Clinical and Pathological Data of 17 Non-Epithelial Pancreatic Tumors in Cats
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Round Cell Tumors, n = 13
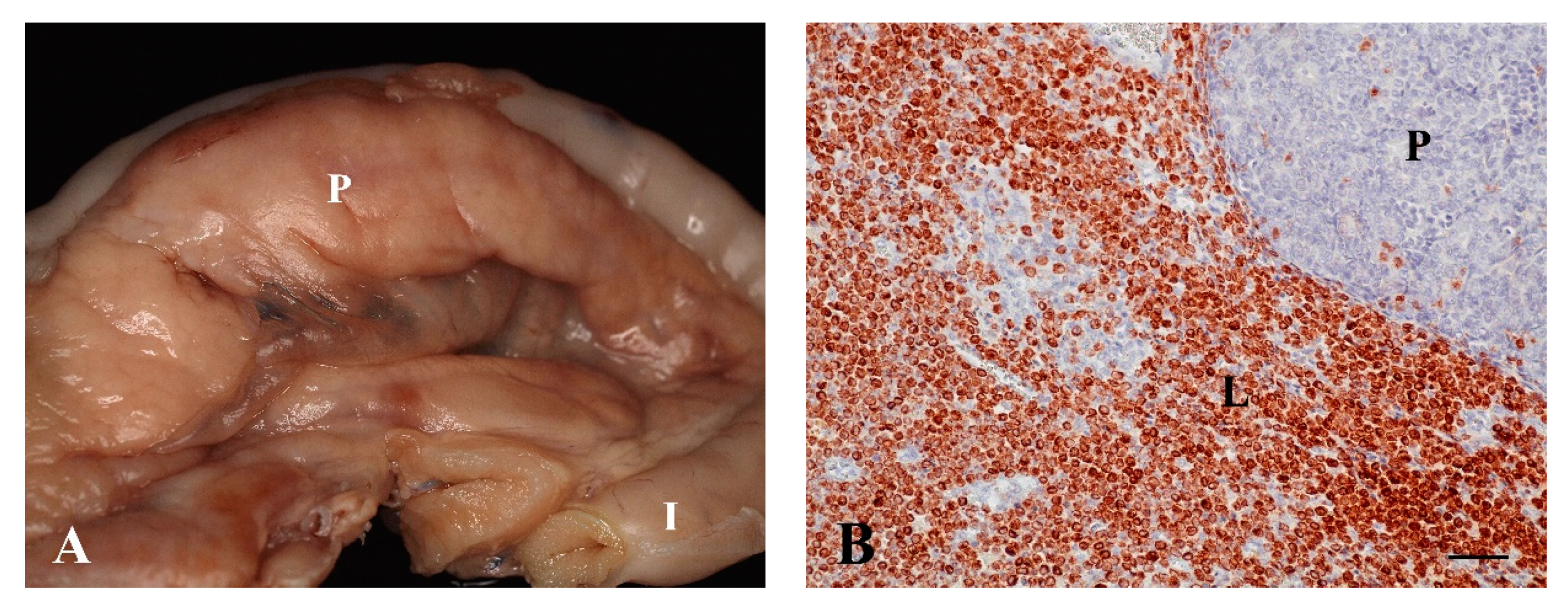
3.1.1. Lymphoma, n = 12
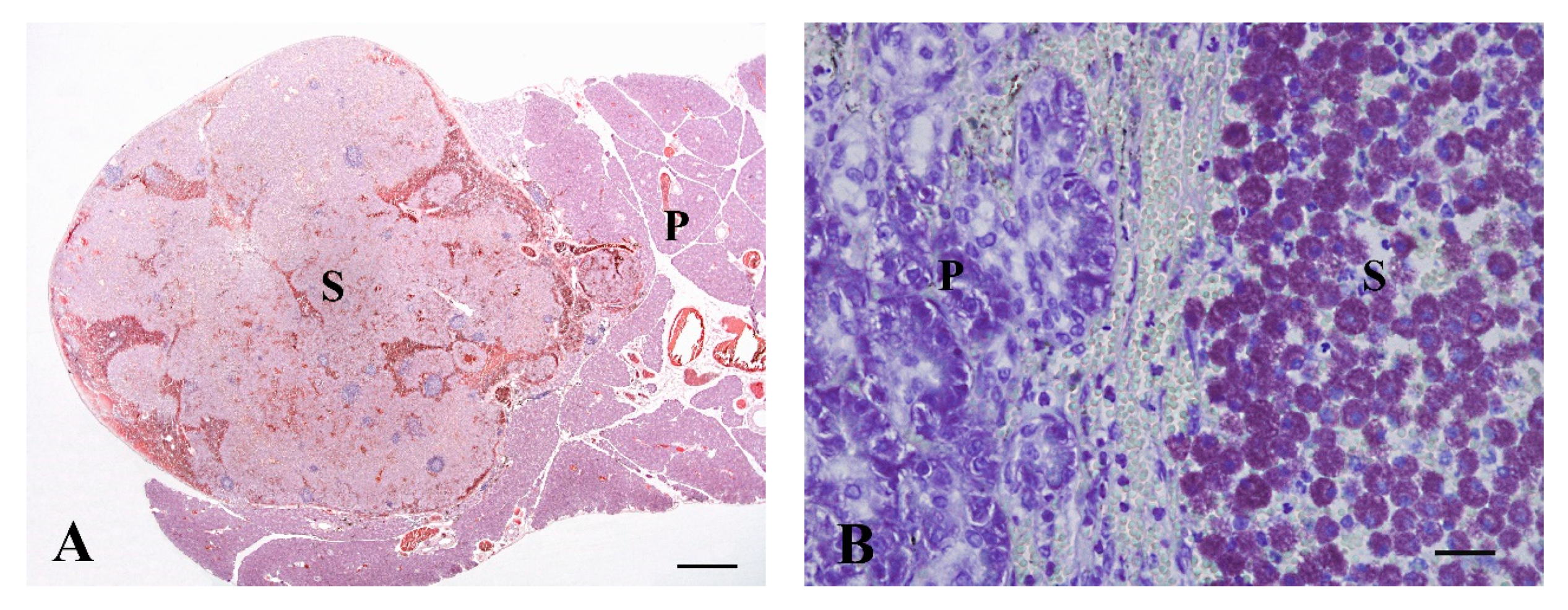
3.1.2. Mast Cell Tumor, n = 1
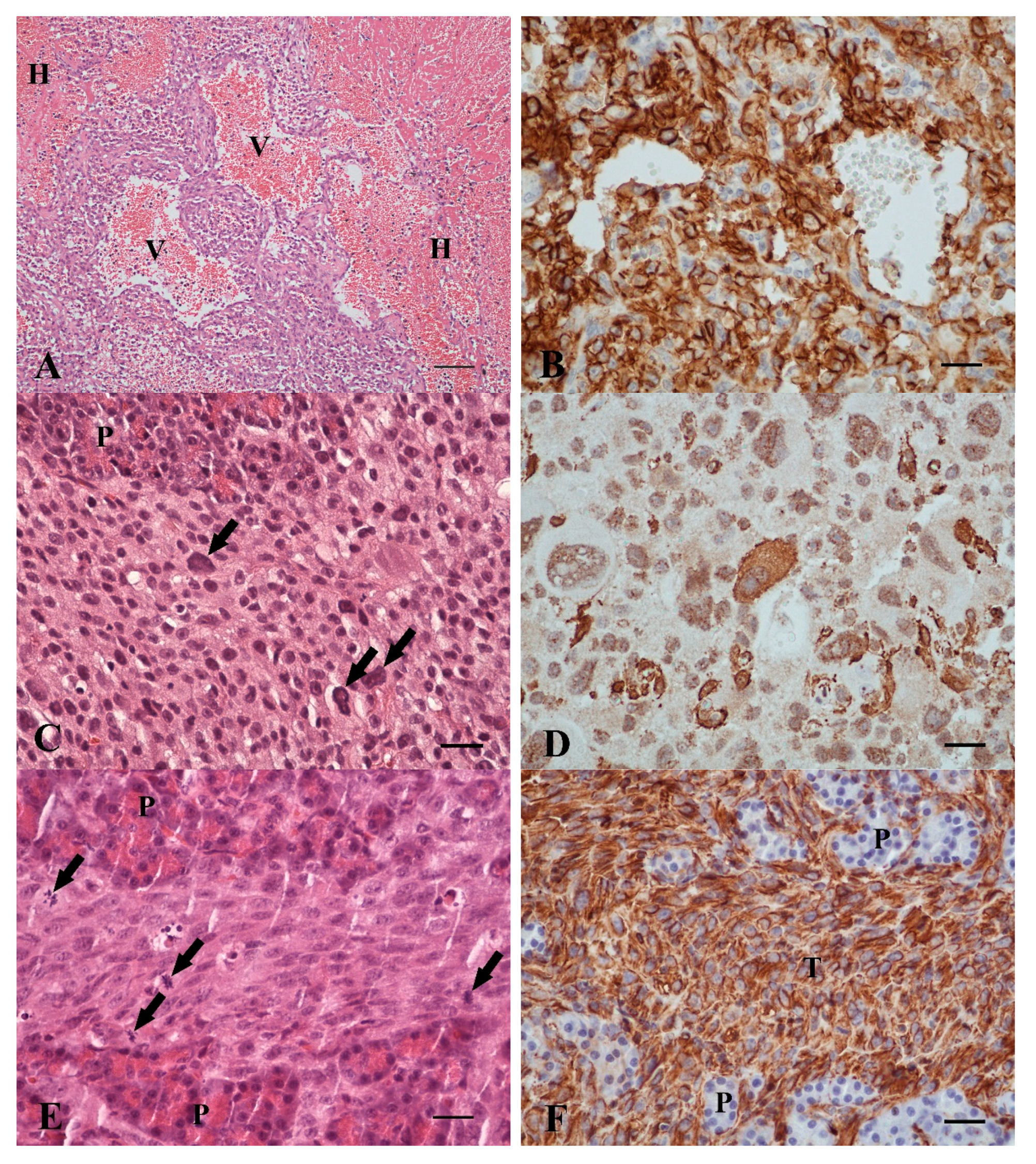
3.2. Spindle Cell Tumors, n = 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Head, K.W.; Cullen, J.M.; Dubielzig, R.R.; Else, R.W.; Misdorp, W.; Patnaik, A.K.; Tateyama, S.; Van der Gaag, I. Histological classification of tumours of the pancreas of domestic animals. In World Health Organization International Histological Classification of Tumors of Domestic Animals, Histological Classification of Tumours of the Alimentary System of Domestic Animals; Head, K.W., Cullen, J.M., Dubielzig, R.R., Else, R.W., Misdorp, W., Patnaik, A.K., Tateyama, S., Van der Gaag, I., Eds.; Armed Forces Institute of Pathology: Washington, DC, USA, 2003; pp. 111–118. [Google Scholar]
- Jubb, K.V.F.; Stent, A.W. Exocrine pancreas. In Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, pp. 353–368. [Google Scholar]
- Munday, J.S.; Loehr, C.V.; Kiupel, M. Tumors of the alimentary tract. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Wiley Blackwell: Ames, IA, USA, 2017; pp. 499–601. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kircher, C.H.; Nielsen, S.W. Tumours of the pancreas. Bull. World Health Organ. 1976, 53, 195–202. [Google Scholar] [PubMed]
- Bennett, P.F.; Hahn, K.A.; Toal, R.L.; Legendre, A.M. Ultrasonographic and cytopathological diagnosis of exocrine pancreatic carcinoma in the dog and cat. J. Am. Anim. Hosp. Assoc. 2001, 37, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Seaman, R.L. Exocrine pancreatic neoplasia in the cat: A case series. J. Am. Anim. Hosp. Assoc. 2004, 40, 238–245. [Google Scholar] [CrossRef]
- Linderman, M.J.; Brodsky, E.M.; De Lorimier, L.P.; Clifford, C.A.; Post, G.S. Feline exocrine pancreatic carcinoma: A retrospective study of 34 cases. Vet. Comp. Oncol. 2013, 11, 208–218. [Google Scholar] [CrossRef]
- Gieger, T. Alimentary lymphoma in cats and dogs. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 419–432. [Google Scholar] [CrossRef]
