Effect of the Age and Body Weight of the Broiler Breeders Male on the Presentation of Oxidative Stress and Its Correlation with the Quality of Testicular Parenchyma and Physiological Antioxidant Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. A Sampling of Testis for Histologic Interpretation and Oxidative Stress Assessment
2.3. Histological Analysis of the Testicular Parenchyma
2.4. Description of the Histologic Assessment
2.5. Oxidative Stress and Antioxidant Capacity in Testicular Tissue
2.5.1. Malondialdehyde (MDA) Content
2.5.2. Non-Enzymatic Antioxidant Capacity
2.5.3. Antioxidant Enzyme Activity Assays
Glutathione Peroxidase (GPx)
Glutathione Reductase (GR)
Glutathione -S-Transferase (GST)
2.6. Total Protein Determination
2.7. Statistical Analysis
3. Results
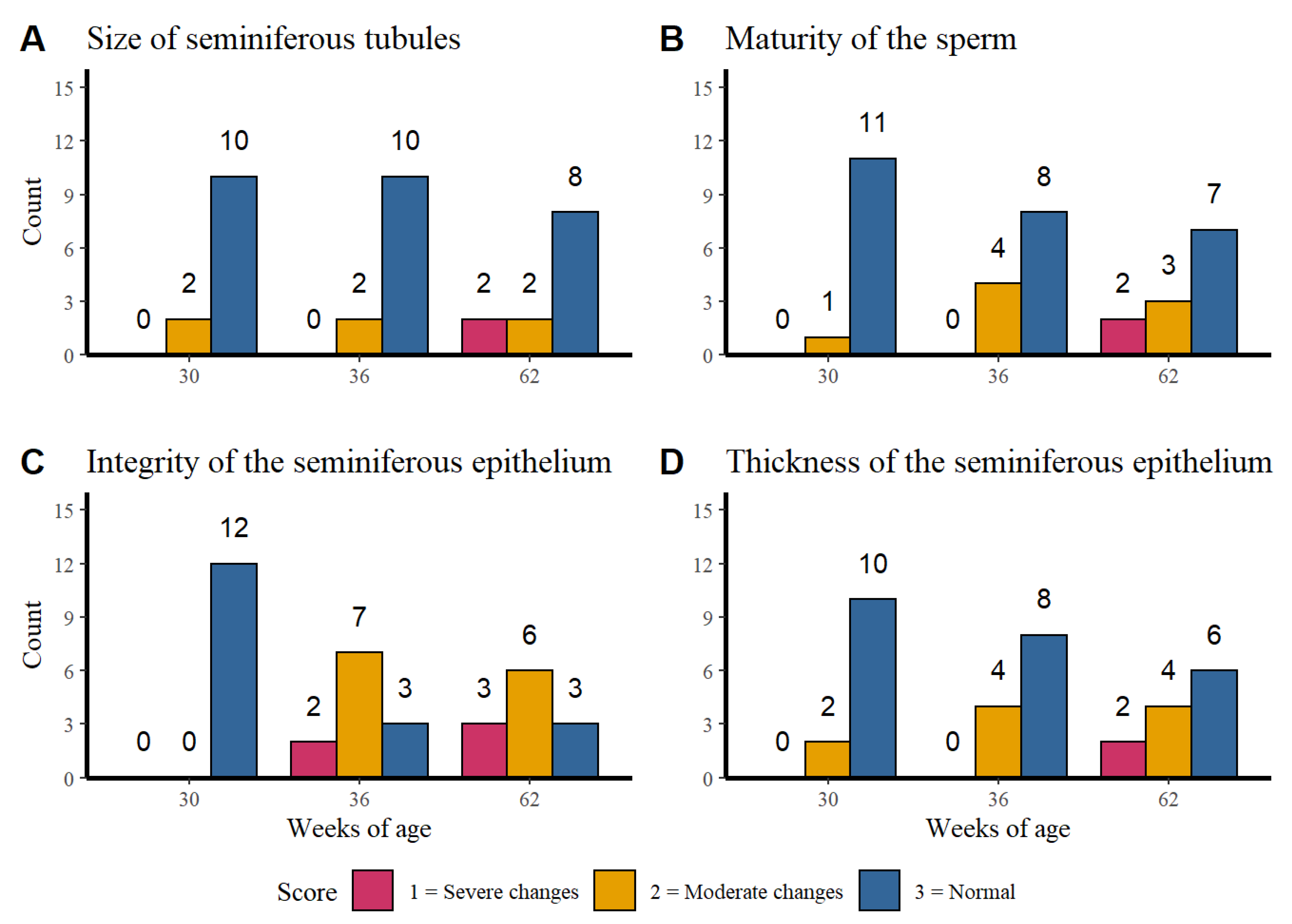
3.1. Corporal Weight, Age, and Integrity of Testicular Parenchyma
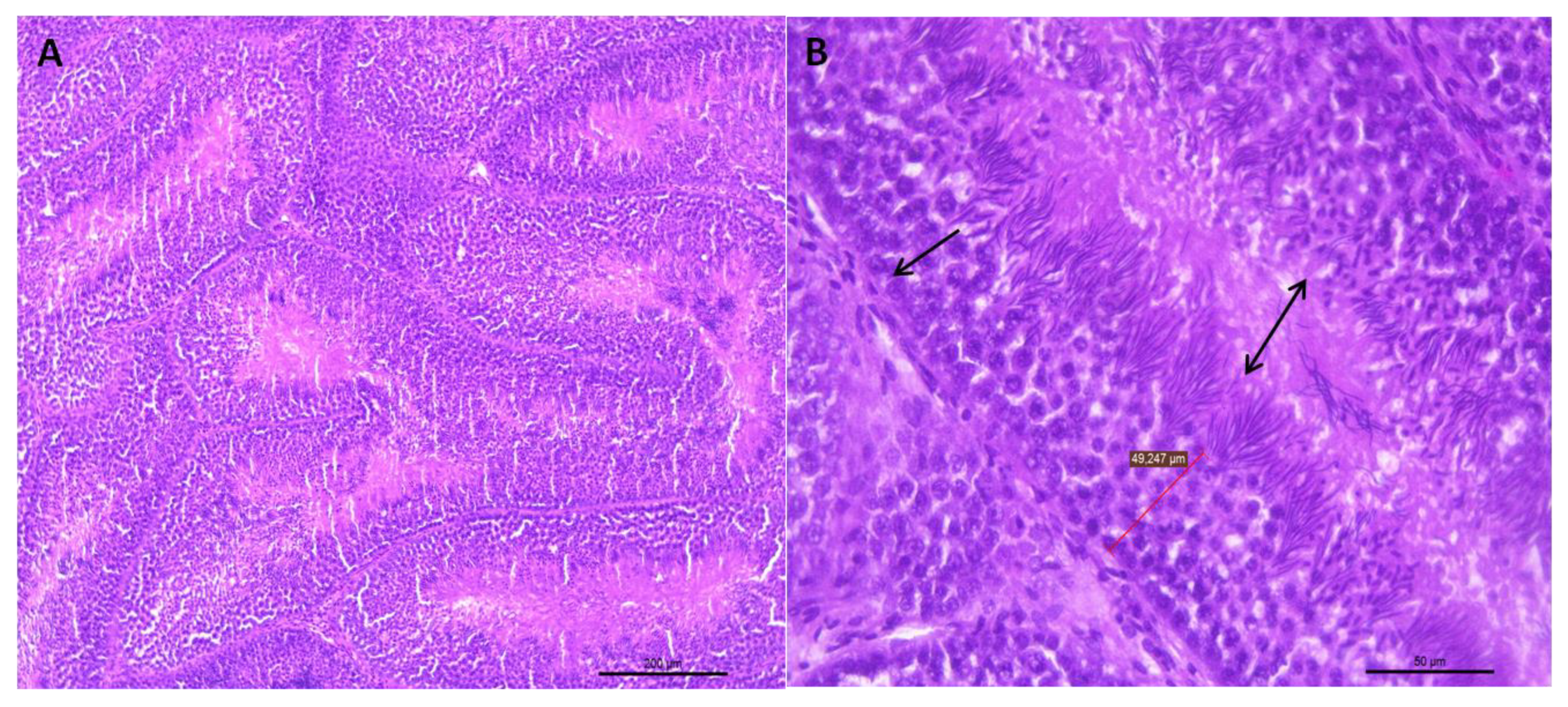
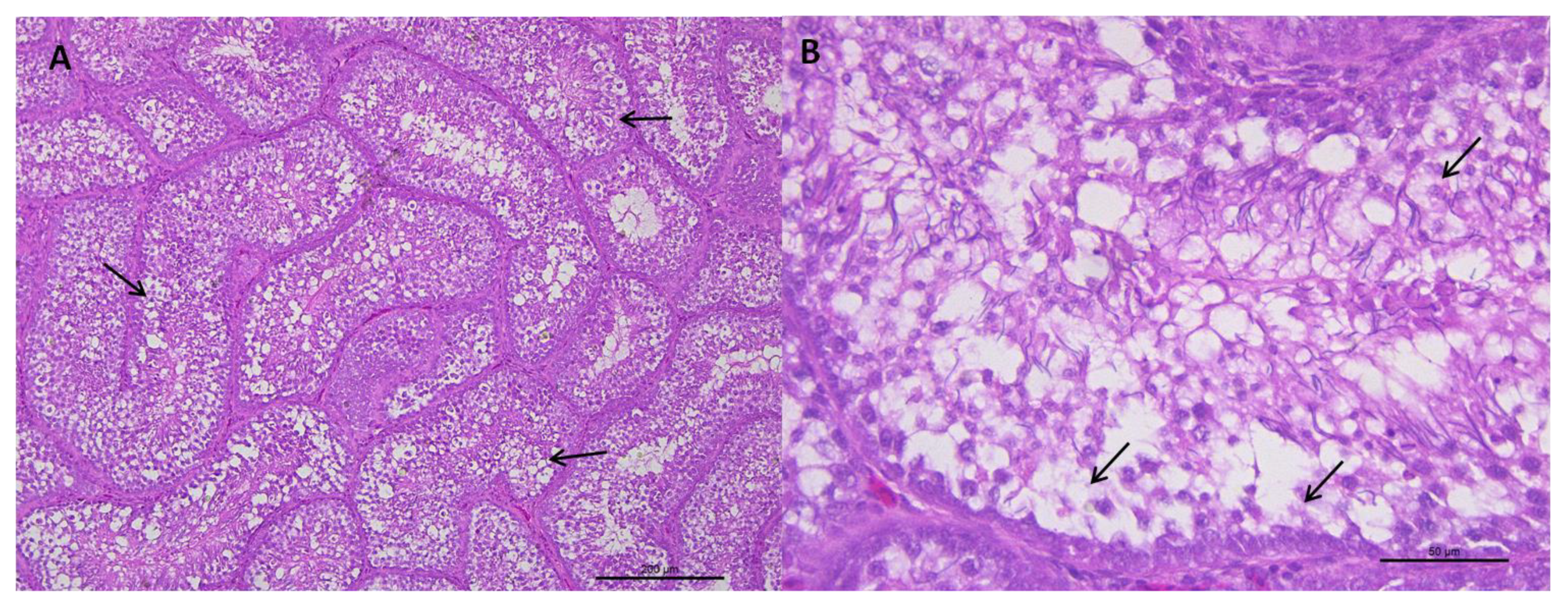
3.1.1. Histological Analysis of the Testicular Parenchyma
3.1.2. Integrity of the Seminiferous Epithelium
