Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing
Abstract
1. Nutrition-Oriented Health Promotion in Animals
2. Natural Presence of PEA in Vegetable and Animal Food Sources
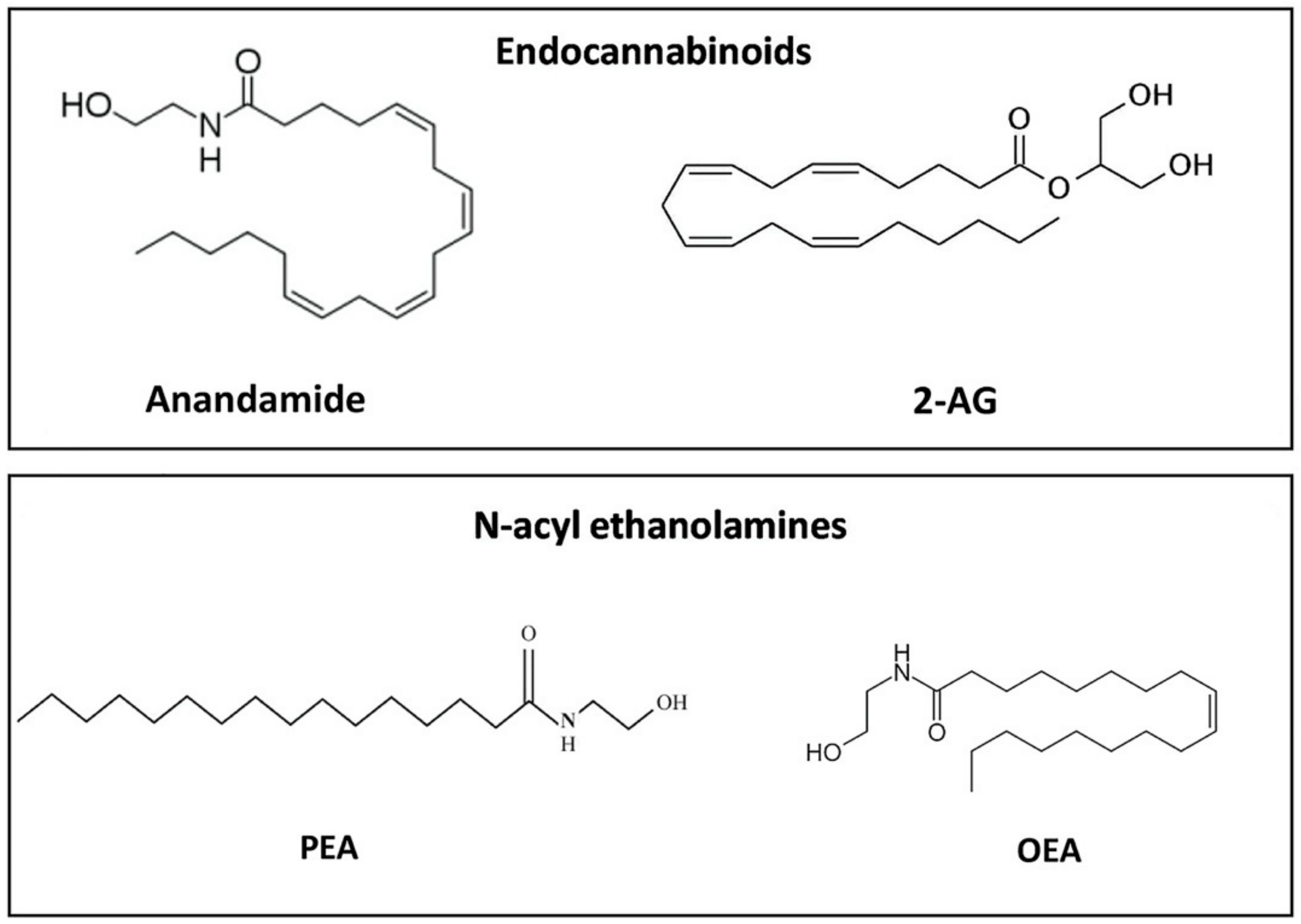
3. ALIAmides and the Highly Conserved N-acylethanolamine Family
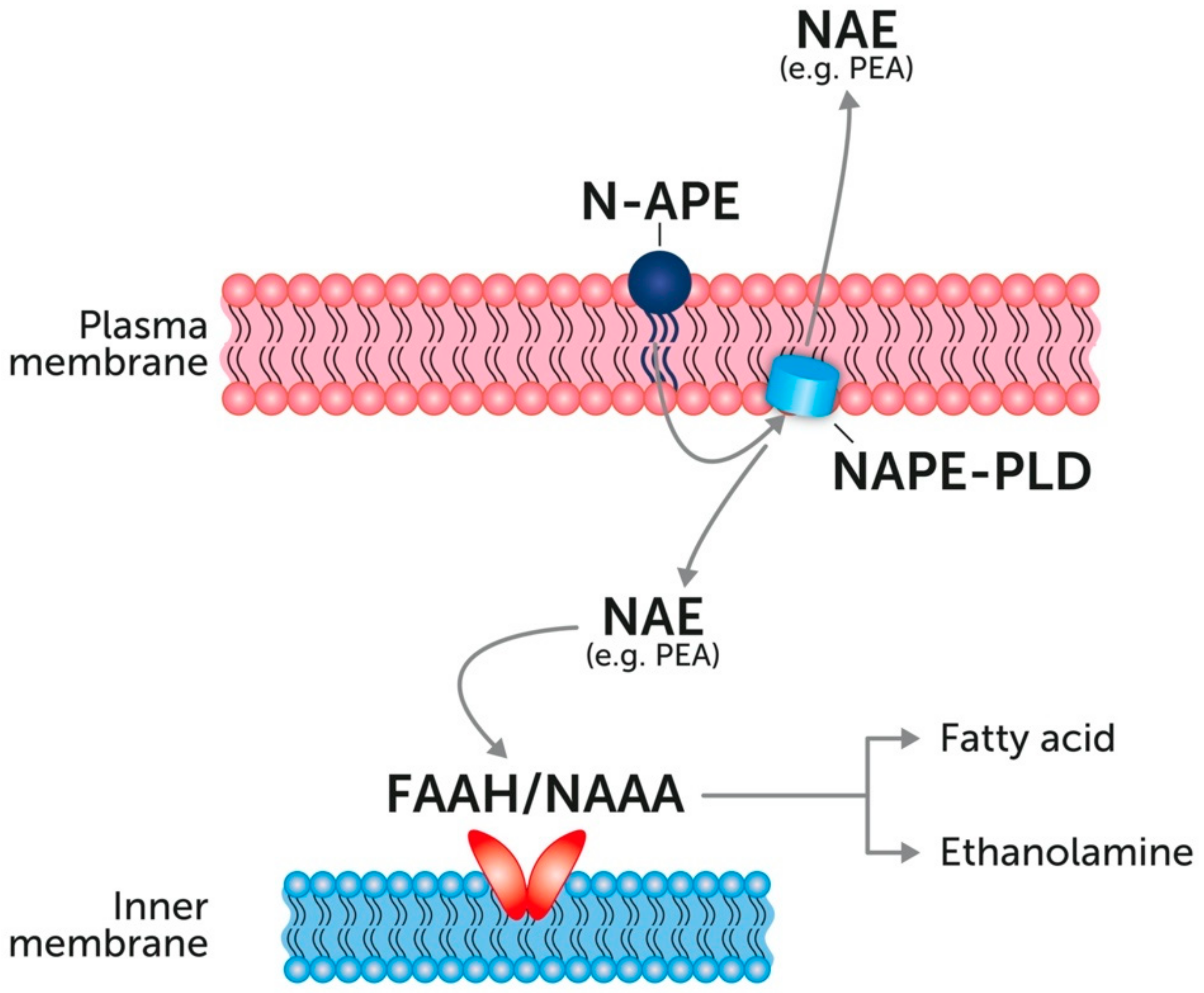
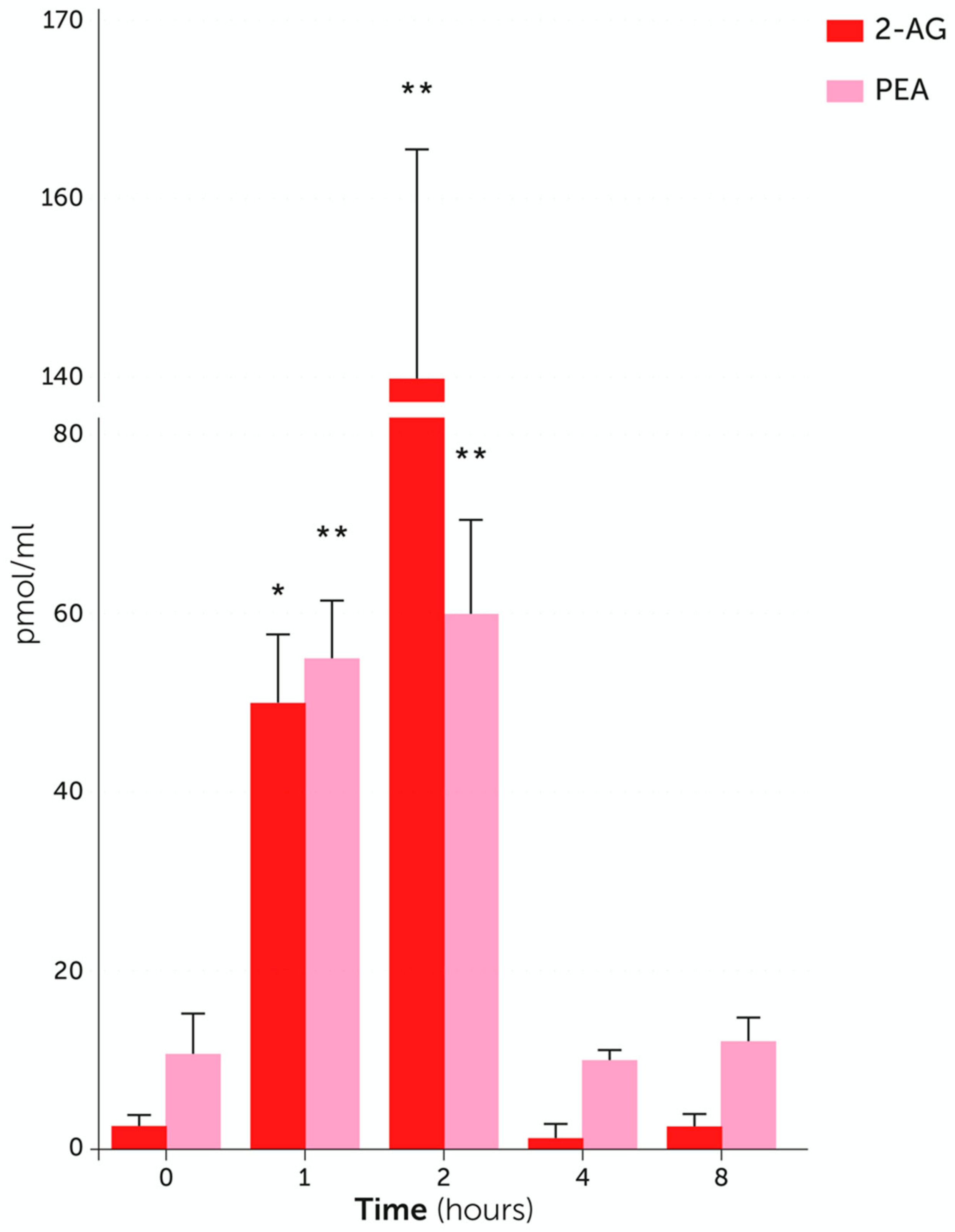
4. Endogenous PEA: Metabolic Pathways and Change in Tissue Levels
5. PEA Mechanism of Action: A Multitarget Redundancy
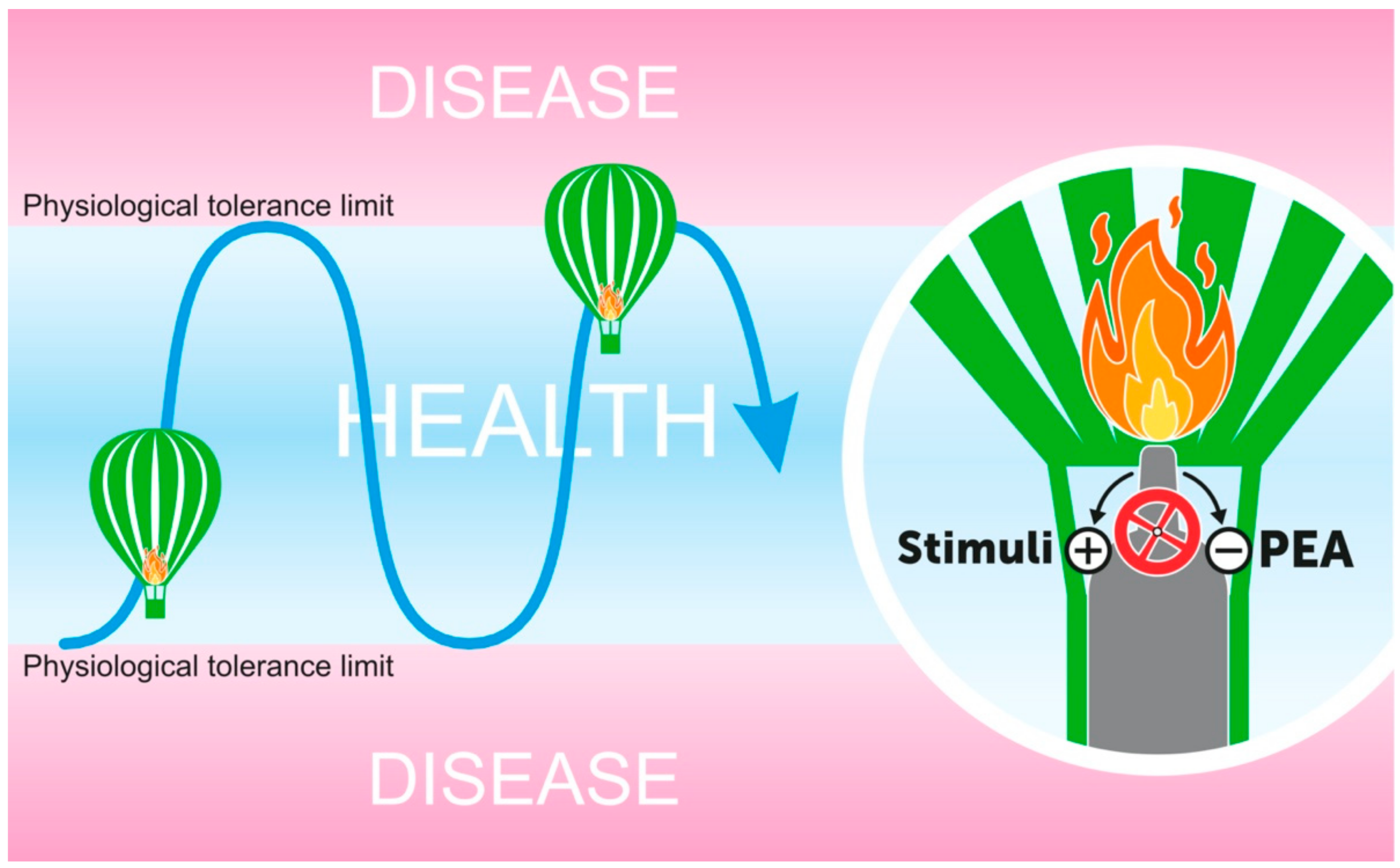
6. PEA as A “Proresolving” Lipid Mediator
7. PEA Bioavailability: A Size Issue
8. Application of ALIAmides to Animal Health and Wellbeing
8.1. Gastrointestinal Tract
8.2. Upper and Lower Urinary Tract
8.3. Nervous System
8.4. Musculoskeletal System
8.5. Mucocutaneous and Skin Sites
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zohoori, F.V. Nutrition and Diet Monogr. Oral. Sci. 2020, 28, 1–13. [Google Scholar]
- Panagiotou, G.; Nielsen, J. Nutritional systems biology: Definitions and approaches. Annu. Rev. Nutr. 2009, 29, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Davies, M. Veterinary clinical nutrition: Success stories: An overview. Proc. Nutr. Soc. 2016, 75, 392–397. [Google Scholar] [CrossRef]
- Gupta, R.C.; Srivastava, A.; Lall, R. Nutraceuticals in Veterinary Medicine, 1st ed.; Springer Nature: Cham, Switzerland, 2019; pp. 7–9. [Google Scholar]
- Saevik, B.K.; Bergvall, K.; Holm, B.R.; Saijonmaa-Koulumies, L.E.; Hedhammar, A.; Larsen, S.; Kristensen, F. A randomized, controlled study to evaluate the steroid sparing effect of essential fatty acid supplementation in the treatment of canine atopic dermatitis. Vet. Dermatol. 2004, 15, 137–145. [Google Scholar] [CrossRef]
- Schumann, J.; Basiouni, S.; Gück, T.; Fuhrmann, H. Treating canine atopic dermatitis with unsaturated fatty acids: The role of mast cells and potential mechanisms of action. J. Anim. Physiol. Anim. Nutr. (Berl) 2014, 98, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Witzel-Rollins, A.; Murphy, M.; Becvarova, I.; Were, S.R.; Cadiergues, M.C.; Meyer, H. Non-controlled, open-label clinical trial to assess the effectiveness of a dietetic food on pruritus and dermatologic scoring in atopic dogs. BMC Vet. Res. 2019, 15, 220. [Google Scholar] [CrossRef]
- Plantinga, E.A.; Everts, H.; Kastelein, A.M.; Beynen, A.C. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet. Rec. 2005, 157, 185–187. [Google Scholar] [CrossRef]
- Brown, S.A.; Brown, C.A.; Crowell, W.A.; Barsanti, J.A.; Allen, T.; Cowell, C.; Finco, D.R. Beneficial effects of chronic administration of dietary omega-3 polyunsaturated fatty acids in dogs with renal insufficiency. J. Lab. Clin. Med. 1998, 131, 447–455. [Google Scholar] [CrossRef]
- Pan, Y.; Landsberg, G.; Mougeot, I.; Kelly, S.; Xu, H.; Bhatnagar, S.; Gardner, C.L.; Milgram, N.W. Efficacy of a therapeutic diet on dogs with signs of cognitive dysfunction syndrome (CDS): A prospective double blinded placebo controlled clinical study. Front. Nutr. 2018, 5, 127. [Google Scholar] [CrossRef]
- Mehler, S.J.; May, L.R.; King, C.; Harris, W.S.; Shah, Z. A prospective, randomized, double blind, placebo-controlled evaluation of the effects of eicosapentaenoic acid and docosahexaenoic acid on the clinical signs and erythrocyte membrane polyunsaturated fatty acid concentrations in dogs with osteoarthritis. Prostaglandins Leukot. Essent. Fatty Acids. 2016, 109, 1–7. [Google Scholar] [CrossRef]
- Fritsch, D.A.; Allen, T.A.; Dodd, C.E.; Jewell, D.E.; Sixby, K.A.; Leventhal, P.S.; Brejda, J.; Hahn, K.A. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J. Am. Vet. Med. Assoc. 2010, 236, 535–539. [Google Scholar] [CrossRef]
- Moreau, M.; Troncy, E.; Del Castillo, J.R.; Bédard, C.; Gauvin, D.; Lussier, B. Effects of feeding a high omega-3 fatty acids diet in dogs with naturally occurring osteoarthritis. J. Anim. Physiol. Anim. Nutr. 2013, 97, 830–837. [Google Scholar] [CrossRef]
- Rialland, P.; Bichot, S.; Lussier, B.; Moreau, M.; Beaudry, F.; del Castillo, J.R.; Gauvin, D.; Troncy, E. Effect of a diet enriched with green-lipped mussel on pain behavior and functioning in dogs with clinical osteoarthritis. Can. J. Vet. Res. 2013, 77, 66–74. [Google Scholar]
- Roush, J.K.; Dodd, C.E.; Fritsch, D.A.; Allen, T.A.; Jewell, D.E.; Schoenherr, W.D.; Richardson, D.C.; Leventhal, P.S.; Hahn, K.A. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Santos, A.M.; Jorge, P.I. Effect of an oral joint supplement when compared to carprofen in the management of hip osteoarthritis in working dogs. Top. Companion. Anim. Med. 2017, 32, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Bhathal, A.; Spryszak, M.; Louizos, C.; Frankel, G. Glucosamine and chondroitin use in canines for osteoarthritis: A review. Open Vet. J. 2017, 7, 36–49. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, G.; O’donovan, J.; Jones, B.; McAllister, H.; Seed, M.; Mooney, C. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet. J. 2007, 174, 54–61. [Google Scholar] [CrossRef]
- Musco, N.; Vassalotti, G.; Mastellone, V.; Cortese, L.; Della Rocca, G.; Molinari, M.L.; Calabrò, S.; Tudisco, R.; Cutrignelli, M.I.; Lombardi, P. Effects of a nutritional supplement in dogs affected by osteoarthritis. Vet. Med. Sci. 2019, 5, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Coburn, A.F.; Trulson, M.F.; Moore, L.V. [Further study of the effect of the administration of egg yolk on susceptibility of children to rheumatic infection]. Minerva Med. 1954, 45, 1534–1536. [Google Scholar]
- Ganley, O.H.; Graessle, O.E.; Robinson, H.J. Anti-inflammatory activity on compounds obtained from egg yolk, peanut oil, and soybean lecithin. J. Lab. Clin. Med. 1958, 51, 709–714. [Google Scholar]
- Kuehl, F.A.; Jacob, T.A.; Ganley, O.H.; Ormond, R.E.; Meisinger, M.A.P. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occuring anti-inflammatory agent. J. Am. Chem. Soc. 1957, 79, 5577–5578. [Google Scholar] [CrossRef]
- Kilaru, A.; Blancaflor, E.B.; Venables, B.J.; Tripathy, S.; Mysore, K.S.; Chapman, K.D. The N-acylethanolamine-mediated regulatory pathway in plants. Chem. Biodivers. 2007, 4, 1933–1955. [Google Scholar] [CrossRef]