- Hecht, S.; Penninck, D.G.; Keating, J.H. Imaging findings in pancreatic neoplasia and nodular hyperplasia in 19 cats. Vet. Radiol. Ultrasound 2007, 48, 45–50. [Google Scholar] [CrossRef]
- Lingard, A.E.; Briscoe, K.; Beatty, J.A.; Moore, A.S.; Crowley, A.M.; Krockenberger, M.; Churcher, R.K.; Canfield, P.J.; Barrs, V.R. Low-grade alimentary lymphoma: Clinicopathological findings and response to treatment in 17 cases. J. Feline Med. Surg. 2009, 11, 692–700. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Takahashi, M.; Tsuboi, M.; Fujino, Y.; Uchida, K.; Ohno, K.; Nakayama, H.; Tsujimoto, H. Intestinal T-cell lymphoma with severe hypereosinophilic syndrome in a cat. J. Vet. Med. Sci. 2012, 74, 1057–1062. [Google Scholar] [CrossRef]
- Kerns, A.T.; Brakel, K.A.; Premanandan, C.; Saffire, A.; Moore, S. Extranodal non-B, non-T-cell lymphoma with bilateral tympanic bulla involvement in a cat. JFMS Open Rep. 2018, 4, 2055116918756724. [Google Scholar] [CrossRef]
- Louwerens, M.; London, C.A.; Pedersen, N.C.; Lyons, L.A. Feline lymphoma in the post-feline leukemia virus era. J. Vet. Intern. Med. 2005, 19, 329–335. [Google Scholar] [PubMed]
- Hendrick, M.J. Mesenchymal tumors of the skin and soft tissues. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Wiley Blackwell: Ames, IA, USA, 2017; pp. 142–175. [Google Scholar]
- Yamamoto, R.; Suzuki, K.; Uchida, K.; Onda, N.; Shibutani, M.; Mitsumori, K. Pancreatic carcinosarcoma in a cat. J. Comp. Pathol. 2012, 147, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Huddy, J.R.; Sodergren, M.H.; Deguara, J.; Thway, K.; Jones, R.L.; Mudan, S.S. Pancreaticoduodenectomy for the management of pancreatic or duodenal metastases from primary sarcoma. Anticancer Res. 2018, 38, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.W.; Tsao-Wei, D.D.; Callegaro, D.; Grignani, G.; D’Ambrosio, L.; Bonvalot, S.; Ethin, C.G.; Cardona, K.; Mullen, J.T.; Canter, R.J.; et al. A collaborative effort from the Trans-Atlantic Retroperitoneal Sarcoma Working Group (TARPSWG). Pancreaticoduodenectomy in the surgical management of primary retroperitoneal sarcoma. Eur. J. Surg. Oncol. 2018, 44, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N. Hemangiosarcoma in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 533–552. [Google Scholar] [CrossRef]
