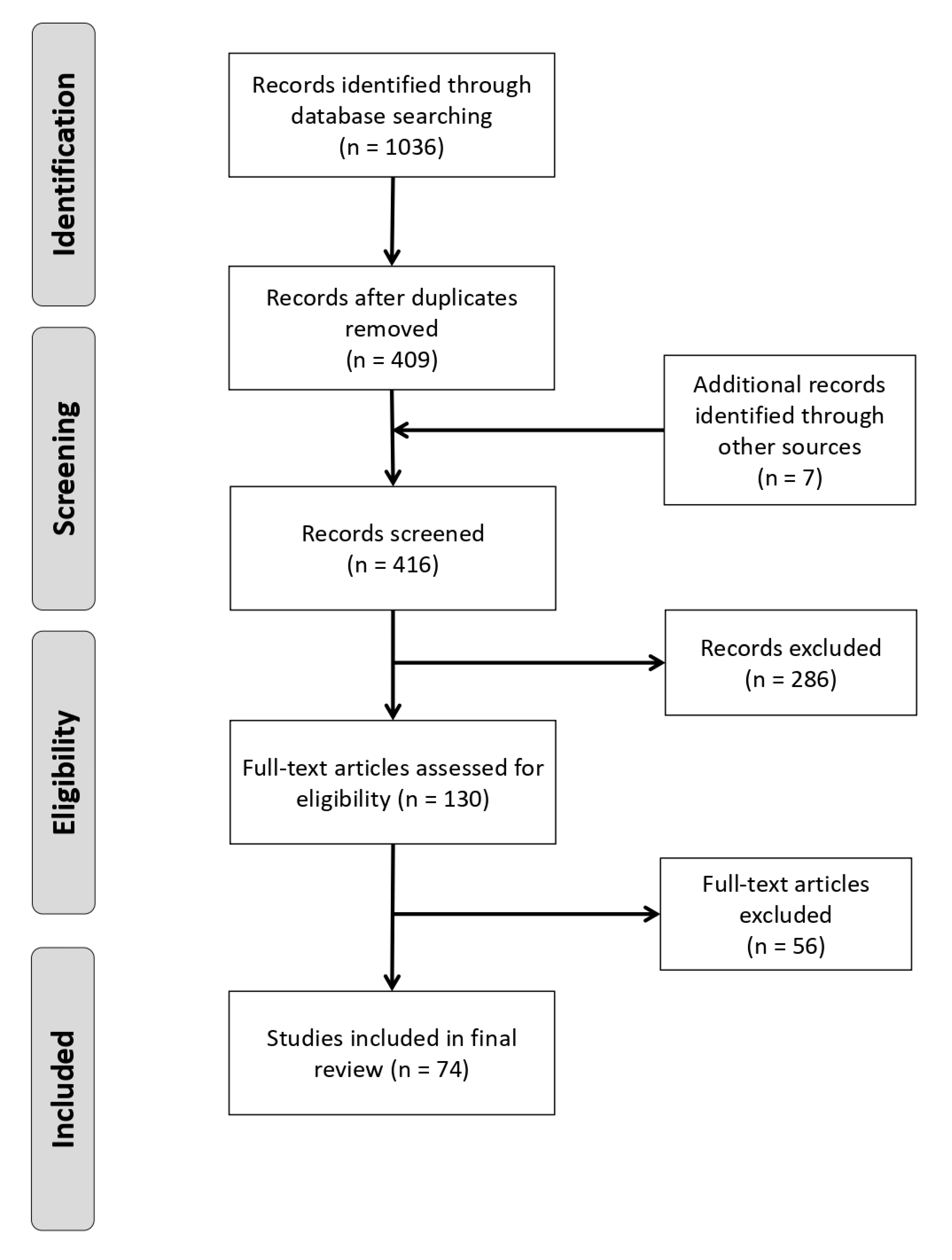
2. Materials and Methods
2.1. Search Strategy and Record Assessment
2.2. Quality Criteria Assessment
3. Results
3.1. Summary
3.2. Viruses
3.3. Bacteria
3.4. Parasites and Fungi
4. Discussion
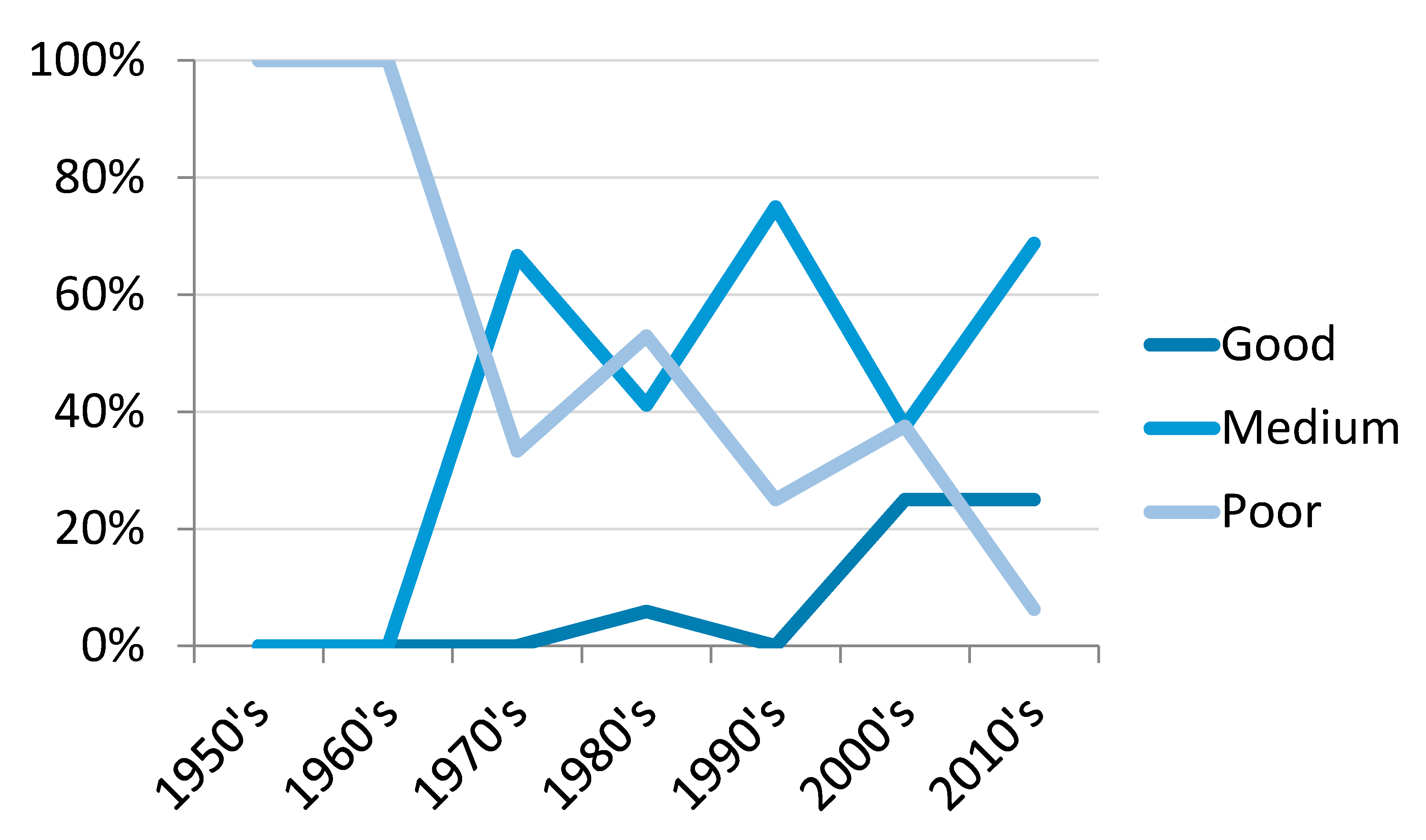
4.1. Trends in Camel Research
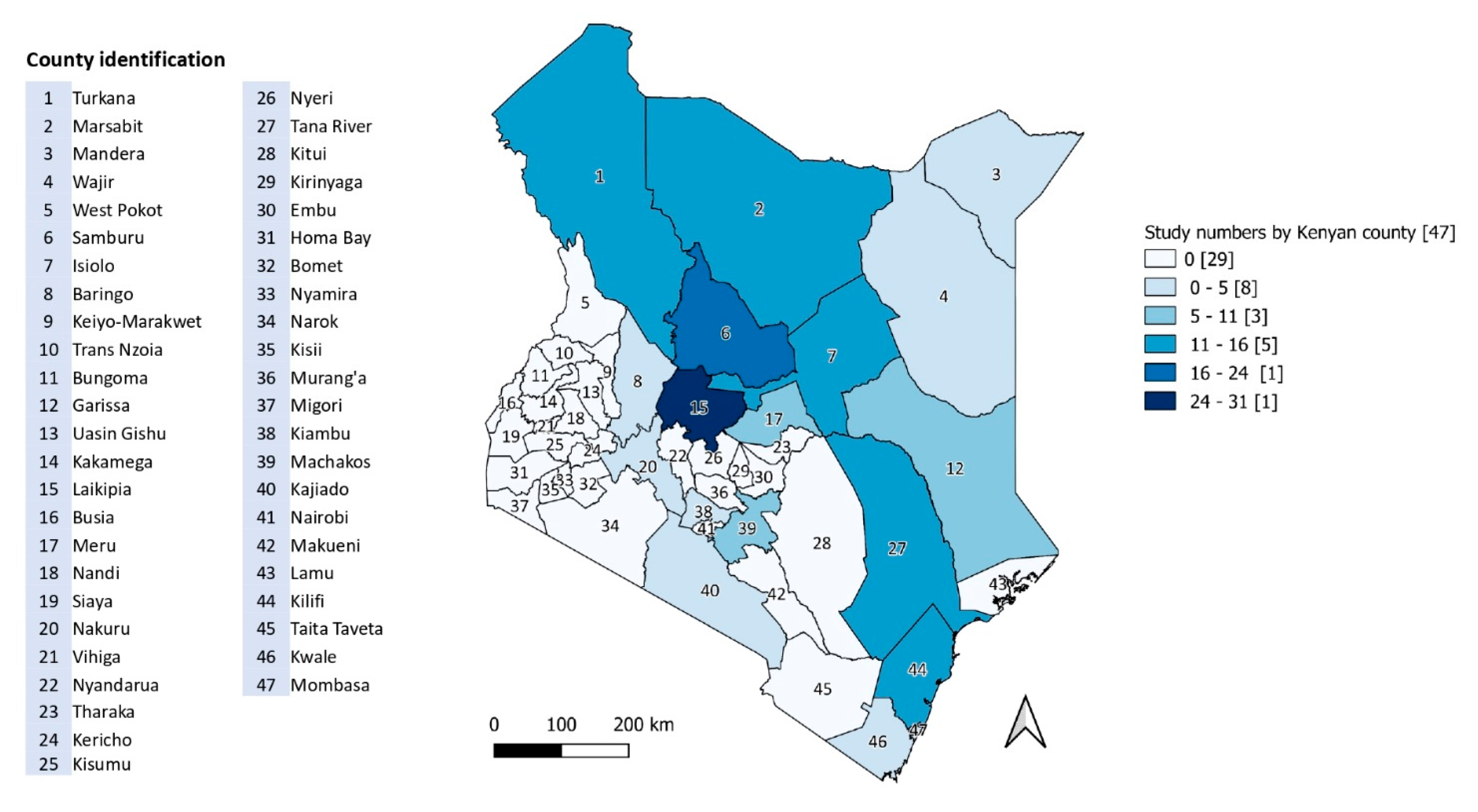
4.2. Study Locations
4.3. Viral Zoonoses
4.4. Bacterial Zoonoses
4.5. Parasitic Zoonoses
4.6. ‘Missing’ Pathogens
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organisation of the United Nations. FAOSTAT Database: Rome, Italy. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 6 June 2019).