- Venables, B.J.; Waggoner, C.A.; Chapman, K.D. N-acylethanolamines in seeds of selected legumes. Phytochemistry 2005, 66, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Ferracane, R.; Vitaglione, P. Food database of N-acyl-phosphatidylethanolamines, N-acylethanolamines and endocannabinoids and daily intake from a Western, a Mediterranean and a vegetarian diet. Food Chem. 2019, 300, 125218. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Scuto, M.; Siracusa, R.; D’amico, R.; Peritore, F.A.; Gugliandolo, E.; Fusco, R.; Crupi, R.; Impellizzeri, D.; Pozzebon, M.; et al. Effect of N-palmitoylethanolamine-oxazoline on comorbid neuropsychiatric disturbance associated with inflammatory bowel disease. FASEB J. 2020, 34, 4085–4106. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Crupi, R.; Pascali, J.; Alfonsi, D.; Marcolongo, G.; Cuzzocrea, S. 2-pentadecyl-2-oxazoline: Identification in coffee, synthesis and activity in a rat model of carrageenan-induced hindpaw inflammation. Pharmacol. Res. 2016, 108, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-Acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 2002, 121, 211–227. [Google Scholar] [CrossRef]
- Lam, P.M.; Marczylo, T.H.; Konje, J.C. Simultaneous measurement of three N-acylethanolamides in human bio-matrices using ultra performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 2089–2097. [Google Scholar] [CrossRef]
- Gouveia-Figueira, S.; Nording, M.L. Development and validation of a sensitive UPLC-ESI-MS/MS method for the simultaneous quantification of 15 endocannabinoids and related compounds in milk and other biofluids. Anal. Chem. 2014, 86, 1186–1195. [Google Scholar] [CrossRef]
- Gaitán, A.V.; Wood, J.T.; Solomons, N.W.; Donohue, J.A.; Ji, L.; Liu, Y.; Nikas, S.P.; Zhang, F.; Allen, L.H.; Makriyannis, A.; et al. Endocannabinoid metabolome characterization of milk from guatemalan women living in the western highlands. Curr. Dev. Nutr. 2019, 3, nzz018. [Google Scholar] [CrossRef]
- Bruun, S.; Gouveia-Figueira, S.; Domellöf, M.; Husby, S.; Neergaard Jacobsen, L.; Michaelsen, K.F.; Fowler, C.J.; Zachariassen, G. Satiety factors oleoylethanolamide, stearoylethanolamide, and palmitoylethanolamide in mother’s milk are strongly associated with infant weight at four months of age-data from the odense child cohort. Nutrients 2018, 10, 1747. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, M.T.; Vázquez, M.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alén, F.; Antón, M.; Decara, J.; Arco, R.; Orio, L.; et al. A moderate diet restriction during pregnancy alters the levels of endocannabinoids and endocannabinoid-related lipids in the hypothalamus, hippocampus and olfactory bulb of rat offspring in a sex-specific manner. PLoS ONE 2017, 12, e0174307. [Google Scholar] [CrossRef] [PubMed]
- Bachur, N.R.; Masek, K.; Melmon, K.L.; Udenfriend, S. Fatty acid amides of ethanolamine in mammalian tissues. J. Biol. Chem. 1965, 240, 1019–1024. [Google Scholar] [PubMed]
- Epps, D.E.; Schmid, P.C.; Natarajan, V.; Schmid, H.H. N-Acylethanolamine accumulation in infarcted myocardium. Biochem. Biophys. Res. Commun. 1979, 90, 628–633. [Google Scholar] [CrossRef]
- Abramo, F.; Campora, L.; Albanese, F.; della Valle, M.F.; Cristino, L.; Petrosino, S.; Di Marzo, V.; Miragliotta, V. Increased levels of palmitoylethanolamide and other bioactive lipid mediators and enhanced local mast cell proliferation in canine atopic dermatitis. BMC Vet. Res. 2014, 10, 21. [Google Scholar] [CrossRef]
- Matias, I.; Wang, J.W.; Moriello, A.S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration. Prostaglandins Leukot. Essent. Fatty Acids. 2006, 75, 413–418. [Google Scholar] [CrossRef]
- Annuzzi, G.; Piscitelli, F.; Di Marino, L.; Patti, L.; Giacco, R.; Costabile, G.; Bozzetto, L.; Riccardi, G.; Verde, R.; Petrosino, S.; et al. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients. Lipids Health Dis. 2010, 9, 43. [Google Scholar] [CrossRef]
- Schreiber, D.; Harlfinger, S.; Nolden, B.M.; Gerth, C.W.; Jaehde, U.; Schomig, E.; Klosterkotter, J.; Giuffrida, A.; Astarita, G.; Piomelli, D.; et al. Determination of anandamide and other fatty acyl ethanolamides in human serum by electrospray tandem mass spectrometry. Anal. Biochem. 2007, 361, 162–168. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Croxford, J.L.; Brown, P.; Pertwee, R.G.; Makriyannis, A.; Khanolkar, A.; Layward, L.; Fezza, F.; Bisogno, T.; et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001, 15, 300–302. [Google Scholar] [CrossRef]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef]
- Bisogno, T.; Martire, A.; Petrosino, S.; Popoli, P.; Di Marzo, V. Symptom-related changes of endocannabinoid and palmitoylethanolamide levels in brain areas of R6/2 mice, a transgenic model of Huntington’s disease. Neurochem. Int. 2008, 52, 307–313. [Google Scholar] [CrossRef]
- Kilaru, A.; Isaac, G.; Tamura, P.; Baxter, D.; Duncan, S.R.; Venables, B.J.; Welti, R.; Koulen, P.; Chapman, K.D. Lipid profiling reveals tissue-specific differences for ethanolamide lipids in mice lacking fatty acid amide hydrolase. Lipids 2010, 45, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.; Parmentier-Batteur, S.; Walter, L.; Greenberg, D.A.; Stella, N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J. Neurosci. 2003, 23, 7767–7775. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Stella, N. An optimized GC-MS method detects nanomolar amounts of anandamide in mouse brain. Anal. Biochem. 2008, 373, 220–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmid, P.C.; Krebsbach, R.J.; Perry, S.R.; Dettmer, T.M.; Maasson, J.L.; Schmid, H.H. Occurrence and postmortem generation of anandamide and other long-chain N-acylethanolamines in mammalian brain. FEBS Lett. 1995, 375, 117–120. [Google Scholar] [CrossRef]
- Richardson, D.; Ortori, C.A.; Chapman, V.; Kendall, D.A.; Barrett, D.A. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal. Biochem. 2007, 360, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; McHugh, D.; Fernández-Ruiz, J.; Bradshaw, H.; Walker, J.M. Short-term exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci. Lett. 2007, 421, 270–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fonseca, B.M.; Correia-da-Silva, G.; Taylor, A.H.; Lam, P.M.; Marczylo, T.H.; Konje, J.C.; Bell, S.C.; Teixeira, N.A. N-acylethanolamine levels and expression of their metabolizing enzymes during pregnancy. Endocrinology 2010, 151, 3965–3974. [Google Scholar] [CrossRef]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar] [CrossRef]
- Valastro, C.; Campanile, D.; Marinaro, M.; Franchini, D.; Piscitelli, F.; Verde, R.; Di Marzo, V.; Di Bello, A. Characterization of endocannabinoids and related acylethanolamides in the synovial fluid of dogs with osteoarthritis: A pilot study. BMC Vet. Res. 2017, 13, 309. [Google Scholar] [CrossRef]