- Culp, W.T.N.; Drobatz, K.J.; Glassman, M.M.; Baez, J.L.; Aronson, L.R. Feline visceral hemangiosarcoma. J. Vet. Intern. Med. 2008, 22, 148–152. [Google Scholar] [CrossRef]
- Sharpe, A.; Cannon, M.J.; Lucke, V.M.; Day, M.J. Intestinal haemangiosarcoma in the cat: Clinical and pathological features of four cases. J. Small Anim. Pract. 2000, 41, 411–415. [Google Scholar] [CrossRef]
- Hendrick, M.J.; Mahaffey, E.A.; Moore, F.M.; Vos, J.H.; Walder, E.J. Tumors of fibrous tissue. In World Health Organization International Histological Classification of Tumors of Domestic Animals, Histological Classification of Mesenchymal Tumors of the Skin and Soft Tissues of Domestic Animals; Schulman, F.Y., Ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1998A; pp. 15–18. [Google Scholar]
- Hendrick, M.J.; Mahaffey, E.A.; Moore, F.M.; Vos, J.H.; Walder, E.J. Tumors of vascular tissue. In World Health Organization International Histological Classification of Tumors of Domestic Animals, Histological Classification of Mesenchymal Tumors of the Skin and Soft Tissues of Domestic Animals; Schulman, F.Y., Ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1998B; pp. 22–25. [Google Scholar]
- Hendrick, M.J.; Mahaffey, E.A.; Moore, F.M.; Vos, J.H.; Walder, E.J. Mast cell tumors. In World Health Organization International Histological Classification of Tumors of Domestic Animals, Histological Classification of mesenchymal tumors of the skin and soft tissues of Domestic Animals; Schulman, F.Y., Ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1998C; pp. 28–29. [Google Scholar]
- Valli, V.E.; Jacobs, R.M.; Parodi, A.L.; Vernau, W.; Moore, P.F. Tumors of lymphoid system. In World Health Organization International Histological Classification of Tumors of Domestic Animals, Histological Classification of Hematopoietic Tumors of Domestic Animals; Armed Forces Institute of Pathology: Washington, DC, USA, 2002; pp. 25–47. [Google Scholar]
- Gabor, L.J.; Canfield, P.J.; Malik, R. Immunophenotypic and histological characterization of 109 cases of feline lymphosarcoma. Aust. Vet. J. 1999, 77, 436–441. [Google Scholar] [CrossRef]