- Guliye, A.Y.; Noor, I.M.; Bebe, B.O.; Kosgey, I.S. Role of camels (Camelus dromedarius) in the traditional lifestyle of Somali pastoralists in northern Kenya. Outlook Agric. 2007, 36, 29–34. [Google Scholar] [CrossRef]
- Anderson, D.M.; Elliott, H.; Kochore, H.H.; Lochery, E. Camel herders, middlewomen, and urban milk bars: The commodification of camel milk in Kenya. J. East. Afr. Stud. 2012, 6, 383–404. [Google Scholar] [CrossRef]
- Noor, I.M.; Guliye, A.Y.; Bebe, B.; Tariq, M. Assessment of camel and camel milk marketing practices in an emerging peri-urban production system in Isiolo County, Kenya. Pastor. Res. Policy Pract. 2013, 3, 28. [Google Scholar] [CrossRef]
- Mahmoud, H.A. Camel Marketing in the Northern Kenya/Southern Ethiopia Borderlands; FAC Pastoralist Theme Research Update; Future Agricultures: Kenya, 2010; Available online: https://www.future-agricultures.org/wp-content/uploads/pdf-archive/FAC_Research_Update_005.pdf (accessed on 21 June 2017).
- Watson, E.E.; Kochore, H.H.; Dabasso, B.H. Camels and climate resilience: Adaptation in Northern Kenya. Hum. Ecol. 2016, 44, 701–713. [Google Scholar] [CrossRef]
- Kagunyu, A.W.; Wanjohi, J. Camel rearing replacing cattle production among the Borana community in Isiolo County of Northern Kenya, as climate variability bites. Pastoralism 2014, 4, 13. [Google Scholar] [CrossRef]
- Plummer, P.; Coatney, J.W. The impact of camel disease on human welfare in East. Africa. CAB Rev. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Government of the Republic of Kenya. Vision 2030 Development Strategy for Northern Kenya and other Arid Lands; Ministry of State for Development of Northern Kenya and other Arid Lands: Nairobi, Kenya, 2012.
- Chemuliti, J.K.; Njiru, Z.K.; Bukachi, S. Disease conditions of camels in non-traditional camel keeping areas of Kajiado District in Kenya: A case study. J. Camel Pract. Res. 2003, 10, 207–210. [Google Scholar]
- Abbas, B.; Omer, O.H. Review of infectious diseases of the camel. Vet. Bull. 2005, 75, 1–16. [Google Scholar]
- Wilson, A.J.; Dolan, R.; Schwartz, H.J.; Field, C.R. Diseases of camels in Kenya, in The Camelid: An All-Purpose animal. In Proceedings of the Khartoum Workshop on Camels, Khartoum, Sudan, 18–20 December 1979; Cockrill, W.R., Ed.; Uppsala, Scandinavian Institute of African Studies: Uppsala, Sweden, 1984; Volume 1, pp. 519–531. [Google Scholar]
- Abbas, B.; Agab, H. A review of camel brucellosis. Prev. Vet. Med. 2002, 55, 47–56. [Google Scholar] [CrossRef]
- Bornstein, S.; Younan, M. Significant veterinary research on the dromedary camels of Kenya: Past and present. J. Camelid Sci. 2013, 6, 1–48. [Google Scholar]
- Roess, A.; Carruth, L.; Lahm, S.; Salman, M. Camels, MERS-CoV, and other emerging infections in east Africa. Lancet Infect. Dis. 2016, 16, 14–15. [Google Scholar] [CrossRef]
- Zumla, A.; Dar, O.; Kock, R.; Muturi, M.; Ntoumi, F.; Kaleebu, P.; Eusebio, M.; Mfinanga, S.; Bates, M.; Mwaba, P.; et al. Taking forward a ‘One Health’ approach for turning the tide against the Middle East Respiratory Syndrome Coronavirus and other zoonotic pathogens with epidemic potential. Int. J. Infect. Dis. 2016, 47, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Grace, D.; Mutua, F.; Ochungo, P.; Kruska, R.L.; Jones, K.; Brierley, L.; Lapar, M.; Said, M.Y.; Herrero, M.T.; Phuc, P.M.; et al. Mapping of Poverty and Likely Zoonoses Hotspots, in Zoonoses Project 4; Report to the UK Department for International Development; ILRI: Nairobi, Kenya, 2012. [Google Scholar]
- Cleaveland, S.; Sharp, J.; Abela-Ridder, B.; Allan, K.J.; Buza, J.; Crump, J.A.; Davis, A.; Vilas, V.D.R.; De Glanville, W.A.; Kazwala, R.R.; et al. One Health contributions towards more effective and equitable approaches to health in low- and middle-income countries. Philos. Trans. R. Soc. Lond B Biol. Sci. 2017, 372, 20160168. [Google Scholar] [CrossRef] [PubMed]
- Kemunto, N.; Mogoa, E.; Osoro, E.M.; Bitek, A.; Njenga, M.K.; Thumbi, S.M. Zoonotic disease research in East. Africa. BMC Infect. Dis. 2018, 18, 545. [Google Scholar] [CrossRef]
- Munyua, P.M.; Njenga, M.K.; Osoro, E.M.; O Onyango, C.; Bitek, A.O.; Mwatondo, A.; Muturi, M.; Musee, N.; Bigogo, G.; Otiang, E.; et al. Successes and challenges of the One Health approach in Kenya over the last decade. BMC Public Health 2019, 19, 465. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- O’Connor, A.; Anderson, K.; Goodell, C.K.; Sargeant, J.M. Conducting systematic reviews of intervention questions I: Writing the review protocol, formulating the question and searching the literature. Zoonoses Public Health 2014, 61, 28–38. [Google Scholar] [CrossRef]
- Sargeant, J.M.; O’Connor, A.M. Conducting systematic reviews of intervention questions II: Relevance screening, data extraction, assessing risk of bias, presenting the results and interpreting the findings. Zoonoses Public Health 2014, 61, 39–51. [Google Scholar] [CrossRef]
- Alonso, S.; Lindahl, J.F.; Roesel, K.; Traore, S.G.; Yobouet, B.A.; Ndour, A.P.N.; Carron, M.; Grace, D. Where literature is scarce: Observations and lessons learnt from four systematic reviews of zoonoses in African countries. Anim. Health Res. Rev. 2016, 17, 28–38. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Zoonotic Disease Unit. The Zoonotic Disease Unit: National One Health Strategic Plan. 2012–2017; Ministry of Health and Ministry of Agriculture, Livestock and Fisheries, Government of Kenya: Nairobi, Kenya, 2012.