3.2. Correlation of Body Weight, Age with Oxidative Stress and Integrity of Testicular Parenchyma
3.3. Biochemical Correlations
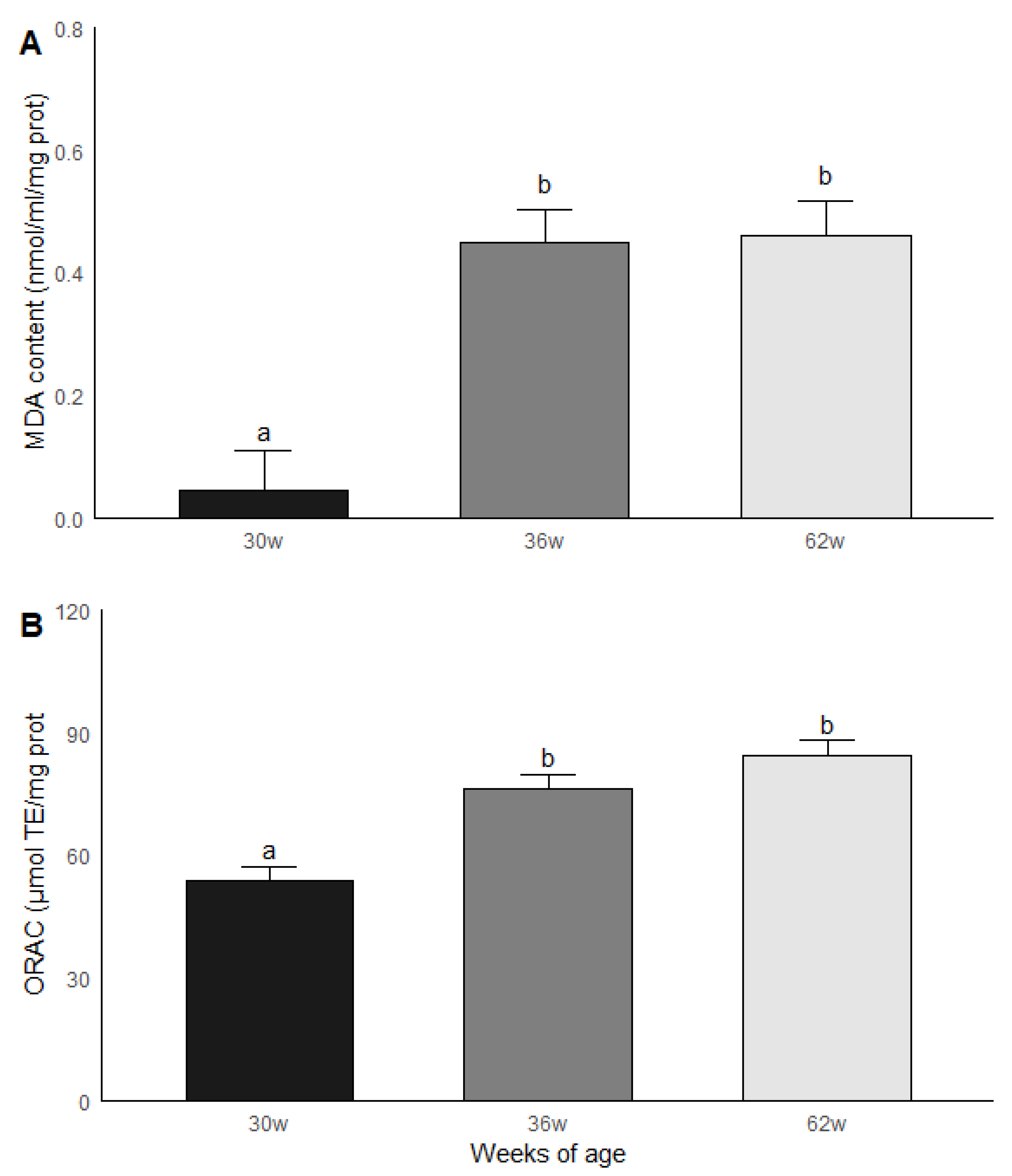
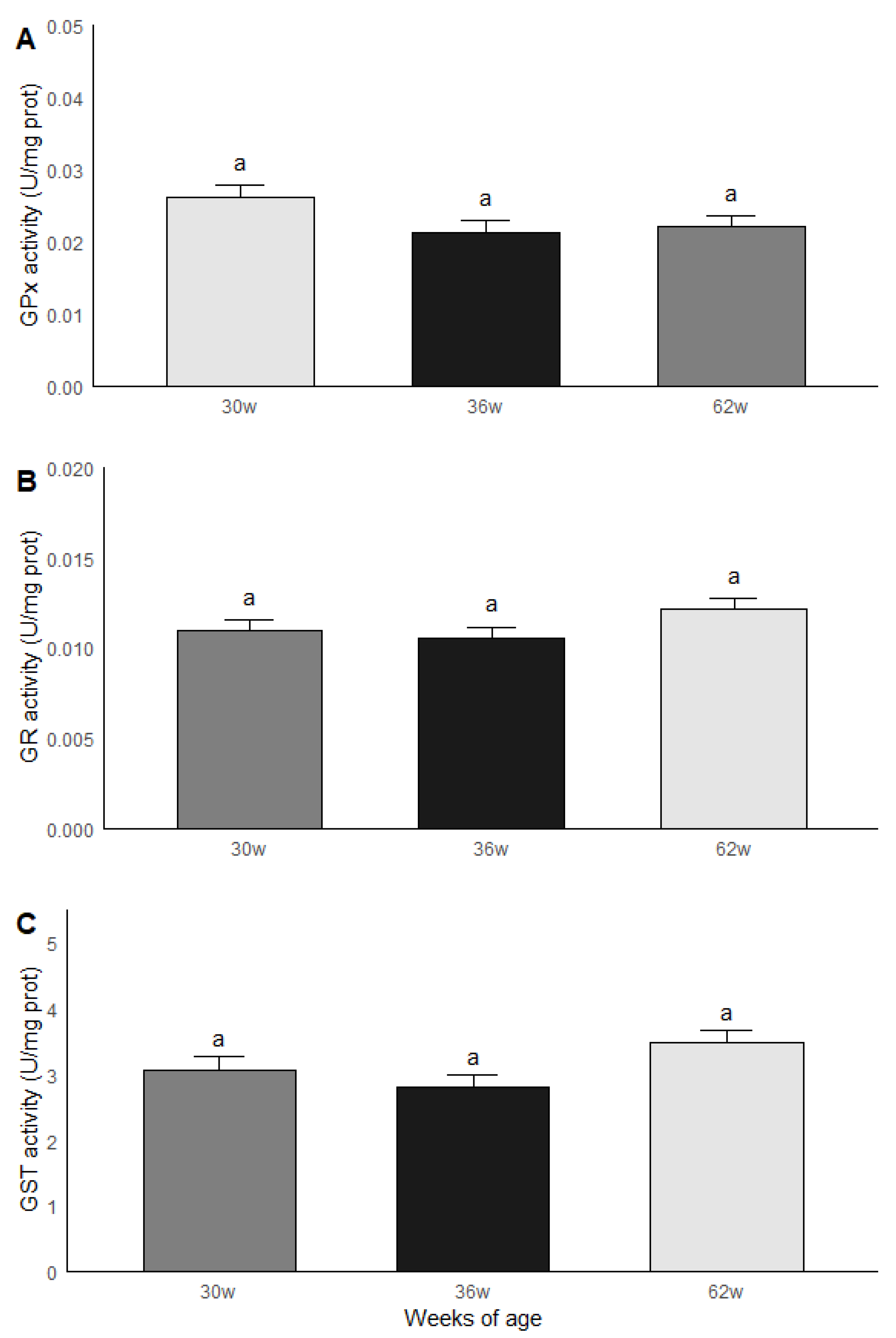
3.4. Age Group Comparisons: Oxidative Stress and Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canela, U.L. Avicultura Info Home Page. Available online: http://www.avicultura.info/factores-que-pueden-afectar-al-desarrollo-testicular-del-gallo (accessed on 6 November 2018).
- Gary, D.M.; Estevez, I.; Bakst, M.R.; Pollock, D.L. Phenotypic traits as reliable indicators of fertility in male broiler breeders. Poult. Sci. 2002, 81, 102–111. [Google Scholar]
- Aviagen Home Page. Available online: http://es.aviagen.com/tech-center/search (accessed on 12 December 2018).