- De Cock, H.E.V.; Forman, M.A.; Farver, T.B.; Marks, S.L. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet. Pathol. 2007, 44, 39–49. [Google Scholar] [CrossRef]
- Priester, W.A. Data from eleven United States and Canadian colleges of veterinary medicine on pancreatic carcinoma in domestic animals. Cancer Res. 1974, 34, 1372–1375. [Google Scholar]
- Törner, K.; Aupperle-Lellbach, H.; Staudacher, A.; Staudacher, M.; Steiger, K. Primary solid and cystic tumours of the exocrine pancreas in cats. J. Comp. Pathol. 2019, 165, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. Tumours of the pancreas. In World Health Organization Classification of Tumours of the Digestive System, 4th ed.; Bosmann, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC Press: Lyon, France, 2010; pp. 279–337. [Google Scholar]
- Vezzali, E.; Parodi, A.L.; Marcato, P.S.; Bettini, G. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet. Comp. Oncol. 2009, 8, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Törner, K.; Staudacher, M.; Tress, U.; Weber, C.N.; Stadler, C.; Grassinger, J.M.; Müller, E.; Aupperle-Lellbach, H. Histopathology and feline pancreatic lipase immunoreactivity in inflammatory, hyperplastic and neoplastic pancreatic diseases in cats. J. Comp. Pathol. 2020, 174, 63–72. [Google Scholar] [CrossRef]
- Lidbury, J.A.; Suchodolski, J.S. New advances in the diagnosis of canine and feline liver and pancreatic disease. Vet. J. 2016, 215, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Waly, N.E.; Gruffydd-Jones, T.J.; Stokes, C.R.; Day, M.J. Immunohistochemical diagnosis of alimentary lymphomas and severe intestinal inflammation in cats. J. Comp. Pathol. 2005, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, M.J.; Bienzle, D. Making sense of lymphoma diagnostics in small animal patients. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1331–1347. [Google Scholar] [CrossRef] [PubMed]
- Pohlman, L.M.; Higginbotham, M.L.; Welles, E.G.; Johnson, C.M. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet. Pathol. 2009, 46, 259–268. [Google Scholar] [CrossRef]