- Truc, P.; Vanhollebeke, B.; Gibson, W.; Herder, S.; Poelvoorde, P.; Pays, A.; Joshi, P.P.; Katti, R.; Shegokar, V.R.; Powar, R.M.; et al. Human infection by Trypanosoma evansi in India: Diagnosis, treatment, genetic and epidemiological investigations. Infect. Genet. Evol. 2008, 8, S29–S30. [Google Scholar]
- Truc, P.; Büscher, P.; Cuny, G.; Gonzatti, M.I.; Jannin, J.; Joshi, P.; Juyal, P.; Lun, Z.-R.; Mattioli, R.; Pays, E.; et al. Atypical human infections by animal trypanosomes. PLoS Negl. Trop. Dis. 2013, 7, e2256. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.V.V.; Chau, L.B.; Desquesnes, M.; Herder, S.; Lan, N.P.H.; Campbell, J.I.; Van Cuong, N.; Yimming, B.; Chalermwong, P.; Jittapalapong, S.; et al. A clinical and epidemiological investigation of the first reported human infection with the zoonotic parasite Trypanosoma evansi in Southeast Asia. Clin. Infect. Dis. 2016, 62, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Liljander, A.; Meyer, B.; Jores, J.; Müller, M.A.; Lattwein, E.; Njeru, I.; Bett, B.; Drosten, C.; Corman, V.M. MERS-CoV antibodies in humans, Africa, 2013–2014. Emerg. Infect. Dis. 2016, 22, 1086–1089. [Google Scholar] [CrossRef]
- Munyua, P.; Corman, V.M.; Bitek, A.; Osoro, E.M.; Meyer, B.; Müller, M.A.; Lattwein, E.; Thumbi, S.M.; Murithi, R.; Widdowson, M.-A.; et al. No serologic evidence of Middle East Respiratory Syndrome Coronavirus infection among camel farmers exposed to highly seropositive camel herds: A household linked study, Kenya, 2013. Am. J. Trop. Med. Hyg. 2017, 96, 1318–1324. [Google Scholar] [CrossRef]
- Corman, V.M.; Jores, J.; Meyer, B.; Younan, M.; Liljander, A.; Said, M.Y.; Gluecks, I.; Lattwein, E.; Bosch, B.J.; Drexler, J.F.; et al. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerg. Infect. Dis. 2014, 20, 1319–1322. [Google Scholar] [CrossRef]
- Deem, S.L.; Fèvre, E.M.; Kinnaird, M.; Browne, A.S.; Muloi, D.; Godeke, G.-J.; Koopmans, M.P.G.; Reusken, C.B. Serological evidence of MERS-CoV antibodies in dromedary camels (Camelus dromedaries) in Laikipia County, Kenya. PLoS ONE 2015, 10, e0140125. [Google Scholar] [CrossRef]
- Bird, B.H.; Githinji, J.W.; Macharia, J.M.; Kasiiti, J.L.; Muriithi, R.M.; Gacheru, S.G.; Musaa, J.O.; Towner, J.S.; Reeder, S.A.; Oliver, J.B.; et al. Multiple virus lineages sharing recent common ancestry were associated with a Large Rift Valley fever outbreak among livestock in Kenya during 2006–2007. J. Virol. 2008, 82, 11152–11166. [Google Scholar] [CrossRef]
- Britch, S.C.; Binepal, Y.S.; Ruder, M.G.; Kariithi, H.M.; Linthicum, K.J.; Anyamba, A.; Small, J.L.; Tucker, C.J.; Ateya, L.O.; Oriko, A.A.; et al. Rift valley fever risk map model and seroprevalence in selected wild ungulates and camels from Kenya. PLoS ONE 2013, 8, e66626. [Google Scholar] [CrossRef]
- Scott, G.R.; Coackley, W.; Roach, R.W.; Cowdy, N.R. Rift valley fever in camels. J. Pathol. Bacteriol. 1963, 86, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G.; Koros, J.; Mbugua, H. Rift Valley fever in Kenya: The presence of antibody to the virus in camels (Camelus dromedarius). J. Hyg. 1985, 94, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Gitao, C.G. An investigation of camelpox outbreaks in two principal camel (Camelus dromedarius) rearing areas of Kenya. Rev. Sci. Tech. Off. Int. Epizoot. 1997, 16, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G.; Mungai, J.N.; Shaw, T. Characteristics of a Kenyan camelpox virus. J. Hyg. 1975, 75, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Munz, E.; Kropp, E.; Pfahler, W.; Reimann, M. Detection of antibodies against the orthopox virus cameli in sera of East. African dromedaries using two different ELISAs. Nuclear and related techniques in animal production and health. In Proceedings of the A Symposium, Vienna, Austria, 10–14 November 1986. [Google Scholar]
- Davies, F.G.; Mbugua, H.; Atema, C.; Wilson, A. The prevalence of antibody to camel pox virus in six different herds in Kenya. J. Comp. Pathol. 1985, 95, 633–635. [Google Scholar] [CrossRef]
- Sang, R.; Lutomiah, J.; Koka, H.; Makio, A.; Chepkorir, E.; Ochieng, C.; Yalwala, S.; Mutisya, J.; Musila, L.; Richardson, J.H.; et al. Crimean-Congo hemorrhagic fever virus in Hyalommid Ticks, Northeastern Kenya. Emerg. Infect. Dis. 2011, 17, 1502–1505. [Google Scholar] [CrossRef]
- Morrill, J.C.; Soliman, A.K.; Imam, I.Z.; A Botros, B.; I Moussa, M.; Watts, D.M. Serological evidence of Crimean-Congo haemorrhagic fever viral infection among camels imported into Egypt. J. Trop. Med. Hyg. 1990, 93, 201–204. [Google Scholar]
- Gitao, C.G. Outbreaks of contagious ecthyma in camels (Camelus dromedarius) in the Turkana district of Kenya. Rev. Sci. Technol. 1994, 13, 939–945. [Google Scholar] [CrossRef]
- Munz, E.; Schillinger, D.; Reimann, M.; Mahnel, H. Electron microscopical diagnosis of ecthyma contagiosum in camels (Camelus dromedarius). First report of the disease in Kenya. J. Vet. Med. B 1986, 33, 73–77. [Google Scholar] [CrossRef]
- Lutomiah, J.; Musila, L.; Makio, A.; Ochieng, C.; Koka, H.; Chepkorir, E.; Mutisya, J.; Mulwa, F.; Khamadi, S.; Miller, B.R.; et al. Ticks and tick-borne viruses from livestock hosts in arid and semiarid regions of the eastern and northeastern parts of Kenya. J. Med. Entomol. 2014, 51, 269–277. [Google Scholar] [CrossRef]
- Salem, E.; Cook, E.A.J.; Lbacha, H.A.; Oliva, J.; Awoume, F.; Aplogan, G.L.; Hymann, E.C.; Muloi, D.; Deem, S.L.; Alali, S.; et al. Serologic evidence for influenza C and D virus among ruminants and Camelids, Africa, 1991–2015. Emerg. Infect. Dis. 2017, 23, 1556–1559. [Google Scholar] [CrossRef] [PubMed]
- DePuy, W.; Benka, V.; Massey, A.; Deem, S.L.; Kinnaird, M.; O’Brien, T.; Wanyoike, S.; Njoka, J.; Butt, B.; Foufopoulos, J.; et al. Q fever risk across a dynamic, heterogeneous landscape in Laikipia County, Kenya. Ecohealth 2014, 11, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.S.; Fèvre, E.M.; Kinnaird, M.; Muloi, D.; Wang, C.A.; Larsen, P.S.; O’Brien, T.; Deem, S.L. Serosurvey of Coxiella burnetii (Q fever) in dromedary camels (Camelus dromedarius) in Laikipia County, Kenya. Zoonoses Public Health 2017, 64, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D. Serological evidence of Q fever infection in domestic animals in Kenya. Bull. Epizoot. Dis. Afr. 1956, 4, 41–45. [Google Scholar]
- Gitao, C.G. The prevalence of Dermatophilus congolensis infection of camels in four rearing areas in Kenya and the presence of a mixed infection with Trichophyton verrucosum. Israel J. Vet. Med. 1998, 53, 89–93. [Google Scholar]
- Gitao, C.G.; Agab, H.; Khalifalla, A.J. A comparison of camel dermatophilosis in Kenya and Sudan. Ann. N. Y. Acad. Sci. 1998, 849, 461–464. [Google Scholar] [CrossRef]
- Gitao, C.G. Dermatophilosis in camels (Camelus dromedarius Linnaeus, 1758) in Kenya. Rev. Sci. Technol. 1992, 11, 1079–1086. [Google Scholar] [CrossRef]
- Gitao, C.G. The epidemiology and control of camel dermatophilosis. Rev. Elev. Med. Vet. Pays Trop. 1993, 46, 309–311. [Google Scholar]
- Gitao, C.G.; Evans, J.O.; Atkins, D.J. Natural Dermatophilus congolensis infection in camels (Camelus dromedarius) from Kenya. J. Comp. Pathol. 1990, 103, 307–313. [Google Scholar] [CrossRef]
- Osoro, E.M.; Bitek, A.O.; Ogola, E.; Njeru, I.; Wanyoike, S.; Mbabu, M.R. Linked human and livestock study on seroprevalence and risk factors for brucellosis in Kenya, 2012. Am. J. Trop. Med. Hyg. 2014, 1, 199. [Google Scholar]
- Kagunya, D.; Waiyaki, P. A serological survey of animal brucellosis in the north-eastern province of Kenya. Kenya Vet. 1978, 2, 35–38. [Google Scholar]