- Aloe, L.; Leon, A.; Levi-Montalcini, R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions 1993, 39, C145–C147. [Google Scholar] [CrossRef] [PubMed]
- Melmon, K.L.; Rocklin, R.E.; Rosenkranz, R.P. Autacoids as modulators of the inflammatory and immune response. Am. J. Med. 1981, 71, 100–106. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Chapman, K.D. Emerging physiological roles for N-acylphosphatidylethanolamine metabolism in plants: Signal transduction and membrane protection. Chem. Phys. Lipids 2000, 108, 221–230. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Mast cells, glia and neuroinflammation: Partners in crime? Immunology 2014, 141, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.; Lone, M.A.; Kaul, T.K.; Reis Rodrigues, P.; Ogungbe, I.V.; Gill, M.S. Characterization of N-acyl phosphatidylethanolamine-specific phospholipase-D isoforms in the nematode Caenorhabditis elegans. PLoS ONE 2014, 9, e113007. [Google Scholar] [CrossRef] [PubMed]
- Schmid, H.H.; Berdyshev, E.V. Cannabinoid receptor-inactive N-acylethanolamines and other fatty acid amides: Metabolism and function. Prostaglandins Leukot. Essent. Fatty Acids. 2002, 66, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D. Occurrence, metabolism, and prospective functions of N-acylethanolamines in plants. Prog. Lipid Res. 2004, 43, 302–327. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Sia, A.; Muchowski, P.J.; Stella, N. Genetic manipulation of palmitoylethanolamide production and inactivation in Saccharomyces cerevisiae. PLoS ONE 2009, 4, e5942. [Google Scholar] [CrossRef]
- Sepe, N.; De Petrocellis, L.; Montanaro, F.; Cimino, G.; Di Marzo, V. Bioactive long chain N-acylethanolamines in five species of edible bivalve molluscs. Possible implications for mollusc physiology and sea food industry. Biochim. Biophys. Acta 1998, 1389, 101–111. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Uyama, T.; Tsuboi, K.; Ueda, N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1546–1561. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T. Endogenous cannabinoids: Structure and metabolism. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 1–9. [Google Scholar] [CrossRef]
- Tsuboi, K.; Ikematsu, N.; Uyama, T.; Deutsch, D.G.; Tokumura, A.; Ueda, N. Biosynthetic pathways of bioactive N-acylethanolamines in brain. CNS Neurol. Disord. Drug Targets 2013, 12, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Balvers, M.G.; Verhoeckx, K.C.; Meijerink, J.; Wortelboer, H.M.; Witkamp, R.F. Measurement of palmitoylethanolamide and other N-acylethanolamines during physiological and pathological conditions. CNS Neurol. Disord. Drug Targets 2013, 12, 23–33. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Palmitoylethanolamide in homeostatic and traumatic central nervous system injuries. CNS Neurol. Disord. Drug Targets 2013, 12, 55–61. [Google Scholar] [CrossRef]
- Hansen, H.S. Effect of diet on tissue levels of palmitoylethanolamide. CNS Neurol. Disord. Drug Targets 2013, 12, 17–25. [Google Scholar] [CrossRef]
- Berdyshev, E.V.; Schmid, P.C.; Dong, Z.; Schmid, H.H. Stress-induced generation of N-acylethanolamines in mouse epidermal JB6 P+ cells. Biochem. J. 2000, 346, 369–374. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov. Today. 2014, 19, 1632–1639. [Google Scholar] [CrossRef]
- Rinne, P.; Guillamat-Prats, R.; Rami, M.; Bindila, L.; Ring, L.; Lyytikäinen, L.P.; Raitoharju, E.; Oksala, N.; Lehtimäki, T.; Weber, C.; et al. Palmitoylethanolamide promotes a proresolving macrophage phenotype and attenuates atherosclerotic plaque formation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2562–2575. [Google Scholar] [CrossRef]
- Roviezzo, F.; Rossi, A.; Caiazzo, E.; Orlando, P.; Riemma, M.A.; Iacono, V.M.; Guarino, A.; Ialenti, A.; Cicala, C.; Peritore, A.; et al. Palmitoylethanolamide supplementation during sensitization prevents airway allergic symptoms in the mouse. Front. Pharmacol. 2017, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Barbierato, M.; Zusso, M.; Bruschetta, G.; Impellizzeri, D.; Cuzzocrea, S.; Giusti, P. N-Palmitoylethanolamine and neuroinflammation: A novel therapeutic strategy of resolution. Mol. Neurobiol. 2015, 52, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Solorzano, C.; Zhu, C.; Battista, N.; Astarita, G.; Lodola, A.; Rivara, S.; Mor, M.; Russo, R.; Maccarrone, M.; Antonietti, F.; et al. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. USA 2009, 106, 20966–20971. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, S.; Brazis, P.; Miolo, A.; della Valle, M.F.; Puigdemont, A. Effects of palmitoylethanolamide on immunologically induced histamine, PGD2 and TNFα release from canine skin mast cells. Vet. Immunol. Immunopathol. 2010, 133, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Abramo, F.; Lazzarini, G.; Pirone, A.; Lenzi, C.; Albertini, S.; della Valle, M.F.; Schievano, C.; Vannozzi, I.; Miragliotta, V. Ultramicronized palmitoylethanolamide counteracts the effects of compound 48/80 in a canine skin organ culture model. Vet. Dermatol. 2017, 28, 456-e104. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, S.; Brazis, P.; della Valle, M.F.; Miolo, A.; Petrosino, S.; Di Marzo, V.; Puigdemont, A. Effects of palmitoylethanolamide on the cutaneous allergic inflammatory response in Ascaris hypersensitive Beagle dogs. Vet. J. 2012, 191, 377–382. [Google Scholar] [CrossRef]
- Scarampella, F.; Abramo, F.; Noli, C. Clinical and histological evaluation of an analogue of palmitoylethanolamide, PLR 120 (comicronized Palmidrol INN) in cats with eosinophilic granuloma and eosinophilic plaque: A pilot study. Vet. Dermatol. 2001, 12, 29–39. [Google Scholar] [CrossRef]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tüting, T.; Bisogno, T.; De Filippis, D.; D’Amico, A.; Saturnino, C.; et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 2010, 65, 698–711. [Google Scholar] [CrossRef]
- Bettoni, I.; Comelli, F.; Colombo, A.; Bonfanti, P.; Costa, B. Non-neuronal cell modulation relieves neuropathic pain: Efficacy of the endogenous lipid palmitoylethanolamide. CNS Neurol. Disord. Drug Targets 2013, 12, 34–44. [Google Scholar] [CrossRef]