- Sato, H.; Fujino, Y.; Chino, J.; Takahashi, M.; Fukushima, K.; Goto-Koshino, Y.; Uchida, K.; Ohno, K.; Tsujimoto, H. Prognostic analyses on anatomical and morphological classification of feline lymphoma. J. Vet. Med. Sci. 2014, 76, 807–811. [Google Scholar] [CrossRef]
- Wolfesberger, B.; Skor, O.; Hammer, S.E.; Flickinger, I.; Kleiter, M.; Rütgen, B.C.; Schwendenwein, I.; Tichy, A.; Hittmair, K.M.; Degasperi, B.; et al. Does categorisation of lymphoma subtypes according to the World Health Organization classification predict clinical outcome in cats? J. Feline Med. Surg. 2017, 19, 897–906. [Google Scholar] [CrossRef]
- Ramírez, G.A.; Altimira, J.; García-González, B.; Vilafranca, M. Intrapancreatic ectopic splenic tissue in dogs and cats. J. Comp. Pathol. 2013, 148, 361–364. [Google Scholar] [CrossRef]
- Mallett, C.L.; Northrup, N.C.; Saba, C.F.; Rodriguez, C.O.; Rassnick, K.M.; Gieger, T.L.; Childress, M.O.; Howerth, E.W. Immunohistochemical characterization of feline mast cell tumors. Vet. Pathol. 2012, 50, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Sabattini, S.; Bettini, G. Prognostic value of histologic and immunohistochemical features in feline cutaneous mast cell tumors. Vet. Pathol. 2010, 47, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.J.; O’Brien, D.; Allstadt, S.D.; Gregor, T.P.; Sorenmo, K.U. Treatment outcomes and prognostic factors of feline splenic mast cell tumors: A multi-institutional retrospective study of 64 cases. Vet. Comp. Oncol. 2018, 16, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, P.C. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J. Vet. Diagn. Investig. 2004, 16, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, M.J.; Brooks, J.J. Postvaccinal sarcomas in the cat: Histology and immunohistochemistry. Vet. Pathol. 1994, 31, 126–129. [Google Scholar] [CrossRef]
- Aberdein, D.; Munday, J.S.; Dyer, C.B.; Knight, C.G.; French, A.F.; Gibson, I.R. Comparison of the histology and immunohistochemistry of vaccination-site and non-vaccination-site sarcomas from cats in New Zealand. N. Z. Vet. J. 2007, 55, 203–207. [Google Scholar] [CrossRef]
- Jelinek, F. Postinflammatory sarcoma in cats. Exp. Toxicol. Pathol. 2003, 55, 167–172. [Google Scholar] [CrossRef]