- Paling, R.W.; Waghela, S.; MacOwan, K.J.; Heath, B.R. The occurrence of infectious diseases in mixed farming of domesticated wild herbivores and livestock in Kenya. II. Bacterial diseases. J. Wildl. Dis. 1988, 24, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wanjohi, M.; Gitao, C.G.; Bebora, L. The prevalence of Brucella spp. in camel milk marketed from North. Eastern Province, Kenya. Res. Opin. Anim. Vet. Sci. 2012, 2, 425–434. [Google Scholar]
- Kimber, K.; Lubroth, J.; Dubovi, E.J.; Berninger, M.L.; Demaar, T.W. Serologic survey of selected viral, bacterial, and protozoal agents in captive and free-ranging ungulates from central Kenya. Ann. N. Y. Acad. Sci. 2002, 969, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Waghela, S.; Fazil, M.A.; Gathuma, J.M.; Kagunya, D.K. A serological survey of brucellosis in camels in north-eastern province of Kenya. Trop. Anim. Health Prod. 1978, 10, 28–29. [Google Scholar] [CrossRef]
- Koka, H.; Sang, R.C.; Kutima, H.L.; Musila, L. The detection of spotted fever group rickettsia DNA in tick samples from pastoral communities in Kenya. J. Med. Entomol. 2017, 54, 774–780. [Google Scholar] [CrossRef]
- Gibson, W.C.; Wilson, A.J.; Moloo, S.K. Characterisation of Trypanosoma (Trypanozoon) evansi from camels in Kenya using isoenzyme electrophoresis. Res. Vet. Sci. 1983, 34, 114–118. [Google Scholar] [CrossRef]
- Masiga, D.K.; Ndung’U, K.; Tweedie, A.; Tait, A.; Turner, C.M.R. Trypanosoma evansi: Genetic variability detected using amplified restriction fragment length polymorphism (AFLP) and random amplified polymorphic DNA (RAPD) analysis of Kenyan isolates. Exp. Parasitol. 2006, 114, 147–153. [Google Scholar] [CrossRef]
- Njiru, Z.; Constantine, C.; Ndung’U, J.; Robertson, I.; Okaye, S.; Thompson, R.; Reid, S.A. Detection of Trypanosoma evansi in camels using PCR and CATT/T. evansi tests in Kenya. Vet. Parasitol. 2004, 124, 187–199. [Google Scholar] [CrossRef]
- Njiru, Z.K.; Constantine, C.C.; Guya, S.; Crowther, J.; Kiragu, J.M.; Thompson, R.C.A.; Davila, A.M.R. The use of ITS1 rDNA PCR in detecting pathogenic African trypanosomes. Parasitol. Res. 2005, 95, 186–192. [Google Scholar] [CrossRef]
- Njiru, Z.K.; Constantine, C.C.; Masiga, D.K.; Reid, S.A.; Thompson, R.C.A.; Gibson, W.C. Characterization of Trypanosoma evansi type B. Infect. Genet. Evol. 2006, 6, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Kwena, A.M.; Olaho, W.M.; Ngaira, J. Characterization of Trypanosoma (Trypanozoon) from camels in Kenya using both starch gel electrophoresis and isoelectric focussing. Bull. Anim. Health Prod. Afr. 1990, 38, 365–368. [Google Scholar]
- Nantulya, V.M. Suratex: A simple latex agglutination antigen test for diagnosis of Trypanosoma evansi infections (surra). Trop. Med. Parasitol. 1994, 45, 9–12. [Google Scholar] [PubMed]
- Nantulya, V.M.; Lindqvist, K.J.; Diall, O. Two simple antigen-detection enzyme immunoassays for the diagnosis of Trypanosoma evansi infections in the dromedary camel (Camelus dromedarius). Trop. Med. Parasitol. 1989, 40, 415–418. [Google Scholar]
- Ngaira, J.M.; Bett, B.; Karanja, S.M. Animal-level risk factors for Trypanosoma evansi infection in camels in eastern and central parts of Kenya. Onderstepoort J. Vet. Res. 2002, 69, 263–271. [Google Scholar]
- Ngaira, J.M.; Bett, B.; Karanja, S.M.; Njagi, E.N.M. Evaluation of antigen and antibody rapid detection tests for Trypanosoma evansi infection in camels in Kenya. Vet. Parasitol. 2003, 114, 131–141. [Google Scholar] [CrossRef]
- Olaho-Mukani, W.; Munyua, W.K.; Njogu, A.R.; Mutugi, M.W.; Omuse, J.K.; Sayer, P.D. Application of an antigen-enzyme linked Immunosorbent assay for the diagnosis of Trypanosomosis in Camels in Kenya. In Proceedings of the First International Camel Conference, Dubai, UAE, 2–6 February 1992. [Google Scholar]
- Olaho-Mukani, W.; Nyang’ao, J.M.N. Evaluation of SuratexReg. for the Field Diagnosis of Trypanosoma evansi Infections in Camels in Kenya; International Scientific Council for Trypanosomiasis Research and Control: Kampala, Uganda, 1993. [Google Scholar]
- Olaho-Mukani, W.; Nyang’ao, J.M.N.; Ouma, J.O. Comparison of SuratexReg. parasite detection and antigen-ELISA for the evaluation of treatment efficacy and diagnosis of surra in dromedary camels. J. Camel Pract. Res. 1996, 3, 1–5. [Google Scholar]
- Olaho-Mukani, W.; Nyang’ao, J.M.N.; Ouma, J.O. Use of Suratex for field diagnosis of patent and non-patent Trypanosoma evansi infections in camels. Br. Vet. J. 1996, 152, 109–111. [Google Scholar] [CrossRef]
- Olaho-Mukani, W.; Mboloi, M.M.; Muriuki, S.P.; Ouma, J.O.; Guya, S.O.; Ndung’u, J.M. Application of pen-side diagnosis in the control of surra in dromedary camels in Kenya. J. Camel Pract. Res. 1997, 4, 281–282. [Google Scholar]
- Waithanji, E.M.; Nantulya, V.M.; Mbiuki, S.M. Use of antigen capture tube enzyme-linked immunosorbent assay for the diagnosis of Trypanosoma evansi infections in dromedary camels (Camelus dromedarius). Rev. Sci. Tech. 1993, 12, 665–672. [Google Scholar] [CrossRef]
- Waitumbi, J.N.Y.; John, R. Electrophoretic karyotyping is a sensitive epidemiological tool for studying Trypanosoma evansi infections. Vet. Parasitol. 1994, 52, 47–56. [Google Scholar] [CrossRef]
- Waitumbi, J.N.; Murphy, N.B.; Peregrine, A.S. Genotype and drug-resistance phenotype of Trypanosoma evansi isolated from camels in northern Kenya. Ann. Trop. Med. Parasitol. 1994, 88, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Schwartz, H.J.; Dolan, R.; Olahu, W.M. A simple classification of different types of trypanosomiasis occurring in four camel herds in selected areas of Kenya. Trop. Parasitol. 1983, 34, 220–224. [Google Scholar]
- Maina, N.W.N.; Otieno, C.; Farah, R.; Ngatia, P.N.; Olaho-Mukani, W.M.; Sutherland, D.V.; Ndung’u, J.M. Treatment failure in camel Trypanosomosis in Uaso region of Kenya. J. Protozool. Res. 1998, 8, 253–257. [Google Scholar]
- Masiga, R.C.; Nyang’ao, J.M.N. Identification of trypanosome species from camel using polymerase chain reaction and procyclic transformation test. J. Camel Pract. Res. 2001, 8, 17–22. [Google Scholar]
- Njiru, Z.K.; Ole-Mapeny, I.M.; Ouma, J.; Ndung’u, J.M.; Olaho-Mukani, W.M. Surra in camel calves in Laikipia district of Kenya. J. Protozool. Res. 2001, 11, 19–25. [Google Scholar]
- Njiru, Z.K.; Kamau, D.L.; Mwendia, C.M.T.; Ndung’u, J.M. The impact of surra on camel husbandry: A pilot study in Laikipia district of Kenya. J. Camel Pract. Res. 2002, 9, 139–144. [Google Scholar]
- Njiru, Z.K.B.; Ole-Mapeny, I.M.; Githiori, J.B.; Ndung’u, J.M. Trypanosomosis and helminthosis in camels: Comparison of ranch and traditional camel management systems in Kenya. J. Camel Pract. Res. 2002, 9, 67–71. [Google Scholar]
- Olaho, W.; Wilson, A.J. The Prevalence of Camel Trypanosomiasis in Selected Areas of Kenya; Seventeenth Meeting of the International Scientific Council for Trypanosomiasis Research and Control: Arusha, Tanzania, 1981. [Google Scholar]
- Oyieke, F.A. Mechanical transmission of camel trypanosomiasis in Northern Kenya and population dynamics of possible vectors. Medical and veterinary dipterology. In Proceedings of the International Conference, Ceske Budejovice, Czechoslovakia, 30 November–4 December 1987; Volume 6, pp. 281–285. [Google Scholar]
- Oyieke, F.A. Occurrence and transmission of camel trypanosomiasis in northern Kenya. J. Camel Pract. Res. 2003, 10, 17–21. [Google Scholar]
- Rutagwenda, T. A study of important camel diseases in northern Kenya with special emphasis on their control. Camel Newsl. 1984, 1, 12–16. [Google Scholar]
- Addy, F.; Wassermann, M.; Kagendo, D.; Ebi, D.; Zeyhle, E.; Elmahdi, I.E.; Umhang, G.; Casulli, A.; Harandi, M.F.; Aschenborn, O. Genetic differentiation of the G6/7 cluster of Echinococcus canadensis based on mitochondrial marker genes. Int. J. Parasitol. 2017, 47, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, C.N.L. An active intermediate host role for man in the life cycle of Echinococcus granulosus in Turkana, Kenya. Am. J. Trop. Med. Hyg. 1983, 32, 397–404. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P. A biochemical study of adult and cystic stages of Echinococcus granulosus of human and animal origin from Kenya. J. Hyg. 1981, 55, 21–27. [Google Scholar] [CrossRef]