- Luongo, L.; Guida, F.; Boccella, S.; Bellini, G.; Gatta, L.; Rossi, F.; de Novellis, V.; Maione, S. Palmitoylethanolamide reduces formalin-induced neuropathic-like behaviour through spinal glial/microglial phenotypical changes in mice. CNS Neurol. Disord. Drug Targets 2013, 12, 45–54. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Marmo, F.; Romano, R.; Iannotta, M.; Napolitano, F.; Belardo, C.; Marabese, I.; D’Aniello, A.; De Gregorio, D.; et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain 2015, 8, 47. [Google Scholar] [CrossRef]
- Gabrielsson, L.; Gouveia-Figueira, S.; Häggström, J.; Alhouayek, M.; Fowler, C.J. The anti-inflammatory compound palmitoylethanolamide inhibits prostaglandin and hydroxyeicosatetraenoic acid production by a macrophage cell line. Pharmacol. Res. Perspect. 2017, 5, e00300. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Smoum, R.; Mechoulam, R.; Maccarrone, M. Bioactive lipids ALIAmides differentially modulate inflammatory responses of distinct subsets of primary human T lymphocytes. FASEB J. 2018, 32, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Verde, R.; Allarà, M.; Imperatore, R.; Ligresti, A.; Mahmoud, A.M.; Peritore, A.F.; Iannotti, F.A.; Di Marzo, V. Palmitoylethanolamide counteracts substance P-induced mast cell activation in vitro by stimulating diacylglycerol lipase activity. J. Neuroinflamm. 2019, 16, 274. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249–255. [Google Scholar] [CrossRef]
- Ho, W.S.; Barrett, D.A.; Randall, M.D. Entourage effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Davis, J.B.; Di Marzo, V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001, 506, 253–256. [Google Scholar] [CrossRef]
- Ambrosino, P.; Soldovieri, M.V.; De Maria, M.; Russo, C.; Taglialatela, M. Functional and biochemical interaction between PPARalpha receptors and TRPV1 channels: Potential role in PPARalpha agonists-mediated analgesia. Pharmacol. Res. 2014, 87, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Soldovieri, M.V.; Russo, C.; Taglialatela, M. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARalpha agonist palmitoylethanolamide. Br. J. Pharmacol. 2013, 168, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.J. The endocannabinoid system of animals. Animals (Basel) 2019, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Barbero, R.; Vercelli, C.; Cuniberti, B.; Martano, M.; della Valle, M.F.; Re, G. Expression of functional TRPV1 receptors in primary culture of canine keratinocytes. J. Vet. Pharmacol. Ther. 2018, 41, 795–804. [Google Scholar] [CrossRef]
- Campora, L.; Miragliotta, V.; Ricci, E.; Cristino, L.; Di Marzo, V.; Albanese, F.; della Valle, M.F.; Abramo, F. Cannabinoid receptor type 1 and 2 expression in the skin of healthy dogs and dogs with atopic dermatitis. Am. J. Vet. Res. 2012, 73, 988–995. [Google Scholar] [CrossRef]
- Dall’Aglio, C.; Mercati, F.; Pascucci, L.; Boiti, C.; Pedini, V.; Ceccarelli, P. Immunohistochemical localization of CB1 receptor in canine salivary glands. Vet. Res. Commun. 2010, 34 (Suppl. 1), S9–S12. [Google Scholar] [CrossRef]
- Fernández-Trapero, M.; Espejo-Porras, F.; Rodríguez-Cueto, C.; Coates, J.R.; Pérez-Díaz, C.; de Lago, E.; Fernández-Ruiz, J. Upregulation of CB2 receptors in reactive astrocytes in canine degenerative myelopathy, a disease model of amyotrophic lateral sclerosis. Dis. Model. Mech. 2017, 10, 551–558. [Google Scholar] [CrossRef]
- Freundt-Revilla, J.; Kegler, K.; Baumgärtner, W.; Tipold, A. Spatial distribution of cannabinoid receptor type 1 (CB1) in normal canine central and peripheral nervous system. PLoS ONE 2017, 12, e0181064. [Google Scholar] [CrossRef]
- Freundt-Revilla, J.; Heinrich, F.; Zoerner, A.; Gesell, F.; Beyerbach, M.; Shamir, M.; Oevermann, A.; Baumgärtner, W.; Tipold, A. The endocannabinoid system in canine steroid-responsive meningitis-arteritis and intraspinal spirocercosis. PLoS ONE 2018, 13, e0187197. [Google Scholar] [CrossRef]
- Galiazzo, G.; Giancola, F.; Stanzani, A.; Fracassi, F.; Bernardini, C.; Forni, M.; Pietra, M.; Chiocchetti, R. Localization of cannabinoid receptors CB1, CB2, GPR55 and PPARalfa in the canine gastrointestinal tract. Histochem. Cell Biol. 2018, 150, 187–205. [Google Scholar] [CrossRef]
- Gebremedhin, D.; Lange, A.R.; Campbell, W.B.; Hillard, C.J.; Harder, D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999, 276, H2085–H2093. [Google Scholar] [CrossRef] [PubMed]
- Mercati, F.; Dall’Aglio, C.; Pascucci, L.; Boiti, C.; Ceccarelli, P. Identification of cannabinoid type 1 receptor in dog hair follicles. Acta Histochem. 2012, 114, 68–71. [Google Scholar] [CrossRef]
- Miragliotta, V.; Ricci, P.L.; Albanese, F.; Pirone, A.; Tognotti, D.; Abramo, F. Cannabinoid receptor types 1 and 2 and peroxisome proliferator-activated receptor-alpha: Distribution in the skin of clinically healthy cats and cats with hypersensitivity dermatitis. Vet. Dermatol. 2018, 29, 316-e111. [Google Scholar] [CrossRef] [PubMed]
- Ndong, C.; O’Donnell, D.; Ahmad, S.; Groblewski, T. Cloning and pharmacological characterization of the dog cannabinoid CB2receptor. Eur. J. Pharmacol. 2011, 669, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pirone, A.; Cantile, C.; Miragliotta, V.; Lenzi, C.; Giannessi, E.; Cozzi, B. Immunohistochemical distribution of the cannabinoid receptor 1 and fatty acid amide hydrolase in the dog claustrum. J. Chem. Neuroanat. 2016, 74, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pirone, A.; Lenzi, C.; Briganti, A.; Abbate, F.; Levanti, M.; Abramo, F.; Miragliotta, V. Spatial distribution of cannabinoid receptor 1 and fatty acid amide hydrolase in the cat ovary and oviduct. Acta Histochem. 2017, 119, 417–422. [Google Scholar] [CrossRef]
- Ponti, W.; Rubino, T.; Bardotti, M.; Poli, G.; Parolaro, D. Cannabinoids inhibit nitric oxide production in bone marrow derived feline macrophages. Vet. Immunol. Immunopathol. 2001, 82, 203–214. [Google Scholar] [CrossRef]