- Haddad, J.L.; Goldschmidt, M.H.; Patel, R.T. Fibrosarcoma arising at the site of a retained surgical sponge in a cat. Vet. Clin. Pathol. 2010, 39, 241–246. [Google Scholar] [CrossRef]
- Coffin, C.M.; Dehner, L.F.; Meis-Kindblom, J.M. Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: An historical review with differential diagnostic considerations. Semin. Diagn. Pathol. 1998, 15, 102–110. [Google Scholar]
- Meis, J.M.; Enzinger, F.M. Inflammatory fibrosarcoma of the mesentery and retroperitoneum a tumor closely simulating inflammatory pseudotumor. Am. J. Surg. Pathol. 1991, 15, 1146–1156. [Google Scholar] [CrossRef]
| Marker | Type of Antibody | Source | Dilution | Pretreatment |
|---|---|---|---|---|
| Vimentin | Monoclonal mouse anti-vimentin (Clone V9) | Dako, Glostrup, Denmark, M0725 | 1:1000 | Peroxidase blocking; steam cook in EDTA buffer |
| Smooth muscle actin | Monoclonal mouse anti-human (Clone 1A4) | Dako, Glostrup, Denmark, M0851 | 1:100 | Peroxidase blocking; pressure cook in citrate buffer |
| Desmin | Monoclonal mouse anti-human (Clone D33) | Dako, Glostrup, Denmark, M0760 | 1:100 | Peroxidase blocking; steam cook in EDTA buffer |
| Von Willebrand factor | Polyclonal rabbit anti-human | Dako, Glostrup, Denmark, A0082 | 1:2000 | Peroxidase blocking; pressure cook in citrate buffer |
| CD31 | Monoclonal mouse anti-human (Clone JC70A) | Dako, Glostrup, Denmark, M0823 | 1:100 | Peroxidase blocking; steam cook in EDTA buffer |
| CD3 | Monoclonal mouse anti-human (Clone F7.2.38) | Dako, Glostrup, Denmark, M7254 | 1:100 | Peroxidase blocking; steam cook in EDTA buffer |
| CD79a | Monoclonal mouse anti-human (Clone HM57) | Bio-Rad Laboratories Inc, Munich, Germany, MCA2538GA | 1:3000 | Peroxidase blocking; steam cook in EDTA buffer |
| CD117/c-kit | Polyclonal rabbit anti-human | Dako, Glostrup, Denmark, A4502 | 1:150 | Peroxidase blocking; steam cook in EDTA buffer |
| Feline Leukemia Virus gp70 | Monoclonal mouse anti-feline leukemia virus (Clone C11D8) | Bio-Rad Laboratories Inc, Munich, Germany, MCA1897 | 1:200 | Peroxidase blocking; steam cook in EDTA buffer |