- McManus, D.P.; Simpson, A.J.G.; Rishi, A.K. Characterization of the Hydatid Disease Organism, Echinococcus granulosus, from Kenya Using Cloned DNA Markers; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1987. [Google Scholar]
- Macpherson, C.N.L.; McManus, D.P. A comparative study of Echinococcus granulosus from human and animal hosts in Kenya using isoelectric focusing and isoenzyme analysis. Int. J. Parasitol. 1982, 12, 515–521. [Google Scholar] [CrossRef]
- Mbaya, H.; Magambo, J.; Njenga, S.; Zeyhle, E.; Mbae, C.; Mulinge, E.; Wassermann, M.; Kern, P.; Romig, T. Echinococcus spp. in central Kenya: A different story. Parasitol. Res. 2014, 113, 3789–3794. [Google Scholar] [CrossRef]
- Casulli, A.; Zeyhle, E.; Brunetti, E.; Pozio, E.; Meroni, V.; Genco, F.; Filice, C. Molecular evidence of the camel strain (G6 genotype) of Echinococcus granulosus in humans from Turkana, Kenya. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 29–32. [Google Scholar] [CrossRef]
- Wachira, T.M.; Bowles, J.; Zeyhle, E.; McManus, D.P. Molecular examination of the sympatry and distribution of sheep and camel strains of Echinococcus granulosus in Kenya. Am. J. Trop. Med. Hyg. 1993, 48, 473–479. [Google Scholar] [CrossRef]
- Dinkel, A.; Njoroge, E.M.; Zimmermann, A.; Waelz, M.; Zeyhle, E.; Elmahdi, I.E.; Mackenstedt, U.; Romig, T. A PCR system for identification of Echinococcus species and genotypes, with reference to the epidemiological situation in eastern Africa. IJMM Int. J. Med. Microbiol. 2004, 293, 48. [Google Scholar]
- Njoroge, E.M.; Mbithi, P.M.F.; Gathuma, J.M.; Wachira, T.M.; Gathura, P.B.; Magambo, J.K.; Zeyhle, E. A study of cystic echinococcosis in slaughter animals in three selected areas of northern Turkana, Kenya. Vet. Parasitol. 2002, 104, 85–91. [Google Scholar] [CrossRef]
- Oryan, A.; Mosadeghhesari, M.; Zibaee, S.; Mohammadi, A. Identification and phylogenetic analysis of contagious ecthyma virus from camels (Camelus dromedarius) in Iran. Onderstepoort J. Vet. Res. 2017, 84, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.J.; Gamawa, A.A.; Chima, N.C.; Ahmed, A.I. First report of camel contagious ecthyma in Nigeria. Open Vet. J. 2018, 8, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Burt, F.J.; Spencer, D.C.; Leman, P.A.; Patterson, B.; Swanepoel, R. Investigation of tick-borne viruses as pathogens of humans in South. Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol. Infect. 2009, 116, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Onyango, C.; Gachoya, J.; Mabinda, E.; Konongoi, S.; Ofula, V.; Dunster, L.; Okoth, F.; Coldren, R.; Tesh, R.; et al. Tickborne arbovirus surveillance in market livestock, Nairobi, Kenya. Emerg. Infect. Dis. 2006, 12, 1074–1080. [Google Scholar] [CrossRef]
- Crabtree, M.B.; Sang, R.; Miller, B.R. Kupe virus, a new virus in the family bunyaviridae, genus nairovirus, kenya. Emerg. Infect. Dis. 2009, 15, 147–154. [Google Scholar] [CrossRef]
- Osoro, E.M.; Munyua, P.; Omulo, S.; Ogola, E.; Ade, F.; Mbatha, P.; Mbabu, M.; Ng’Ang’A, Z.; Kairu, S.; Maritim, M.; et al. Strong association between human and animal Brucella Seropositivity in a linked study in Kenya, 2012–2013. Am. J. Trop. Med. Hyg. 2015, 93, 224–231. [Google Scholar] [CrossRef]
- Memish, Z.A.; Cotten, M.; Meyer, B.; Watson, S.J.; Alsahafi, A.J.; Al Rabeeah, A.A.; Corman, V.M.; Sieberg, A.; Makhdoom, H.Q.; Assiri, A.; et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg. Infect. Dis. 2014, 20, 1012–1015. [Google Scholar] [CrossRef]
- Reusken, C.B.; Haagmans, B.L.; A Mueller, M.; Gutiérrez, C.; Godeke, G.-J.; Meyer, B.; Muth, D.; Raj, V.S.; Vries, L.S.-D.; Corman, V.M.; et al. Middle East Respiratory Syndrome Coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect. Dis. 2013, 13, 859–866. [Google Scholar] [CrossRef]
- Reusken, C.B.; Messadi, L.; Feyisa, A.; Ularamu, H.; Godeke, G.J.; Danmarwa, A.; Dawo, F.; Jemli, M.; Melaku, S.; Shamaki, D.; et al. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg. Infect. Dis. 2014, 20, 1370–1374. [Google Scholar] [CrossRef]
- Muller, M.A.; Meyer, B.; Corman, V.M.; Al-Masri, M.; Turkestani, A.; Ritz, D.; Alhakeem, R.F. Presence of Middle East Respiratory Syndrome Coronavirus antibodies in Saudi Arabia: A nationwide, cross-sectional, serological study. Lancet Infecti. Dis. 2015, 15, 559–564. [Google Scholar] [CrossRef]
- Clarke, D.H. Further studies on antigenic relationships among the viruses of the group b tick-borne complex. Bull. World Health Organ. 1964, 31, 45–56. [Google Scholar] [PubMed]
- Madani, T.A.; Al-Mazrou, Y.Y.; Al-Jeffri, M.H.; Mishkhas, A.A.; Al-Rabeah, A.M.; Turkistani, A.M.; Al-Sayed, M.O.; Abodahish, A.A.; Khan, A.S.; Ksiazek, T.G.; et al. Rift Valley fever epidemic in Saudi Arabia: Epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis. 2003, 37, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Paweska, J.T.; Mortimer, E.; Leman, P.A.; Swanepoel, R. An inhibition enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in humans, domestic and wild ruminants. J. Virol. Methods 2005, 127, 10–18. [Google Scholar] [CrossRef]
- Dinkel, A.; Njoroge, E.M.; Zimmermann, A.; Wälz, M.; Zeyhle, E.; Elmahdi, I.E.; Romig, T. A PCR system for detection of species and genotypes of the Echinococcus granulosus-complex, with reference to the epidemiological situation in eastern Africa. Int. J. Parasitol. 2004, 34, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Bosire, C.; Krol, M.S.; Mekonnen, M.M.; Joseph, O.O.; De Leeuw, J.; Lannerstad, M.; Hoekstra, A. Meat and milk production scenarios and the associated land footprint in Kenya. Agric. Syst. 2016, 145, 64–75. [Google Scholar] [CrossRef]
- Elhadi, Y.A.; Nyariki, D.M.; Wasonga, O.V. Role of camel milk in pastoral livelihoods in Kenya: Contribution to household diet and income. Pastoralism 2015, 5, 8. [Google Scholar] [CrossRef]
- Elhadi, Y.A.; Wasonga, O.V. Economic and Nutritional Contribution of Camel Milk in Northern Kenya: A field study in Isiolo County, in IED Country Report; IIED (International Institute for Environment and Development): London, UK, 2015. [Google Scholar]
- Rathinasabapathy, G.; Rajendran, L. Mapping of world-wide camel research publications: A scientometric analysis. J. Libr. Inf. Commun. Technol. 2013, 5, 35–40. [Google Scholar]
- Gupta, B.M.; Ahmed, M.; Gupta, R.; Tiwari, R. World camel research: A scientometric assessment, 2003–2012. Scientometrics 2015, 102, 957–975. [Google Scholar] [CrossRef]
- Kinnaird, M.F.; O’Brien, T.G. Effects of private-land use, livestock management, and human tolerance on diversity, distribution, and abundance of large African mammals. Conserv. Biol. 2012, 26, 1026–1039. [Google Scholar] [CrossRef]
- Azzarri, C. Human Welfare: Poverty, in Atlas of African Agriculture, Research and Development: Revealing Agriculture’s Place in Africa; Sebastian, K., Ed.; International Food Policy Research Institute: Washington, DC, USA, 2014. [Google Scholar]
- WHO (World Health Organisation). Middle East Respiratory Syndrome Coronavirus (MERS-CoV); WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Hemida, M.G.; Elmoslemany, A.; Al-Hizab, F.; Alnaeem, A.; Almathen, F.; Faye, B.; Chu, D.K.; Perera, R.A.P.M.; Peiris, M. Dromedary camels and the transmission of Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Transbound. Emerg. Dis. 2017, 64, 344–353. [Google Scholar] [CrossRef]
- Memish, Z. Mers-CoV: From camels to humans. Int. J. Infect. Dis. 2016, 45, 7–8. [Google Scholar] [CrossRef]