- Blesbois, E. Biological features of the avian male gamete and their application to biotechnology of conservation. J. Poult. Sci. 2012, 49, 141–149. [Google Scholar] [CrossRef]
- Herrera, A.; Shuster, S.G.; Perriton, C.L.; Cohn, M.J. Developmental basics of phallus reduction during bird evolution. Curr. Biol. 2013, 23, 1065–1074. [Google Scholar] [CrossRef]
- Sarabia, F.J.; Pizarro, D.M.; Abad, M.J.C.; Casanovas, I.P.; Rodriguez-Bertos, A.; Barger, K. Relationships between fertility and some parameters in male broiler breeders (body and testicular weight, histology and immunohistochemistry of testes, spermatogenesis and hormonal levels). Reprod. Domest. Anim. 2013, 48, 345–352. [Google Scholar] [CrossRef]
- Catt, J.K.; Harwood, J.P.; Clayton, R.N.; Davies, T.F.; Chan, V.; Katikineni, M.; Nozu, K.; Dufau, M.L. Regulation of peptide hormone receptors and gonadal steroidogenesis. Recent Prog. Horm. Res. 1980, 36, 557–662. [Google Scholar]
- De Reviers, M.; Hochereau-de Reviers, M.T.; Blanc, M.R.; Brillard, J.P.; Courot, M.; Pelletier, J. Control of Sertoli and germ cell populations in the cock and sheep testes. Reprod. Nutr. Dev. 1980, 20, 241–249. [Google Scholar] [CrossRef]
- Vizcarra, J.A.; Kirby, J.D.; Kreider, D.L. Testis development and gonadotropin secretion in broiler breeder males. Poult. Sci. 2010, 89, 328–333. [Google Scholar] [CrossRef]
- Faure, M.; Guibert, E.; Crochet, S.; Chartrin, P.; Brillard, J.P.; Collin, A.; Froment, P. Differential proliferation and metabolic activity of Sertoli cells in the testes of broiler and layer breeder chickens. Poult. Sci. 2017, 96, 2459–2470. [Google Scholar] [CrossRef]
- Fulbert, J.C.; Cals, M.J. Free radicals in clinical biology. Origin, pathogenic effect and defense mechanisms. Pathol. Biol. 1992, 40, 66–77. [Google Scholar]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Pruchniak, M.P.; Arzna, M.; Demkow, U. Biochemistry of oxidative stress. Adv. Exp. Med. Biol. 2016, 878, 9–19. [Google Scholar]
- Agarwal, A.; Virk, G.; Ong, C.; Plessis, S.S.D. Effect of oxidative stress on male reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. Effect on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992, 13, 368–378. [Google Scholar]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 40, 183–197. [Google Scholar] [CrossRef]
- Eid, Y.; Ebeid, T.; Younis, H. Vitamin E supplementation reduces dexamethasone-induced oxidative stress in chicken semen. Br. Poult. Sci. 2006, 47, 350–356. [Google Scholar] [CrossRef]
- Gobierno de México Home Page. Available online: https://www.gob.mx/busqueda?utf8= (accessed on 29 May 2019).
- Prophet, E.B.; Mills, B.; Arrington, J.B.; Sobin, L.H. Laboratory Methods in Histotechnology, 1st ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1992; p. 279. ISBN 188-104-100-X. [Google Scholar]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudiere, J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Prior, R. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- R core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org (accessed on 12 December 2019).
- Zitzmann, M. Effects of age on male fertility. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 617–628. [Google Scholar] [CrossRef]
- Rodriguez, I.; Ody, C.; Araki, I.; Vassalli, P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997, 16, 2262–2270. [Google Scholar] [CrossRef]
- Alonso-Alvarez, C.; Pérez-Rodríguez, L.; García, J.T.; Viñuela, J.; Mateo, R. Age and breeding effort as sources of individual variability in oxidative stress markers in a bird species. Physiol. Biochem. Zool. 2010, 83, 110–118. [Google Scholar] [CrossRef]
- Wilson, F.D.; Johnson, D.I.; Magee, D.L.; Hoerr, F.J. Testicular histomorphometrics including Sertoli cell quantitation for evaluating hatchability and fertility issues in commercial breeder-broiler roosters. Poult. Sci. 2018, 97, 1738–1747. [Google Scholar] [CrossRef]
- Hocking, P.M. The relationship between dietary crude protein, and fertility in naturally mated broiler breeder males. Br. Poult. Sci. 1990, 31, 743–757. [Google Scholar] [CrossRef]