| Feline Leukemia Virus p27 | Monoclonal mouse anti-feline leukemia virus (Clone PF12J-10A) | Bio-Rad Laboratories Inc, Munich, Germany, MCA2551 | 1:100 | Peroxidase blocking; steam cook in EDTA buffer |
| Case No. | Age (years) | Sex | Breed | Clinical Signs | Pancreatic Sample Size (cm) | Diagnosis | Mi/ HPF | Further Infiltrated Organs | Staining, IHC Expression | Survival Time | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | ||||||||||
| 1 | 1 | MN | DSH | Inappetence, ascites | TS 0.8 × 0.3 × 0.3 | Large BCL | 13 | Kidney, liver, LN | CD79a | CD3, FeLV | 2 months |
| 2 | 12 | F | SB | Inappetence | CM 3.0 × 3.0 × 3.0 | Large BCL | 5 | Om | CD79a | CD3, FeLV | U |
| 3 * | 10 | MN | BSH | Vomiting, inappetence, palpable abd. mass | CM 4.0 × 4.0 × 4.0 | Large BCL | 9 | Stomach, mAT | CD79a | CD3, FeLV | Eutha |
| 4 | 13 | MN | Si | Vomiting, diarrhea | TS 1.0 × 0.4 × 0.3 | Small TCL | 0 | Intestine | CD3 | CD79a, FeLV | 7 months |
| 5 * | 15 | MN | DSH | Lethargy, painful abd. | TS 1.5 × 1.5 × 1.5 | Intermediate TCL | 0 | Intestine, mLN | CD3, FeLV | CD79a | Eutha |
| 6 | 11 | F | DSH | Diarrhea | TS 0.3 × 0.3 × 0.3 | Intermediate TCL | 0 | - | CD3 | CD79a | Eutha |
| 7 * | 16 | MN | DSH | Lethargy, ascites | CP 8.5 × 7.0 × 2.6 | Intermediate TCL | 3 | Intestine, pAT, mLN | CD3 | CD79a, FeLV | Eutha |
| 8 | 10 | MN | DSH | U | CP 9.0 × 6.0 × 3.0 | Intermediate TCL | 4 | Liver, intestine, mLN | CD3 | CD79a | Eutha |
| 9 * | 15 | MN | DSH | Lethargy, seizures | CP 7.0 × 6.5 × 0.5 with two masses 0.5 × 0.5 × 0.5 | Large TCL | 1 | Intestine | CD3 | CD79a, FeLV | Eutha |
| 10 | 11 | M | Cart | U | Two TS 1.0 × 0.5 × 0.1 and 1.1 × 0.5 × 0.4 | Large TCL | 3 | Om, spleen | CD3 | CD79a, FeLV | Eutha |
| 11 | 8 | FS | DSH | U | Two TS 3.4 × 3.0 × 3.0 and 5.0 × 3.4 × 2.4 | Large TCL | 3 | Intestine, mAT | CD3, FeLV | CD79a | U |
| 12 * | 10 | MN | DSH | Vomiting, painful abd. | TS 0.4 × 0.4 × 0.4 | Large TCL | 4 | - | CD3 | CD79a, FeLV | U |
| 13 | 16 | F | BLH | Palpable abd. mass | CM 0.3 × 0.3 × 0.3 and 1.0 × 0.6 × 0.5 | Mast cell tumor | 1 | Spleen | Giemsa | c-kit, FeLV | 2 months |
| 14 | 13 | FS | DSH | Palpable abd. mass | CM 2.0 × 1.3 × 1.0 | Hemangio-sarcoma | 3 | - | Vim, vWF, CD31 | Eutha | |