- Muller, M.A.; Corman, V.M.; Jores, J.; Meyer, B.; Younan, M.; Liljander, A.; Bornstein, S. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerg. Infect. Dis. 2014, 20, 2093–2095. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; O Oladipo, J.; Perera, R.A.P.M.; A Kuranga, S.; Chan, S.M.; Poon, L.L.; Peiris, M. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in dromedary camels in Nigeria, 2015. Eurosurveillance 2015, 20, 30086. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Poon, L.L.; Gomaa, M.; Shehata, M.M.; Perera, R.A.P.M.; Abu Zeid, D.; El Rifay, A.S.; Siu, L.Y.; Guan, Y.; Webby, R.J.; et al. MERS coronaviruses in dromedary camels, Egypt. Emerg. Infect. Dis. 2014, 20, 1049–1053. [Google Scholar] [CrossRef]
- Miguel, E.; Chevalier, V.; Ayelet, G.; Ben Bencheikh, M.N.; Boussini, H.; Chu, D.K.W.; El Berbri, I.; Fassi-Fihri, O.; Faye, B.; Fekadu, G.; et al. Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015. Eurosurveillance 2017, 22, 15–24. [Google Scholar] [CrossRef]
- Gossner, C.M.; Danielson, N.; Gervelmeyer, A.; Berthe, F.; Faye, B.; Aaslav, K.K.; Adlhoch, C.; Zeller, H.; Penttinen, P.; Coulombier, D. Human-dromedary camel interactions and the risk of acquiring zoonotic Middle East Respiratory Syndrome Coronavirus infection. Zoonoses Public Health 2016, 63, 1–9. [Google Scholar] [CrossRef]
- Ommeh, S.C.; Zhang, W.; Zohaib, A.; Chen, J.; Zhang, H.; Hu, B.; Ge, X.-Y.; Yang, X.-L.; Masika, M.; Obanda, V.; et al. Genetic evidence of Middle East Respiratory Syndrome Coronavirus (MERS-Cov) and widespread Seroprevalence among Camels in Kenya. Virol. Sin. 2018, 33, 484–492. [Google Scholar] [CrossRef]
- Munyua, P.; Bitek, A.; Osoro, E.M.; Pieracci, E.G.; Muema, J.; Mwatondo, A.; Kungu, M.; Nanyingi, M.; Gharpure, R.; Njenga, K.; et al. Prioritization of zoonotic diseases in Kenya, 2015. PLoS ONE 2016, 11, e0161576. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, S.; Eiden, M.; El Mamy, B.O.; Isselmou, K.; Rodríguez, A.V.; Doumbia, B.; Groschup, M.H. Molecular and serological studies on the rift valley fever outbreak in Mauritania in 2010. Trans. Emerg. Dis. 2013, 60, 31–39. [Google Scholar] [CrossRef]
- Kamal, S.A. Observations on rift valley fever virus and vaccines in Egypt. Virol. J. 2011, 8, 1–9. [Google Scholar]
- El-Harrak, M.; Martín-Folgar, R.; Llorente, F.; Fernández-Pacheco, P.; Brun, A.; Figuerola, J.; Jiménez-Clavero, M.A. Rift Valley and West. Nile virus antibodies in camels, North. Africa. Emerg. Infect. Dis. 2011, 17, 2372–2374. [Google Scholar] [PubMed]
- Abdi, I.H.; Affognon, H.D.; Wanjoya, A.K.; Onyango-Ouma, W.; Sang, R. Knowledge, Attitudes and Practices (KAP) on rift valley fever among pastoralist communities of Ijara District, North Eastern Kenya. PLoS Negl. Trop. Dis. 2015, 9, e0004239. [Google Scholar] [CrossRef] [PubMed]
- Kriz, B. A study of camelpox in Somalia. J. Comp. Pathol. 1982, 92, 1–8. [Google Scholar] [CrossRef]
- Khalafalla, A.I.; Mohamed, M.E.H. Clinical and epizootiological features of camelpox in Eastern Sudan. J. Camel Pract. Res. 1996, 3, 99–102. [Google Scholar]
- Jezek, Z.; Kriz, B.; Rothbauer, V. Camelpox and its risk to the human population. J. Hyg. Epidemiol. Microbiol. Immunol. 1983, 27, 29–42. [Google Scholar]
- Balamurugan, V.; Venkatesan, G.; Bhanuprakash, V.; Singh, R.K. Camelpox, an emerging orthopox viral disease. Indian J. Virol. 2013, 24, 295–305. [Google Scholar] [CrossRef]
- Bera, B.; Shanmugasundaram, K.; Barua, S.; Venkatesan, G.; Virmani, N.; Riyesh, T.; Gulati, B.; Bhanuprakash, V.; Vaid, R.; Kakker, N.; et al. Zoonotic cases of camelpox infection in India. Vet. Microbiol. 2011, 152, 29–38. [Google Scholar] [CrossRef]
- Khalafalla, A.I.; Abdelazim, F. Human and dromedary camel infection with Camelpox virus in Eastern Sudan. Vector Borne Zoonotic Dis. 2017, 17, 281–284. [Google Scholar] [CrossRef]
- Shchelkunov, S.N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013, 9, 4. [Google Scholar] [CrossRef]
- Lwande, O.W.; Irura, Z.; Tigoi, C.; Chepkorir, E.; Orindi, B.; Musila, L.; Venter, M.; Fischer, A.; Sang, R. Seroprevalence of crimean congo hemorrhagic fever Virus in Ijara District, Kenya. Vector-Borne Zoonotic Dis. 2012, 12, 727–732. [Google Scholar] [CrossRef]
- Bente, D.A.; Forrester, N.L.; Watts, U.M.; McAuley, A.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 2013, 100, 159–189. [Google Scholar] [CrossRef] [PubMed]
- Dunster, L.; Dunster, M.; Ofula, V.; Beti, D.; Kazooba-Voskamp, F.; Burt, F.; Swanepoel, R.; Decock, K.M. First documentation of human Crimean-Congo hemorrhagic fever, Kenya. Emerg. Infect. Dis. 2002, 8, 1005–1006. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Kumar, B. Emerging influenza D virus threat: What we know so far! J. Clin. Med. 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.S.; Choi, J.Y.; Fieldhouse, J.K.; Borkenhagen, L.K.; Zemke, J.; Zhang, D.; Gray, G.C. The continual threat of influenza virus infections at the human-animal interface: What is new from a one health perspective? Evol. Med. Public Health 2018, 2018, 192–198. [Google Scholar] [CrossRef]
- Ohwada, K.; Kitame, F.; Sugawara, K.; Nishimura, H.; Homma, M.; Nakamura, K. Distribution of the antibody to influenza C virus in dogs and pigs in Yamagata Prefecture, Japan. Microbiol. Immunol. 1987, 31, 1173–1180. [Google Scholar] [CrossRef]
- Njeru, J.; Melzer, F.; Wareth, G.; El-Adawy, H.; Henning, K.; Pletz, M.W.; Heller, R.; Kariuki, S.; Fèvre, E.M.; Neubauer, H. Human brucellosis in febrile patients seeking treatment at remote hospitals, Northeastern Kenya, 2014–2015. Emerg. Infect. Dis. 2016, 22, 2160–2164. [Google Scholar] [CrossRef]
- McDermott, J.; Grace, D.; Zinsstag, J. Economics of brucellosis impact and control in low-income countries. Rev. Sci. Tech. Off. Int. Epizoot. 2013, 32, 249–261. [Google Scholar] [CrossRef]
- Sprague, L.D.; Al-Dahouk, S.; Neubauer, H. A review on camel brucellosis: A zoonosis sustained by ignorance and indifference. Pathog. Glob. Health 2012, 106, 144–149. [Google Scholar] [CrossRef][Green Version]
- Njeru, J.; Henning, K.; Pletz, M.W.; Heller, R.; Neubauer, H. Q fever is an old and neglected zoonotic disease in Kenya: A systematic review. BMC Public Health 2016, 16, 8. [Google Scholar] [CrossRef]
- Schelling, E.; Diguimbaye, C.; Daoud, S.; Nicolet, J.; Boerlin, P.; Tanner, M.; Zinsstag, J. Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev. Vet. Med. 2003, 61, 279–293. [Google Scholar] [CrossRef]
- Asmare, K.; Abayneh, T.; Sibhat, B.; Shiferaw, D.; Szonyi, B.; I Krontveit, R.; Skjerve, E.; Wieland, B. Major vectors and vector-borne diseases in small ruminants in Ethiopia: A systematic review. Acta Trop. 2017, 170, 95–104. [Google Scholar] [CrossRef]
- Burd, E.M.; Juzych, L.A.; Rudrik, J.T.; Habib, F. Pustular dermatitis caused by Dermatophilus congolensis. J. Clin. Microbiol. 2007, 45, 1655–1658. [Google Scholar] [CrossRef]
- Ndhlovu, D.N.; Masika, P.J. Bovine dermatophilosis: Awareness, perceptions and attitudes in the small-holder sector of north-west Zimbabwe. Onderstepoort J. Vet. Res. 2016, 83, 7. [Google Scholar] [CrossRef][Green Version]
- Baldacchino, F.; Muenworn, V.; Desquesnes, M.; Desoli, F.; Charoenviriyaphap, T.; Duvallet, G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): A review. Parasite 2013, 20, 13. [Google Scholar] [CrossRef]
- Gitao, C.G.; Agab, H.; Khalifalla, A.J. Outbreaks of Dermatophilus congolensis infection in camels (Camelus dromedarius) from the Butana region in Eastern Sudan. Rev. Sci. Tech. Off. Int. Epizoot. 1998, 17, 743–748. [Google Scholar] [CrossRef]
- Khodakaram-Tafti, A.; Khordadmehr, M.; Ardiyan, M. Prevalence and pathology of dermatophilosis in camels (Camelus dromedaries) in Iran. Trop. Anim. Health Prod. 2012, 44, 145–148. [Google Scholar] [CrossRef]