- Stanzani, A.; Galiazzo, G.; Giancola, F.; Tagliavia, C.; De Silva, M.; Pietra, M.; Fracassi, F.; Chiocchetti, R. Localization of cannabinoid and cannabinoid related receptors in the cat gastrointestinal tract. Histochem. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Feehan, K.T.; Gilroy, D.W. Is resolution the end of inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Flower, R.J. Prostaglandins, bioassay and inflammation. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S182–S192. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Cuzzocrea, S.; Crupi, R. An update of palmitoylethanolamide and luteolin effects in preclinical and clinical studies of neuroinflammatory events. Antioxidants (Basel) 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Lerner, R.; Pascual Cuadrado, D.; Post, J.M.; Lutz, B.; Bindila, L. Broad lipidomic and transcriptional changes of prophylactic PEA administration in adult mice. Front. Neurosci. 2019, 13, 527. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on nature: The role of nanomedicine in the development of clinical natural drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral ultramicronized palmitoylethanolamide: Plasma and tissue levels and spinal anti-hyperalgesic effect. Front Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Takano, R.; Furumoto, K.; Shiraki, K.; Takata, N.; Hayashi, Y.; Aso, Y.; Yamashita, S. Rate-limiting steps of oral absorption for poorly water-soluble drugs in dogs; prediction from a miniscale dissolution test and a physiologically-based computer simulation. Pharm. Res. 2008, 25, 2334–2344. [Google Scholar] [CrossRef]
- Leleux, J.; Williams, R.O. Recent advancements in mechanical reduction methods: Particulate systems. Drug. Dev. Ind. Pharm. 2014, 40, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Song, Y.; Peddie, F.; Evans, A.M. Particle size reduction to the nanometer range: A promising approach to improve buccal absorption of poorly water-soluble drugs. Int. J. Nanomed. 2011, 6, 1245–1251. [Google Scholar]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflamm. 2014, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Ultramicronized palmitoylethanolamide reduces inflammation in a Th1-mediated model of colitis. Eur. J. Inflamm. 2015, 13, 14–31. [Google Scholar] [CrossRef]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of palmitoylethanolamide for pain: A meta-analysis. Pain Phys. 2017, 20, 353–362. [Google Scholar] [PubMed]
- Re, G.; Barbero, R.; Miolo, A.; Di Marzo, V. Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animals. Vet. J. 2007, 173, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Fusco, M.; della Valle, M.F.; Zusso, M.; Costa, B.; Giusti, P. Palmitoylethanolamide, a naturally-occurring disease modifying agent in neuropathic pain. Inflammopharmacology 2014, 22, 79–94. [Google Scholar] [CrossRef]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef]
- Palazzo, E.; Luongo, L.; Guida, F.; de Novellis, V.; Boccella, S.; Marabese, I.; Maione, S.; Cristiano, C. Role of N-Acylethanolamines in the neuroinflammation: Ultramicronized palmitoylethanolamide in the relief of chronic pain and neurodegenerative diseases. Neuropsychiatry (London) 2018, 8, 739–744. [Google Scholar]
- Nestmann, E.R. Safety of micronized palmitoylethanolamide (microPEA): Lack of toxicity and genotoxic potential. Food Sci. Nutr. 2016, 5, 292–309. [Google Scholar] [CrossRef]
- Wise, L.E.; Cannavacciulo, R.; Cravatt, B.F.; Marun, B.F.; Lichtman, A.H. Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology 2008, 54, 181–188. [Google Scholar] [CrossRef]
- LoVerme, J.; Russo, R.; La Rana, G.; Fu, J.; Farthing, J.; Raso, G.; Meli, R.; Hohmann, A.; Calignano, A.; Piomelli, D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J. Pharmacol. Exp. Ther. 2006, 319, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Bottemanne, P.; Subramanian, K.V.; Lambert, D.M.; Makriyannis, A.; Cani, P.D.; Muccioli, G.G. N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. FASEB J. 2015, 29, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Plovier, H.; Hul, M.V.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids-at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Matias, I.; Lutz, B.; Borrelli, F.; Capasso, F.; Marsicano, G.; Mascolo, N.; Petrosino, S.; Monory, K.; Valenti, M.; et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology 2005, 129, 941–951. [Google Scholar] [CrossRef]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-brain axis: Role of lipids in the regulation of inflammation, pain and CNS diseases. Curr. Med. Chem. 2018, 25, 3930–3952. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alfa activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef]
- Karwad, M.A.; Macpherson, T.; Wang, B.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARalpha. FASEB J. 2017, 31, 469–481. [Google Scholar] [CrossRef]
- Sarnelli, G.; Seguella, L.; Pesce, M.; Lu, J.; Gigli, S.; Bruzzese, E.; Lattanzi, R.; D’Alessandro, A.; Cuomo, R.; Steardo, L.; et al. HIV-1 Tat-induced diarrhea is improved by the PPARalpha agonist, palmitoylethanolamide, by suppressing the activation of enteric glia. J. Neuroinflamm. 2018, 15, 94. [Google Scholar] [CrossRef]
- Pengo, G.; Miolo, A. Utilizzo di Palmitoiletanolamide micronizzata nell’infiammazione gastrointestinale idiopatica (IBD) del cane: Descrizione di 7 casi clinici. In Proceedings of the 72 International SCIVAC Congress, Milan, Italy, 23–25 March 2012; pp. 299–301. [Google Scholar]
- Carta, G.; Murru, E.; Vargiu, R.; Collu, M.; Carta, M.; Banni, S.; Stancampiano, R. Essential fatty acids deficient diet modulates N-Acylethanolamide profile in rat’s tissues. Prostaglandins Leukot. Essent. Fatty Acids. 2020, 153, 102053. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.A. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Izzo, A. Palmitoylethanolamide, a naturally-occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015, 172, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Orlando, P.; Pagano, E.; Aveta, T.; Buono, L.; Borrelli, F.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: Involvement of CB1 receptors and TRPV1 channels. Br. J. Pharmacol. 2014, 171, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and cannabidiol prevent inflammation-induced hyperpermeability of the human gut in vitro and in vivo-a randomized, placebo-controlled, double-blind controlled trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Impellizzeri, D.; Torre, A.; Mazzon, E.; Cappellani, A.; Faggio, C.; Esposito, E.; Trischitta, F.; Cuzzocrea, S. Effects of palmitoylethanolamide on intestinal injury and inflammation caused by ischemia-reperfusion in mice. J. Leukoc. Biol. 2012, 91, 911–920. [Google Scholar] [CrossRef]
- Hasenoehrl, C.; Storr, M.; Schicho, R. Cannabinoids for treating inflammatory bowel diseases: Where are we and where do we go? Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 329–337. [Google Scholar] [CrossRef]