| 15 | 12 | FS | DSH | U | TS 5.5 × 2.0 × 0.4 | Hemangio-sarcoma | 1 | Om, intestine, LN | Vim, CD31, vWF | Eutha | |
| 16 * | 17 | F | DSH | Palpable abd. mass, lethargy | CP 17.0 × 9.0 × 5.0 with multiple masses up to 2.0 × 2.0 × 2.0 | PS with myofibro-blastic parts | 9 | Om, adherent on capsule of spleen, intestine, liver | Vim, actin | desmin, c-kit | Eutha |
| 17 | 13 | MN | DSH | Inappetence, ascites | Two TS 1.2 × 0.8 × 0.6 and 3.0 × 3.0 × 2.0 | Fibrosarcoma | 2 | Spleen | Vim | 2 weeks | |
| Case No. | Diagnosis | fPLI (<3.5 µg/ L) | fTLI (12–82 µg/ L) | DGGR Lipase (<26 U/L) | Alpha Amylase (<1850 U/L) | SAA (<6.7 µg/mL) | Leuko (6.0–11.0 G/L) | Neutro (3.0–11.0 G/L) | Lymph (1.0–4.0 G/L) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | BCL, mild purulent pancreatitis | 4.7 | 30.7 | 25.9 | 922 | 128.83 | 16.1 | 15.0 | 0.6 |
| 5 | TCL, mild purulent pancreatitis | 4.2 | 25.6 | 30.1 | 552 | 41.06 | |||
| 9 | TCL, mild purulent pancreatitis | 5.6 | 33.0 | 15.4 | 891 | 47.51 | 11.4 | 9.2 | 1.0 |
| 12 | TCL, mild purulent pancreatitis | 22.9 | 43.2 | 40.6 | 839 | 62.40 | |||
| 7 | TCL, mild lymphocytic pancreatitis | >40.0 | 14.1 | 268.7 | 405 | 4.78 | 53.1 | 31.9 | 10.1 |
| 16 | Pleomorphic sarcoma, mild mixed pancreatitis | 1.8 | 38.5 | 8.7 | 701 | 86.12 | 19.3 | 18.5 | 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törner, K.; Staudacher, M.; Steiger, K.; Aupperle-Lellbach, H. Clinical and Pathological Data of 17 Non-Epithelial Pancreatic Tumors in Cats. Vet. Sci. 2020, 7, 55. https://doi.org/10.3390/vetsci7020055
Törner K, Staudacher M, Steiger K, Aupperle-Lellbach H. Clinical and Pathological Data of 17 Non-Epithelial Pancreatic Tumors in Cats. Veterinary Sciences. 2020; 7(2):55. https://doi.org/10.3390/vetsci7020055
Chicago/Turabian StyleTörner, Katrin, Marlies Staudacher, Katja Steiger, and Heike Aupperle-Lellbach. 2020. "Clinical and Pathological Data of 17 Non-Epithelial Pancreatic Tumors in Cats" Veterinary Sciences 7, no. 2: 55. https://doi.org/10.3390/vetsci7020055
APA StyleTörner, K., Staudacher, M., Steiger, K., & Aupperle-Lellbach, H. (2020). Clinical and Pathological Data of 17 Non-Epithelial Pancreatic Tumors in Cats. Veterinary Sciences, 7(2), 55. https://doi.org/10.3390/vetsci7020055