- Amor, A.; Enríquez, A.; Corcuera, M.T.; Toro, C.; Herrero, D.; Baquero, M. Is infection by Dermatophilus congolensis underdiagnosed? J. Clin. Microbiol. 2011, 49, 449–451. [Google Scholar] [CrossRef][Green Version]
- Towersey, L.; Martins, E.D.C.S.; Londero, A.T.; Hay, R.J.; Filho, P.J.S.; Takiya, C.M.; Martins, C.C.; Gompertz, O.F. Dermatophilus congolensis human infection. J. Am. Acad. Dermatol. 1993, 29, 351–354. [Google Scholar] [CrossRef]
- Hyslop, N.S.G. Dermatophilosis (streptothricosis) in animals and man. Comp. Immunol. Microbiol. Infect. Dis. 1979, 2, 389–404. [Google Scholar] [CrossRef]
- Omballa, V.; Musyoka, R.N.; Vittor, A.Y.; Wamburu, K.B.; Wachira, C.M.; Waiboci, L.W.; Abudo, M.U.; Juma, B.W.; Kim, A.A.; Montgomery, J.M.; et al. Serologic evidence of the geographic distribution of bacterial zoonotic agents in Kenya, 2007. Am. J. Trop. Med. Hyg. 2016, 94, 43–51. [Google Scholar] [CrossRef]
- Mwamuye, M.; Kariuki, E.; Omondi, D.; Kabii, J.; Odongo, D.; Masiga, D.K.; Villinger, J. Novel Rickettsia and emergent tick-borne pathogens: A molecular survey of ticks and tick-borne pathogens in Shimba Hills National Reserve, Kenya. Ticks Tick Borne Dis. 2017, 8, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Mwamuye, M.; Kariuki, E.; Omondi, D.; Kabii, J.; Odongo, D.; Masiga, D.; Villinger, J. Novel tick-borne Rickettsia sp. from wild ticks of Kenya: Implications for emerging vector-borne disease outbreaks. Int. J. Infect. Dis. 2016, 45, 60. [Google Scholar] [CrossRef]
- Maina, A.N.; Farris, C.M.; Odhiambo, A.; Jiang, J.; Laktabai, J.; Armstrong, J.; O’Meara, W.P. Q fever, scrub typhus, and rickettsia! Diseases in Children, Kenya, 2011–2012. Emerg. Infect. Dis. 2016, 22, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Sheik-Mohamed, A.; Velema, J.P. Where health care has no access: The nomadic populations of sub-Saharan Africa. Trop. Med. Int. Health 1999, 4, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Kudil, A.C.; Bello, A.; Ndukum, A.J. Prevalence of bovine tuberculosis in camels in northern Nigeria. J. Camel Pract. Res. 2012, 19, 81–86. [Google Scholar]
- Craig, P.S.; Mastin, A.J.; Van Kesteren, F.; Boufana, B. Echinococcus granulosus: Epidemiology and state-of-the-art of diagnostics in animals. Vet. Parasitol. 2015, 213, 132–148. [Google Scholar] [CrossRef]
- Romig, T.; Ebi, D.; Wassermann, M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet. Parasitol. 2015, 213, 76–84. [Google Scholar] [CrossRef]
- Elmahdi, I.E.; Ali, Q.; Magzoub, M.; Ibrahim, A.; Saad, M.; Romig, T. Cystic echinococcosis of livestock and humans in central Sudan. Ann. Trop. Med. Parasitol. 2004, 98, 473–479. [Google Scholar] [CrossRef]
- Nakao, M.; Lavikainen, A.; Yanagida, T.; Ito, A. Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae). Int. J. Parasitol. 2013, 43, 1017–1029. [Google Scholar] [CrossRef]
- Omer, R.; Dinkel, A.; Romig, T.; Mackenstedt, U.; Elnahas, A.; Aradaib, I.; Ahmed, M.; Elmalik, K.; Adam, A. A molecular survey of cystic echinococcosis in Sudan. Vet. Parasitol. 2010, 169, 340–346. [Google Scholar] [CrossRef]
- Rojas, C.A.A.; Romig, T.; Lightowlers, W.M. Echinococcus granulosus sensu lato genotypes infecting humans—review of current knowledge. Int. J. Parasitol. 2014, 44, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.S. Hydatid disease: Research and control in Turkana, Kenya. 1. Epidemiological observations. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 177–182. [Google Scholar] [CrossRef]
- Magambo, J.N.E.; Zeyhle, E. Epidemiology and control of echinococcosis in sub-Saharan Africa. Parasitol. Int. 2006, 55, S193–S195. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, Z.B.M.; Dargantes, A.; Lai, D.-H.; Lun, Z.-R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma evansi and Surra: A review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. BioMed Res. Int. 2013, 2013, 1–20. [Google Scholar] [CrossRef]
- Joshi, P.P.; Truc, P.; Salkar, H.R.; Dani, V.S.; Shegokar, V.R.; Bhargava, A.; Powar, R.M.; Herder, S.; Jannin, J.; Katti, R. Human Trypanosomiasis caused by Trypanosoma Evansi in India: The first case report. Am. Soc. Trop. Med. Hyg. 2005, 73, 491–495. [Google Scholar] [CrossRef]
- Haridy, F.M.; El-Metwally, M.T.; Khalil, H.H.M.; A Morsy, T. Trypanosoma evansi in dromedary camel: With a case report of zoonosis in greater Cairo, Egypt. J. Egypt Soc. Parasitol. 2011, 41, 65–76. [Google Scholar]
- Vanhollebeke, B.; Truc, P.; Poelvoorde, P.; Pays, A.; Joshi, P.P.; Katti, R.; Jannin, J.G.; Pays, E. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-I. New Engl. J. Med. 2006, 355, 2752–2756. [Google Scholar] [CrossRef]
- Aregawi, W.G.; Agga, G.E.; Abdi, R.D.; Büscher, P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasites Vectors 2019, 12, 67. [Google Scholar] [CrossRef]
- Kamau, P.; Jaoko, W.; Gontier, C. Seroepidemiolgy of Toxoplasma gondii in ante-natal women attending Kenyatta National Hospital, Kenya. Int. J. Infect. Dis. 2012, 16, e162. [Google Scholar] [CrossRef][Green Version]
- Tonouhewa, A.B.N.; Akpo, Y.; Sessou, P.; Adoligbe, C.; Yessinou, R.E.; Hounmanou, Y.M.G.; Assogba, M.N.; Youssao, I.; Farougou, S. Toxoplasma gondii infection in meat animals from Africa: Systematic review and meta-analysis of sero-epidemiological studies. Vet. World 2017, 10, 194–208. [Google Scholar] [CrossRef]
- Gebremedhin, E.Z.; Dima, N.; Beyi, A.F.; Dawo, F.; Feyissa, N.; Jorga, E.; Di Marco, V.; Vitale, M. Toxoplasmosis in camels (Camelus dromedarius) of Borana zone, Oromia region, Ethiopia: Seroprevalence and risk factors. Trop. Anim. Health Prod. 2016, 48, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Babelhadj, B.; Di Bari, M.A.; Pirisinu, L.; Chiappini, B.; Gaouar, S.B.S.; Riccardi, G.; Marcon, S.; Agrimi, U.; Nonno, R.; Vaccari, G. Prion disease in dromedary camels, Algeria. Emerg. Infect. Dis. 2018, 24, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Kaindi, D.W.M.; Schelling, E.; Wangoh, J.M.; Imungi, J.K.; Farah, Z.; Meile, L. Risk factors for symptoms of gastrointestinal illness in rural town Isiolo, Kenya. Zoonoses Public Health 2012, 59, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Matofari, J.W.; Shitandi, A.; Shalo, P.L.; Nanua, N.J.; Younan, M. A survey of Salmonella enterica contamination of camel milk in Kenya. Afr. J. Microbiol. Res. 2007, 1, 46–50. [Google Scholar]
- Alonso, S.; Dohoo, I.; Lindahl, J.F.; Verdugo, C.; Akuku, I.; Grace, D. Prevalence of tuberculosis, brucellosis and trypanosomiasis in cattle in Tanzania: A systematic review and meta-analysis. Anim. Health Res. Rev. 2016, 17, 16–27. [Google Scholar] [CrossRef]
- Njeru, J.; Wareth, G.; Melzer, F.; Henning, K.; Pletz, M.W.; Heller, R.; Neubauer, H. Systematic review of brucellosis in Kenya: Disease frequency in humans and animals and risk factors for human infection. BMC Public Health 2016, 16, 853. [Google Scholar] [CrossRef]

| Zoonotic Infections/Agents of Relevance | Search Terms and Synonyms (* Indicates Wildcard Search Function) |
|---|---|
| Brucella spp. | “brucel *” |
| Camelpox | “camelpox”, “pox” “poxvir *”, “orthopox *” |
| Crimean-Congo haemorrhagic fever virus | “Crimean-Congo haemorrhagic fever *” |
| Echinococcus granulosa sensu lato | “echinococ *”, “* hydatid *” |
| Emerging pathogens | “emerging” AND “infection *” OR “virus *” OR “bacteria *” |
| Middle Eastern respiratory syndrome virus | “Middle East respiratory”, “Middle Eastern respiratory”, “MERS”, “MERS-CoV”, “coronavir *” |
| Q fever (Coxiella burnetii) | “q fever *”, “coxiell *” |
| Rift Valley fever virus | “Rift Valley fever”, “RVF” |
| Sarcoptes | “sarcopt *”, “mange *” |
| Toxoplasmosis | “toxoplasma *” |
| Trypanosome spp. | “* trypanos *”, “African trypan *” |