- Renema, R.A.; Robinson, F.E.; Zuidhof, M.J. Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 2. Sexual maturation. Poult. Sci. 2007, 86, 2267–2277. [Google Scholar] [CrossRef]
- Gunes, S.; Neslihan, G.; Hekimi, T.; Alper, M.; Asci, R. Effects of aging on the male reproductive system. J. Assist. Reprod. Genet. 2016, 33, 441–454. [Google Scholar] [CrossRef]
- Paniagua, R. Ultrastructure of the aging human testis. J. Electron Microsc. Tech. 1991, 19, 241–260. [Google Scholar] [CrossRef]
- Monnier, V.M. Nonezymatic Glycosylation, the Maillard Reaction and the Aging Process. J. Gerontol. Biol. Sci. 1990, 45, B105–B111. [Google Scholar] [CrossRef]
- Gutiérrez, J.S.; Sabat, P.; Castañeda, L.E.; Contreras, C.; Navarrete, L.; Peña-Villalobos, I.; Navedo, J.G. Oxidative status and metabolic profile in a long-lived bird preparing for extreme endurance migration. Sci. Rep. 2019, 9, 17616–17626. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, P.; Fraczed, M.; Szumala-Kakol, A.; Taszarek-Hauke, G.; Pawelczyk, L.; Kurpisz, M. Consequences of semen inflammation and lipid peroxidation on fertilization capacity of spermatozoa in vitro conditions. Int. J. Androl. 2005, 5, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.; Kasimanickam, V.; Thatcher, C.D.; Nebel, R.L.; Cassell, B.C. Relationships among lipid peroxidation, glutathione peroxidase, superoxide dismutase, sperm parameters, and competitive index in dairy bulls. Theriogenology 2007, 67, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Lukaszewicz, E.; Niżański, W. Lipid peroxidation and antioxidant enzymes activity in avian semen. Anim. Reprod. Sci. 2012, 134, 184–190. [Google Scholar] [CrossRef] [PubMed]
| Age (Weeks) | 30 | 36 | 60 |
| Weight (kg) | 4.150 | 4.330 | 5.050 |
| Variable Evaluated | Weeks of Age | |||||
|---|---|---|---|---|---|---|
| 30 | 36 | 62 | ||||
| r | p | r | p | r | p | |
| MDA | 0.760 | 0.029 * | −0.246 | 0.442 | −0.370 | 0.237 |
| ORAC | −0.200 | 0.532 | 0.154 | 0.632 | 0.017 | 0.959 |
| GPx | −0.117 | 0.718 | 0.125 | 0.699 | 0.138 | 0.669 |
| GR | −0.252 | 0.429 | 0.160 | 0.620 | −0.015 | 0.963 |
| GST | −0.320 | 0.311 | −0.002 | 0.995 | −0.050 | 0.877 |
| Variable Evaluated | Probabilistic Variable | MDA | ORAC | GPx | GR | GST |
|---|---|---|---|---|---|---|
| MDA | r | 1 | ||||
| p | ||||||
| ORAC | r | 0.528 | 1 | |||
| p | 0.002 ** | |||||
| GPx | r | −0.320 | 0.191 | 1 | ||
| p | 0.074 | 0.265 | ||||
| GR | r | 0.180 | 0.635 | 0.674 | 1 | |
| p | 0.325 | <0.001 ** | <0.001 ** | |||
| GST | r | 0.130 | 0.595 | 0.658 | 0.843 | 1 |
| p | 0.478 | <0.001 ** | <0.001 ** | <0.001 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escorcia, M.; Sánchez-Godoy, F.; Ramos-Vidales, D.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Effect of the Age and Body Weight of the Broiler Breeders Male on the Presentation of Oxidative Stress and Its Correlation with the Quality of Testicular Parenchyma and Physiological Antioxidant Levels. Vet. Sci. 2020, 7, 69. https://doi.org/10.3390/vetsci7020069
Escorcia M, Sánchez-Godoy F, Ramos-Vidales D, Medina-Campos ON, Pedraza-Chaverri J. Effect of the Age and Body Weight of the Broiler Breeders Male on the Presentation of Oxidative Stress and Its Correlation with the Quality of Testicular Parenchyma and Physiological Antioxidant Levels. Veterinary Sciences. 2020; 7(2):69. https://doi.org/10.3390/vetsci7020069
Chicago/Turabian StyleEscorcia, Magdalena, Félix Sánchez-Godoy, David Ramos-Vidales, Omar Noel Medina-Campos, and José Pedraza-Chaverri. 2020. "Effect of the Age and Body Weight of the Broiler Breeders Male on the Presentation of Oxidative Stress and Its Correlation with the Quality of Testicular Parenchyma and Physiological Antioxidant Levels" Veterinary Sciences 7, no. 2: 69. https://doi.org/10.3390/vetsci7020069
APA StyleEscorcia, M., Sánchez-Godoy, F., Ramos-Vidales, D., Medina-Campos, O. N., & Pedraza-Chaverri, J. (2020). Effect of the Age and Body Weight of the Broiler Breeders Male on the Presentation of Oxidative Stress and Its Correlation with the Quality of Testicular Parenchyma and Physiological Antioxidant Levels. Veterinary Sciences, 7(2), 69. https://doi.org/10.3390/vetsci7020069