- Karwad, M.A.; Couch, D.G.; Wright, K.L.; Tufarelli, C.; Larvin, M.; Lund, J.; O’Sullivan, S.E. Endocannabinoids and endocannabinoid-like compounds modulate hypoxia-induced permeability in CaCo-2 cells via CB1, TRPV1, and PPARa. Biochem. Pharmacol. 2019, 168, 465–472. [Google Scholar] [CrossRef]
- Cristiano, C.; Pirozzi, C.; Coretti, L.; Cavaliere, G.; Lama, A.; Russo, R.; Lembo, F.; Mollica, M.P.; Meli, R.; Calignano, A.; et al. Palmitoylethanolamide counteracts autistic-like behaviours in BTBR T+tf/J mice: Contribution of central and peripheral mechanisms. Brain Behav. Immun. 2018, 74, 166–175. [Google Scholar] [CrossRef]
- Pesce, M.; D’Alessandro, A.; Borrelli, O.; Gigli, S.; Seguella, L.; Cuomo, R.; Esposito, G.; Sarnelli, G. Endocannabinoid-related compounds in gastrointestinal diseases. J. Cell Mol. Med. 2018, 22, 706–715. [Google Scholar] [CrossRef]
- Pesce, M.; Esposito, G.; Sarnelli, G. Endocannabinoids in the treatment of gastrointestinal inflammation and symptoms. Curr. Opin. Pharmacol. 2018, 43, 81–86. [Google Scholar] [CrossRef]
- Sarnelli, G.; Gigli, S.; Capoccia, E.; Iuvone, T.; Cirillo, C.; Seguella, L.; Nobile, N.; D’Alessandro, A.; Pesce, M.; Steardo, L.; et al. Palmitoylethanolamide exerts antiproliferative effect and downregulates VEGF signaling in Caco-2 human colon carcinoma cell line through a selective PPAR-a-dependent inhibition of Akt/mTOR pathway. Phytother. Res. 2016, 30, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, J.; Kulkarni, A.; Wang, W.; Garg, S.; Prather, P.L.; Hauer-Jensen, M. Palmitoylethanolamide regulates development of intestinal radiation injury in a mast cell-dependent manner. Dig. Dis. Sci. 2014, 59, 2693–2703. [Google Scholar] [CrossRef]
- Cremon, C.; Stanghellini, V.; Barbaro, M.R.; Cogliandro, R.F.; Bellacosa, L.; Santos, J.; Vicario, M.; Pigrau, M.; Cotoner, C.A.; Lobo, B.; et al. Randomised clinical trial: The analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment Pharmacol. Ther. 2017, 45, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Naslain, D.; Backhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Geurts, L.; Everard, A.; Van Hul, M.; Essaghir, A.; Duparc, T.; Matamoros, S.; Plovier, H.; Castel, J.; Denis, R.G.; Bergiers, M.; et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat. Commun. 2015, 6, 6495. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Piscitelli, F.; De Filippis, F.; Paino, S.; Ricciardi, F.; Siniscalco, D.; Marabese, I.; et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav. Immun. 2020, 85, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Bonechi, E.; Provensi, G.; Costa, A.; Clarke, G.; Ballerini, C.; De Filippo, C.; Passani, M.B. Oleoylethanolamide treatment affects gut microbiota composition and the expression of intestinal cytokines in Peyer’s patches of mice. Sci. Rep. 2018, 8, 14881. [Google Scholar] [CrossRef] [PubMed]
- Fornelos, N.; Franzosa, E.A.; Bishai, J.; Annand, J.W.; Oka, A.; Lloyd-Price, J.; Arthur, T.D.; Garner, A.; Avila-Pacheco, J.; Haiser, H.J.; et al. Growth effects of N-acylethanolamines on gut bacteria reflect altered bacterial abundances in inflammatory bowel disease. Nat. Microbiol. 2020, 5, 486–497. [Google Scholar] [CrossRef]
- Barutta, F.; Bruno, G.; Mastrocola, R.; Bellini, S.; Gruden, G. The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int. 2018, 94, 252–258. [Google Scholar] [CrossRef]
- Izzo, A.A.; Muccioli, G.G.; Ruggieri, M.R.; Schicho, R. Endocannabinoids and the digestive tract and bladder in health and disease. Handb. Exp. Pharmacol. 2015, 231, 423–447. [Google Scholar]
- Merriam, F.V.; Wang, Z.Y.; Guerios, S.D.; Bjorling, D.E. Cannabinoid receptor 2 is increased in acutely and chronically inflamed bladder of rats. Neurosci. Lett. 2008, 445, 130–134. [Google Scholar] [CrossRef]
- Petrosino, S.; Puigdemont, A.; della Valle, M.F.; Fusco, M.; Verde, R.; Allarà, M.; Orlando, P.; Aveta, T.; Di Marzo, V. Adelmidrol increases the endogenous concentrations of palmitoylethanolamide in canine keratinocytes and down-regulates an inflammatory reaction in an in vitro model of contact allergic dermatitis. Vet. J. 2016, 207, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Piscitelli, F.; Pinach, S.; Bruno, G.; Gambino, R.; Rastaldi, M.P.; Salvidio, G.; Di Marzo, V.; Cavallo Perin, P.; Gruden, G. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 2011, 60, 2386–2396. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Locatelli, M.; Corna, D.; Villa, S.; Rottoli, D.; Nava, V.; Verde, R.; Piscitelli, F.; Di Marzo, V.; Fingerle, J.; et al. Therapy with a selective cannabinoid receptor type 2 agonist limits albuminuria and renal injury in mice with type 2 diabetic nephropathy. Nephron 2016, 132, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L.S.; Iannotti, F.A.; Veneziani, L.; Borelli-Tôrres, R.T.; De Maio, F.; Piscitelli, F.; Reis, R.A.M.; Di Marzo, V.; Einicker-Lamas, M. Experimental ischemia/reperfusion model impairs endocannabinoid signaling and Na+/K+ ATPase expression and activity in kidney proximal tubule cells. Biochem. Pharmacol. 2018, 154, 482–491. [Google Scholar] [CrossRef]
- Di Paola, R.; Impellizzeri, D.; Mondello, P.; Velardi, E.; Aloisi, C.; Cappellani, A.; Esposito, E.; Cuzzocrea, S. Palmitoylethanolamide reduces early renal dysfunction and injury caused by experimental ischemia and reperfusion in mice. Shock 2012, 38, 356–366. [Google Scholar] [CrossRef]
- Godlewski, G.; Offertáler, L.; Wagner, J.A.; Kunos, G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009, 89, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Esposito, E.; Attley, J.; Cuzzocrea, S. Targeting inflammation: New therapeutic approaches in Chronic Kidney Disease (CKD). Pharmacol. Res. 2014, 81, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Smart, D.; Jonsson, K.O.; Vandevoorde, S.; Lambert, D.M.; Fowler, C.J. ‘Entourage’ effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br. J. Pharmacol. 2002, 136, 452–458. [Google Scholar] [CrossRef]