| Tuberculosis | “TB”, “tubercul *”, “mycobact *” |
| Good Quality (Low Risk of Bias) | Medium Quality (Moderate Risk of Bias) | Poor Quality (High Risk of Bias) |
|---|---|---|
| Unbiased selection of subjects, evidence of randomisation | Bias in subject selection is acknowledged and accounted for or is unavoidable | Bias in subject selection is not acknowledge or accounted for |
| Appropriate data analysis | Data analysis limitations are acknowledged | Inappropriate data analysis |
| Scientifically sound methods | Methods are sound but may not be the most appropriate | Methods are inappropriate |
| Accurately described methods | Methods are comprehensible and valid even if details are lacking | Methods are unclear or incomplete |
| Accurate and complete reporting of results | Results are reported accurately | Results are inaccurate or incomplete |
| Pathogen or Disease | Number of Studies | Host or Vector of the Pathogen Identified | References | ||||
|---|---|---|---|---|---|---|---|
| Quality Score | |||||||
| Camel | Tick † | Human ‡ | Good | Medium | Poor | ||
| Viruses | |||||||
| MERS-CoV | 4 | X | X | [32,33] | [34,35] | ||
| Rift Valley fever virus | 4 | X | [36,37] | [38,39] | |||
| Camelpox | 4 | X | [40,41] | [42,43] | |||
| Crimea-Congo haemorrhagic fever virus | 2 | X | X | [44,45] | |||
| Contagious ecthyma | 2 | X | [46,47] | ||||
| Dugbe virus | 1 | X | [48] | ||||
| Dhori virus | 1 | X | [48] | ||||
| Influenza viruses (ICV and IDV) | 1 | X | [49] | ||||
| Bacteria | |||||||
| Coxiella burnetii (Q fever) | 3 | X | [50,51] | [52] | |||
| Dermatophilus congolensis | 5 | X | [53] | [54,55,56,57] | |||
| Brucella spp. | 7 | X | [58] | [59] | [12,60,61,62,63] | ||
| Mycobacterium spp. | 1 | X | [60] | ||||
| Rickettsia spp. | 1 | X | [64] | ||||
| Parasites and Fungi | |||||||
| Trypanosoma spp. | 28 | X | [65,66,67,68,69] | [10,70,71,72,73,74,75,76,77,78,79,80,81,82,83] | [84,85,86,87,88,89,90,91,92] | ||
| Echinococcus spp. | 10 | X | X | [93] | [94,95,96,97,98,99,100,101,102] | ||
| Trichophyton verrucosum | 1 | X | [53] | ||||
| Type | Pathogen | Species | Dates Sampled | Test Used | Number Tested | County or Region | Prevalence % (95% CI) † | Quality | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Virus | Middle Eastern respiratory coronavirus (MERS-CoV) | Camel | 1992–2013 | Recombinant MERS-CoV spike protein subunit 1-based ELISA (rELISA) described by Memish et al., 2014 [109] | 162 | North-eastern region | 56.2 | Medium | [34] |
| 154 | Eastern region | 17–100 | |||||||
| 458 | Rift Valley region | 0–18 | |||||||
| Camels | 2013 | Spike protein subunit 1 protein microarray [110,111] | 335 | Laikipia county | 46.9 | Medium | [35] | ||
| Camels | 2013 | AntiMERS-CoV Camel IgG ELISA kit (EUROIMMUN AG, Lübeck, Germany) | 879 | Marsabit county | 90 (95% CI 88–92) | Good | [33] | ||
| Humans | AntiMERS-CoV Camel IgG ELISA kit (EUROIMMUN AG, Lübeck, Germany) followed by plaque reduction neutralisation test (PRNT) [112] | 760 | Marsabit county | 0 | |||||
| Humans | 2013–2014 | rELISA (EUROIMMUN AG, Lübeck, Germany) followed by PRNT [112] | 559 | Garissa county | 0 | Good | [32] | ||
| 563 | Tana River county | 0.36 | |||||||
| Crimean-Congo haemorrhagic fever virus | Camels | 1986–1987 | Agar gel diffusion (AGD) test [113] | 499 | Not specified | 26 | Medium | [45] | |
| Contagious ecthyma | Camels | Not specified | Clinical examination and electron microscopy | 600 | Turkana | 11.2 | Medium | [46] | |
| Rift Valley fever virus | Camels | 2006–2007 (epidemic period) | In-house IgG ELISA [114] | 110 | Not specified | 20.9 | Medium | [36] | |
| Camels | 2000 (pre-epidemic period) | In-house inhibition ELISA [115] | 15 | Galana county | 6.7 | Medium | [37] | ||
| 13 | Garissa county | 7.7 | |||||||
| 2007 (epidemic period) | 28 | Isiolo county | 57.1 | ||||||
| Camelpox | Camels | 1992 | Clinical examination, electron microscopy, virus neutralisation | 1000 | Samburu county | 27 | Medium | [40] | |
| 1200 | Turkana county | 6 | |||||||
| Influenza D virus (IDV) | Camels | 2015 | Hemagglutination inhibition (HI), post-ICV hemadsorption | 293 | Not specified | 8.2 | Medium | [49] | |
| Influenza C virus (ICV) | Camels | 2015 | HI, post-IDV hemadsorption | 293 | Not specified | 10.6 | Medium | [49] | |
| Bacteria | Brucella spp. | Camels | Not specified | Rose Bengal plate test (RBPT) | 174 | Warir, Garissa and Mandera counties | 4.6 | Medium | [59] |
| Serum agglutination test (SAT) | 10.34 | ||||||||
| Complement fixation test (CFT) | 9.77 | ||||||||
| Camels | 2013 | Brucella-Ab C-ELISA kit (SVANOVIR, Uppsala, Sweden) | 1605 | Marsabit county | 11.1 (95% CI 9.4–15.0) | Good | [108] | ||
| Coxiella burnetii (Q fever) | Camels | 2011 | ELISA CHEKIT Q fever test kit (IDEXX, Westbrook, ME, USA | 72 | Laikipia county | Adults (3–9 years) 46 Young (<6 m) 5 | Medium | [50] | |
| Camels | 2013 | ELISA CHEKIT Q fever test kit (IDEXX, Hoofddorp, The Netherlands) | 334 | Laikipia county | 19 | Medium | [51] | ||
| Dermatophilus congolensis | Camels | 1993 | Clinical examination and bacterial isolation | 3200 | Samburu county | Wet season, 20.9 Dry season, 13.6 | Medium | [53] | |
| 600 | Laikipia county | Wet season, 22.7 Dry season, 14.3 | |||||||
| Parasites | Echinococcus spp. | Camels | 1998–2000 | Post-mortem examinations | 70 | Turkana county | 60.1 | Medium | [102] |
| Camels | 2013 | Post-mortem examination and RFLP-PCR [98] | 219 | Meru and Isiolo counties | 6.94 | Medium | [98] | ||
| Trypanosoma spp. | Camels | 1996–1997 | Haematocrit centrifugation technique (HCT) Mouse inoculation test (MIT) Suratex® latex agglutination test (Brentec Diagnostics, Nairobi, Kenya) [71] | 103 | Athi River (Machakos county) | 2.9 (95% CI 0–6.2) | Medium | [73] | |
| 749 | Isiolo county | 25.4 (95% CI 22.3–28.5) | |||||||
| 86 | Mugwoni (Laikipia county) | 18.6 (95% CI 10.4–26.8) | |||||||
| Camels | Not specified | Phase contrast buffy coat technique (BCT) MIT | 347 | Kajiado county | 33.8 | Medium | [10] |
| Echinococcus granulosus Species/Genotype | Host Species | Method of Confirmation | County/Location | Reference |
|---|---|---|---|---|
| E. granulosus Type B | Camel | Electrophoresis: isoelectric focusing | Turkana | [97] |
| E. granulosus Type A | Human | Electrophoresis: isoelectric focusing | Turkana | [97] |
| E. granulosus ‘Common sheep strain’ | Camel | PCR and electrophoresis | Turkana | [100] |
| E. granulosus ‘Camel strain’ | Camel | PCR and electrophoresis | Turkana | [100] |
| E. granulosus G1 | Camel Humans | PCR | Turkana/Maasai | [116] |
| E. granulosus G6 (G6/7) | Camel Human | PCR | Turkana/Maasai | [116] |
| E. granulosus G1 | Human | PCR | Turkana | [99] |
| E. granulosus G6 | Human | PCR | Turkana | [99] |
| E. granulosus sensu stricto (s.s.) | Camel | PCR | Meru/Isiolo | [98] |
| E. canadensis (formally G7) | Camel | PCR | Meru/Isiolo | [98] |
| E. Canadensis G6/7 cluster | Camels Human | PCR | Not specified | [93] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, E.C.; Anderson, N.E. Zoonotic Pathogens of Dromedary Camels in Kenya: A Systematised Review. Vet. Sci. 2020, 7, 103. https://doi.org/10.3390/vetsci7030103
Hughes EC, Anderson NE. Zoonotic Pathogens of Dromedary Camels in Kenya: A Systematised Review. Veterinary Sciences. 2020; 7(3):103. https://doi.org/10.3390/vetsci7030103
Chicago/Turabian StyleHughes, Ellen Clare, and Neil Euan Anderson. 2020. "Zoonotic Pathogens of Dromedary Camels in Kenya: A Systematised Review" Veterinary Sciences 7, no. 3: 103. https://doi.org/10.3390/vetsci7030103
APA StyleHughes, E. C., & Anderson, N. E. (2020). Zoonotic Pathogens of Dromedary Camels in Kenya: A Systematised Review. Veterinary Sciences, 7(3), 103. https://doi.org/10.3390/vetsci7030103