- Cordaro, M.; Impellizzeri, D.; Bruschetta, G.; Siracusa, R.; Crupi, R.; Di Paola, R.; Esposito, E.; Cuzzocrea, S. A novel protective formulation of Palmitoylethanolamide in experimental model of contrast agent induced nephropathy. Toxicol. Lett. 2016, 240, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Mattace Raso, G.; Simeoli, R.; Russo, R.; Santoro, A.; d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Paciello, O.; Orefice, N.; Meli, R.; Calignano, A. N-palmitoylethanolamide protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress. Pharmacol. Res. 2013, 76, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Bruschetta, G.; Ahmad, A.; Crupi, R.; Siracusa, R.; Di Paola, R.; Paterniti, I.; Prosdocimi, M.; Esposito, E.; Cuzzocrea, S. Effects of Palmitoylethanolamide and silymarin combination treatment in an animal model of kidney ischemia and reperfusion. Eur. J. Pharmacol. 2015, 762, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Merriam, F.V.; Wang, Z.Y.; Hillard, C.J.; Stuhr, K.L.; Bjorling, D.E. Inhibition of fatty acid amide hydrolase suppresses referred hyperalgesia induced by bladder inflammation. BJU Int. 2011, 108, 1145–1149. [Google Scholar] [CrossRef]
- Pessina, F.; Capasso, R.; Borrelli, F.; Aveta, T.; Buono, L.; Valacchi, G.; Fiorenzani, P.; Di Marzo, V.; Orlando, P.; Izzo, A.A. Protective effect of palmitoylethanolamide, a naturally-occurring molecule, in a rat model of cystitis. J. Urol. 2015, 193, 1401–1408. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Jaggar, S.I.; Rice, A.S. Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB(1) and CB(2)-like receptors. Pain 2002, 97, 11–21. [Google Scholar] [CrossRef]
- Jaggar, S.I.; Hasnie, F.S.; Sellaturay, S.; Rice, A.S. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain 1998, 76, 189–199. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Rice, A.S. A novel neuroimmune mechanism in cannabinoid-mediated attenuation of nerve growth factor-induced hyperalgesia. Anesthesiology 2003, 99, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Farquhar-Smith, W.P.; Rice, A.S. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology 2001, 94, 507–513. [Google Scholar] [CrossRef]
- Petrini, D.; Di Giuseppe, M.; Deli, G.; De Caro Carella, C. Cystolithiasis in a Syrian hamster: A different outcome. Open Vet. J. 2016, 6, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Lamont, L.A. Multimodal pain management in veterinary medicine: The physiologic basis of pharmacologic therapies. Vet. Clin. North Am. Small Anim. Pract. 2008, 38, 1173–1186. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D.; Sasso, O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 2014, 17, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Koltyn, K.F.; Brellenthin, A.G.; Cook, D.B.; Sehgal, N.; Hillard, C. Mechanisms of exercise-induced hypoalgesia. J. Pain 2014, 15, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a special food for medical purposes, in the treatment of chronic pain: A pooled data meta-analysis. Pain Phys. 2016, 19, 11–24. [Google Scholar]
- Cruccu, G.; Di Stefano, G.; Marchettini, P.; Truini, A. Micronized palmitoylethanolamide: A post-hoc analysis of a controlled study in over 600 patients with low back pain-sciatica. CNS Neurol. Disord. Drug Targets 2019, 18, 491–495. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Luongo, L.; Cipriano, M.; Palazzo, E.; Cinelli, M.P.; de Novellis, V.; Maione, S.; Iuvone, T. Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol. Pain 2011, 7, 3. [Google Scholar] [CrossRef]
- Esposito, E.; Paterniti, I.; Mazzon, E.; Genovese, T.; Di Paola, R.; Galuppo, M.; Cuzzocrea, S. Effects of palmitoylethanolamide on release of mast cell peptidases and neurotrophic factors after spinal cord injury. Brain Behav. Immun. 2011, 25, 1099–1112. [Google Scholar] [CrossRef]
- Genovese, T.; Esposito, E.; Mazzon, E.; Di Paola, R.; Meli, R.; Bramanti, P.; Piomelli, D.; Calignano, A.; Cuzzocrea, S. Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. J. Pharmacol. Exp. Ther. 2008, 326, 12–23. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol. Neurobiol. 2013, 48, 340–352. [Google Scholar] [CrossRef]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef]
- Vezzoni, A.; Crupi, F.; Boiocchi, S.; Boano, S. Effect of palmitoylethanolamide co-ultra micronized with quercetin in dogs with osteoarthritis by means of dynamic gate analysis and canine brief pain inventory questionnaire. In Proceedings of the 5th World Veterinary Orthopaedic Congress ESVOT-VOS, Barcelona, Spain, 12–15 September 2018; pp. 771–772. [Google Scholar]
- Neugebauer, V.; Han, J.S.; Adwanikar, H.; Fu, Y.; Ji, G. Techniques for assessing knee joint pain in arthritis. Mol. Pain 2007, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Orita, S.; Ishikawa, T.; Miyagi, M.; Ochiai, N.; Inoue, G.; Eguchi, Y.; Kamoda, H.; Arai, G.; Toyone, T.; Aoki, Y.; et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet. Disord. 2011, 12, 134. [Google Scholar] [CrossRef]
- Della Valle, M.F.; Mortellaro, C.M.; Miolo, A.; Costa, B. Aliamides for pain management of osteoarthritis. In Proceedings of the 3rd Vepra Conference, Rimini, Italy, 31 May–1 June 2013; pp. 37–44. [Google Scholar]
- Cordaro, M.; Siracusa, R.; Impellizzeri, D.; D’Amico, R.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Fusco, R.; Di Paola, R.; Schievano, C.; et al. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. Ther. 2019, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Miolo, A.; della Valle, M.F.; Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Di Paola, R.; Cuzzocrea, S. Micronized palmitoyl-glucosamine, alone or co-micronized with curcumin, decreases inflammation, chondrodegeneration and pain: A preclinical study. In Proceedings of the 5th World Veterinary Orthopaedic Congress ESVOT-VOS, Barcelona, Spain, 12–15 September 2018; pp. 635–636. [Google Scholar]
- De Filippis, D.; D’Amico, A.; Cinelli, M.P.; Esposito, G.; Di Marzo, V.; Iuvone, T. Adelmidrol, a palmitoylethanolamide analogue, reduces chronic inflammation in a carrageenin-granuloma model in rats. J. Cell Mol. Med. 2009, 13, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Di Paola, R.; Cordaro, M.; Gugliandolo, E.; Casili, G.; Morittu, V.M.; Britti, D.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 2016, 119, 27–41. [Google Scholar] [CrossRef]
- Abramo, F.; Salluzzi, D.; Leotta, R.; Auxilia, S.; Noli, C.; Miolo, A.; Mantis, P.; Lloyd, D.H. Mast cell morphometry and densitometry in experimental skin wounds treated with a gel containing adelmidrol: A placebo controlled study. Wounds 2008, 20, 149–157. [Google Scholar]
- Mantis, P.; Lloyd, D.H.; Pfeiffer, D.; Stevens, K.; Auxilia, S.; Noli, C.; Abramo, F.; Miolo, A. Assessment of the effect of an aliamide-containing topical gel by evaluation of the reduction of wound volume measured by high resolution ultrasound biomicroscopy. Wounds 2007, 19, 113–119. [Google Scholar]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Gugliandolo, E.; Peritore, A.F.; Di Paola, R.; Cuzzocrea, S. Topical application of adelmidrol + trans-traumatic acid enhances skin wound healing in a streptozotocin-induced diabetic mouse model. Front. Pharmacol. 2018, 9, 871. [Google Scholar] [CrossRef]
- Pulvirenti, N.; Nasca, M.R.; Micali, G. Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: A pilot study. Acta Dermatovenerol. Croat. 2007, 15, 80–83. [Google Scholar]
- Karatzi, C.; Stefanidou, M.; Chaniotis, V.; Evangelou, G.; Krueger-Krasagakis, S.; Krasagakis, K. Treatment of giant vulvar syringomas with topical adelmidrol: The role of mast cells. Australas. J. Dermatol. 2018, 59, e305–e307. [Google Scholar] [CrossRef]
- Cerrato, S.; Brazis, P.; della Valle, M.F.; Miolo, A.; Puigdemont, A. Inhibitory effect of topical Adelmidrol on antigen-induced skin wheal and mast cell behavior in a canine model of allergic dermatitis. BMC Vet. Res. 2012, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, F.; Leone, F. Topical adelmidrol (2%) in the management of pruritus associated with atopic dermatitis in dogs-An observational study. Veterinaria 2013, 27, 27–35. [Google Scholar]
- Bonello, D.; Squarzoni, P. Effect of a mucoadhesive gel and dental scaling on gingivitis in dogs. J. Vet. Dent. 2008, 25, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Zerweck, C.; Grove, G.; Fraser, J. Efficacy of S236 cream in promoting barrier repair of razor-induced skin trauma. J. Am. Acad. Dermatol. 2006, 54 (Suppl. 3), AB81. [Google Scholar]
- Vaia, M.; Petrosino, S.; De Filippis, D.; Negro, L.; Guarino, A.; Carnuccio, R.; Di Marzo, V.; Iuvone, T. Palmitoylethanolamide reduces inflammation and itch in a mouse model of contact allergic dermatitis. Eur. J. Pharmacol. 2016, 791, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R.; Joyce, J.; Nicklin, C.; Lopez, J. Evaluation of the effects of Palmitoylethanolamide on clinical signs in house dust mite allergic high IgE Beagle dogs using a randomized, double blinded, placebo controlled design. Vet. Dermatol. 2005, 16, 202. [Google Scholar]
- Noli, C.; della Valle, M.F.; Miolo, A.; Medori, C.; Schievano, C.; Skinalia Clinical Group. Efficacy of ultra-micronized palmitoylethanolamide in canine atopic dermatitis: An open-label multi-centre study. Vet. Dermatol. 2015, 26, 432-e101. [Google Scholar] [CrossRef]
- Noli, C.; della Valle, M.F.; Miolo, A.; Medori, C.; Schievano, C.; Skinalia Clinical Group. Effect of dietary supplementation with ultramicronized palmitoylethanolamide in maintaining remission in cats with nonflea hypersensitivity dermatitis: A double blind, multicentre, randomized, placebo-controlled study. Vet. Dermatol. 2019, 30, 387-e117. [Google Scholar] [CrossRef]



| Food Source | ng/g f.w. | Reference |
|---|---|---|
| Soy lecithin | 950,000 | [23] |
| Soybean (Glycine max) | 6700 | [23,24] |
| Green coffee (depending on the variety) | 2830-11,940 | [27] |
| Raw peanuts | ~7770 | [26] |
| Roasted coffee | 7200 | [27] |
| Peanuts (Arachis hypogaea) | 3730 | [23,24] |
| Alfalfa (Medicago sativa) | 1150 | [24] |
| Refined wheat flour | ~ 800 * | [25] |
| Whole wheat flour | ~ 400 * | [25] |
| Raw pearl barley | ~330 | [26] |
| Walnuts | ~ 250 * | [25] |
| Toasted pearl barley | ~220 | [26] |
| Corn | 200 | [23] |
| Black-eyed peas (Vigna unguiculata) | 138 | [24] |
| Broccoli | ~130 * | [25] |
| Tuna fish | ~120 * | [25] |
| Chicken | ~120 * | [25] |
| Carrots | ~110 * | [25] |
| Eggs | ~ 100 * | [25] |
| Tomato | 100 | [23] |
| Garden pea (Pisum sativum) | 100 | [23,24] |
| Beans | ~90 * | [25] |
| Lettuce | ~70 * | [25] |
| Beef | ~ 60 * | [25] |
| Codfish | ~ 60 * | [25] |
| Common bean (Phaseolus vulgaris) | 53.5 | [24] |
| Cauliflowers | ~50 * | [25] |
| Chickpeas | ~40 * | [25] |
| Anchovies | ~ 40 * | [25] |
| Cow’s milk | ~ 30 * | [25] |
| Almonds | ~ 10 * | [25] |
| Grapes | ~10 * | [25] |
| Oranges | ~10 * | [25] |
| Apples | ~8 * | [25] |
| Lentils | ~6 * | [25] |
| Potatoes | 5 * | [25] |
| Elk milk | 1.81 | [30] |
| Bovine milk | 0.25 | [30] |
| SYSTEM/TISSUE | AREA/CELL | CB1 | CB2 | GPR55 | PPARα | TRPV1 |
|---|---|---|---|---|---|---|
| CNS | Hippocampus | ⬤ | ||||
| Claustrum | ◓ | |||||
| Cerebral cortex | ◓ | |||||
| Cornu Ammonis | ◓ | |||||
| Midbrain | ◓ | |||||
| Cerebellum | ◓ | |||||
| Medulla oblongata | ◓ | |||||
| Spinal cord | ◓ | |||||
| Spinal glial cells | ◓ | |||||
| Astrocytes | ◓ | ◓ | ||||
| Cerebral arterial smooth muscle cells | ◒ | |||||
| PNS | Dorsal root ganglia (neurons, satellite cells) | ◓ | ||||
| Schwann cells | ◓ | |||||
| SKIN | Dermal papillae | ◒ | ||||
| Hair follicles | ◓ | ◓ | ◒ | |||
| Hair bulb cells | ◒ | ◒ | ||||
| Sebaceous glands | ⬤ | ⬤ | ||||
| Sweat glands | ◓ | ⬤ | ||||
| Keratinocytes | ⬤ | ⬤ | ◒ | ◓ | ||
| Mast cells | ◓ | ◓ | ||||
| Fibroblasts | ◓ | ◓ | ||||
| GI TRACT | Salivary glands | ◓ | ||||
| Lamina propria cells | ◓ | |||||
| Enterocytes | ◓ | ◒ | ||||
| Mast cells | ◓ | |||||
| Immunocytes | ◓ | ◓ | ◒ | ⬤ | ||
| Smooth muscle cells | ◓ | ⬤ | ⬤ | |||
| Macrophages | ◒ | ⬤ | ||||
| Submucosal plexus (neurons and glial cells) | ◓ | |||||
| Myenteric plexus glial cells | ◓ | |||||
| Myenteric plexus neurons | ◒ | |||||
| Intestinal enteroendocrine cells | ◒ | ◒ | ◒ | |||
| Goblet cells | ◒ | |||||
| Enteric neurons | ◒ | |||||
| Enteroglial cells | ◒ | |||||
| CIRCULATORY SYSTEM | Lymph nodes | ⬤ | ||||
| B cells | ◓ | |||||
| Endothelial cells | ◓ | ◓ | ||||
| Spleen | ◓ | |||||
| GENITAL TRACT | Ovary | ◒ | ||||
| Oviducts | ◒ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugliandolo, E.; Peritore, A.F.; Piras, C.; Cuzzocrea, S.; Crupi, R. Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing. Vet. Sci. 2020, 7, 78. https://doi.org/10.3390/vetsci7020078
Gugliandolo E, Peritore AF, Piras C, Cuzzocrea S, Crupi R. Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing. Veterinary Sciences. 2020; 7(2):78. https://doi.org/10.3390/vetsci7020078
Chicago/Turabian StyleGugliandolo, Enrico, Alessio Filippo Peritore, Cristian Piras, Salvatore Cuzzocrea, and Rosalia Crupi. 2020. "Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing" Veterinary Sciences 7, no. 2: 78. https://doi.org/10.3390/vetsci7020078
APA StyleGugliandolo, E., Peritore, A. F., Piras, C., Cuzzocrea, S., & Crupi, R. (2020). Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing. Veterinary Sciences, 7(2), 78. https://doi.org/10.3390/vetsci7020078








