A Case of Sebaceous Adenitis and Concurrent Meibomian Gland Dysfunction in a Dog
Abstract
1. Introduction
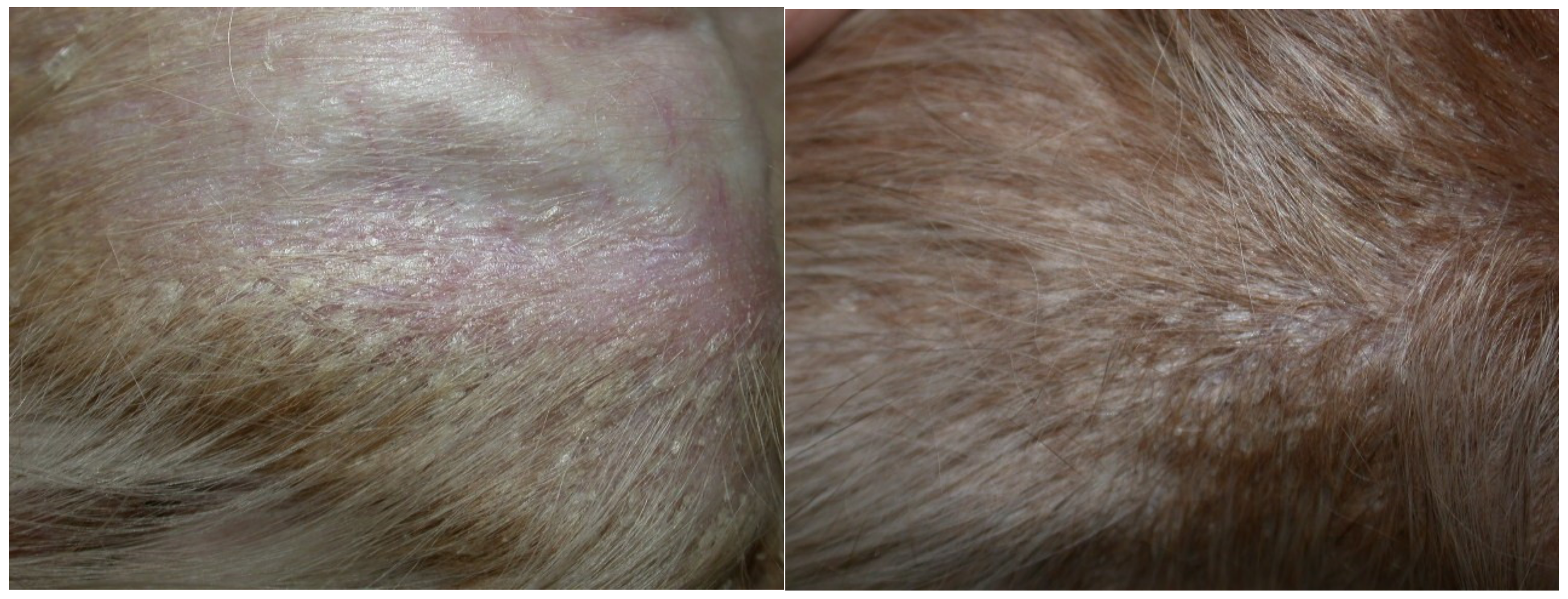
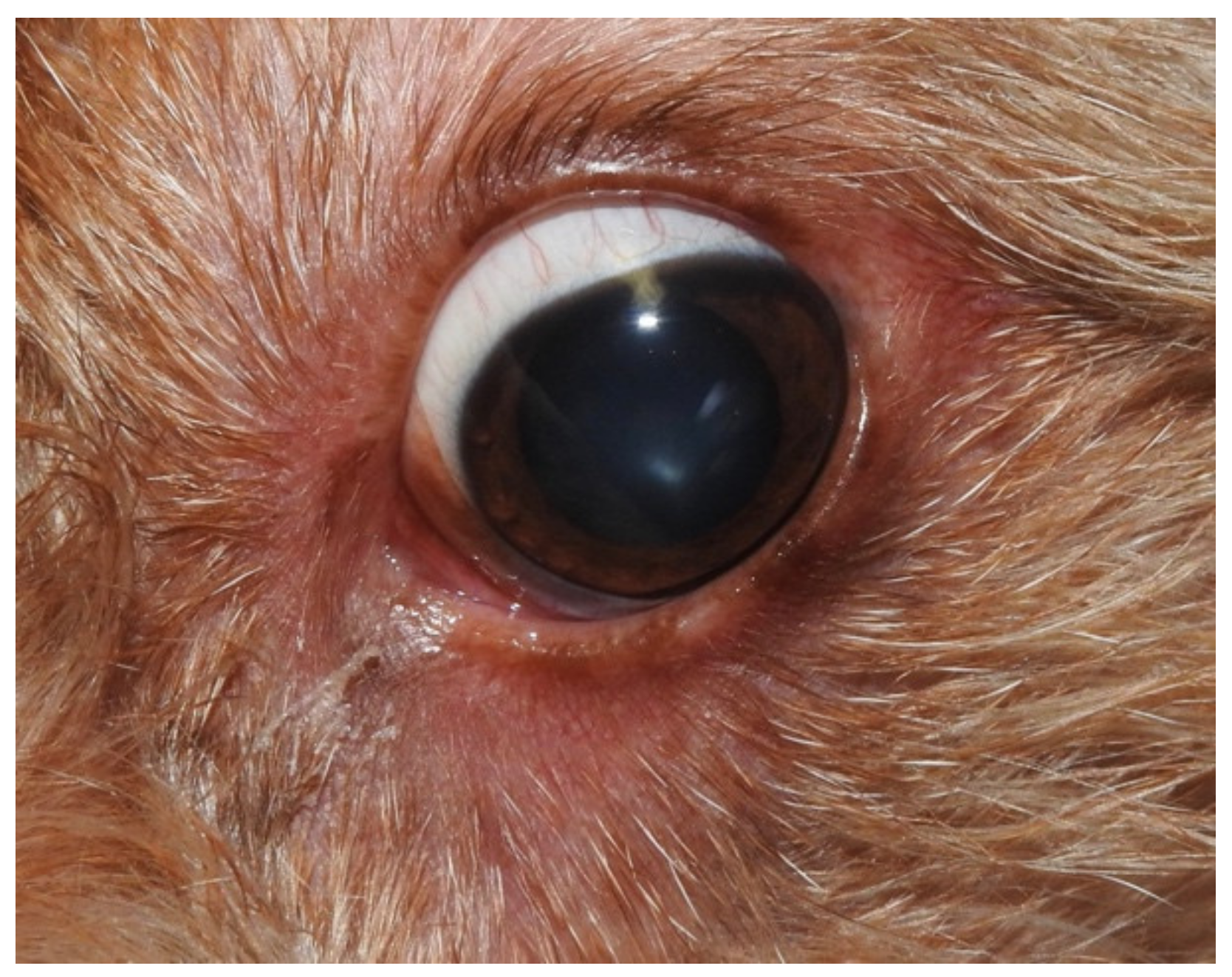
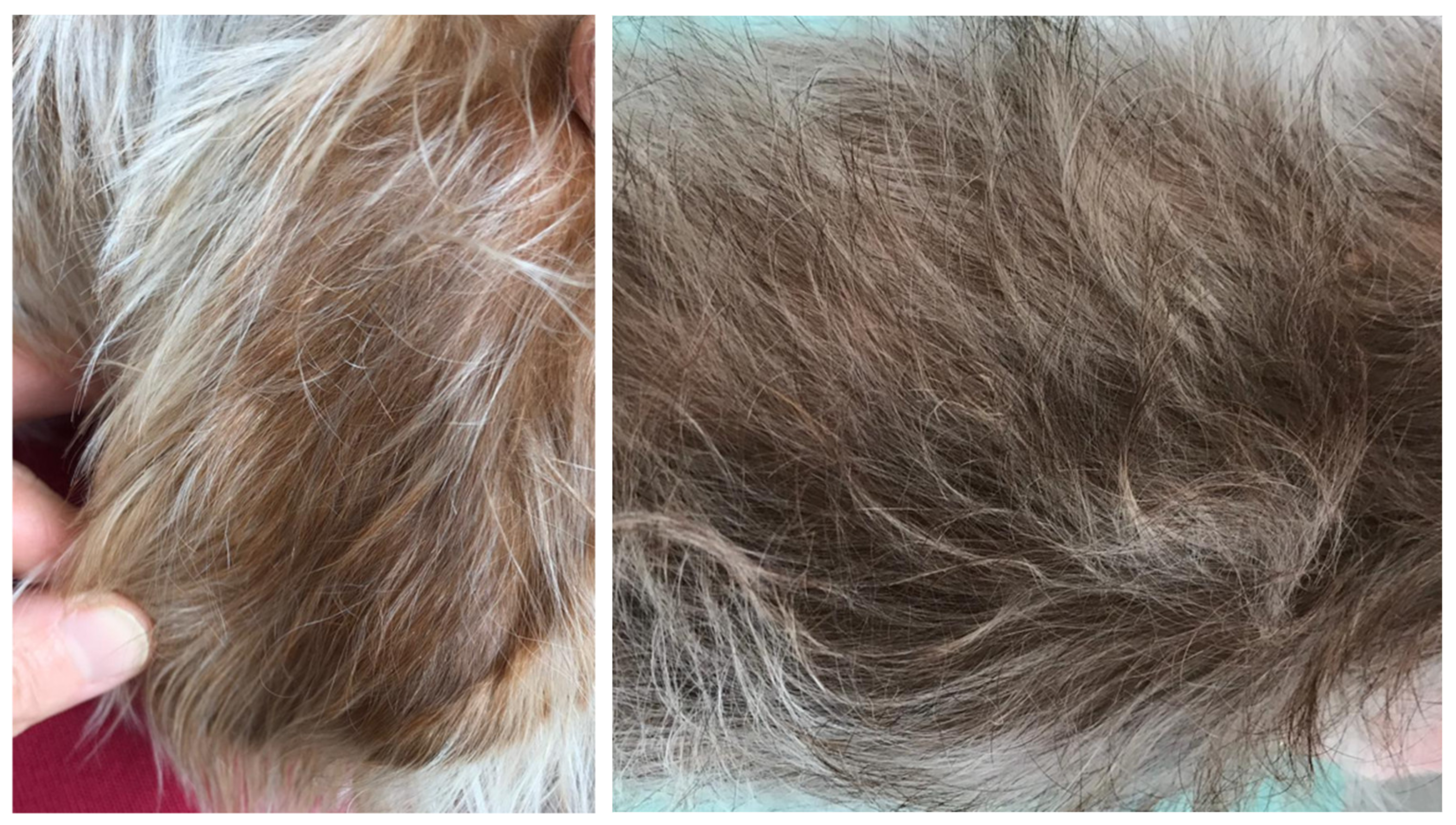
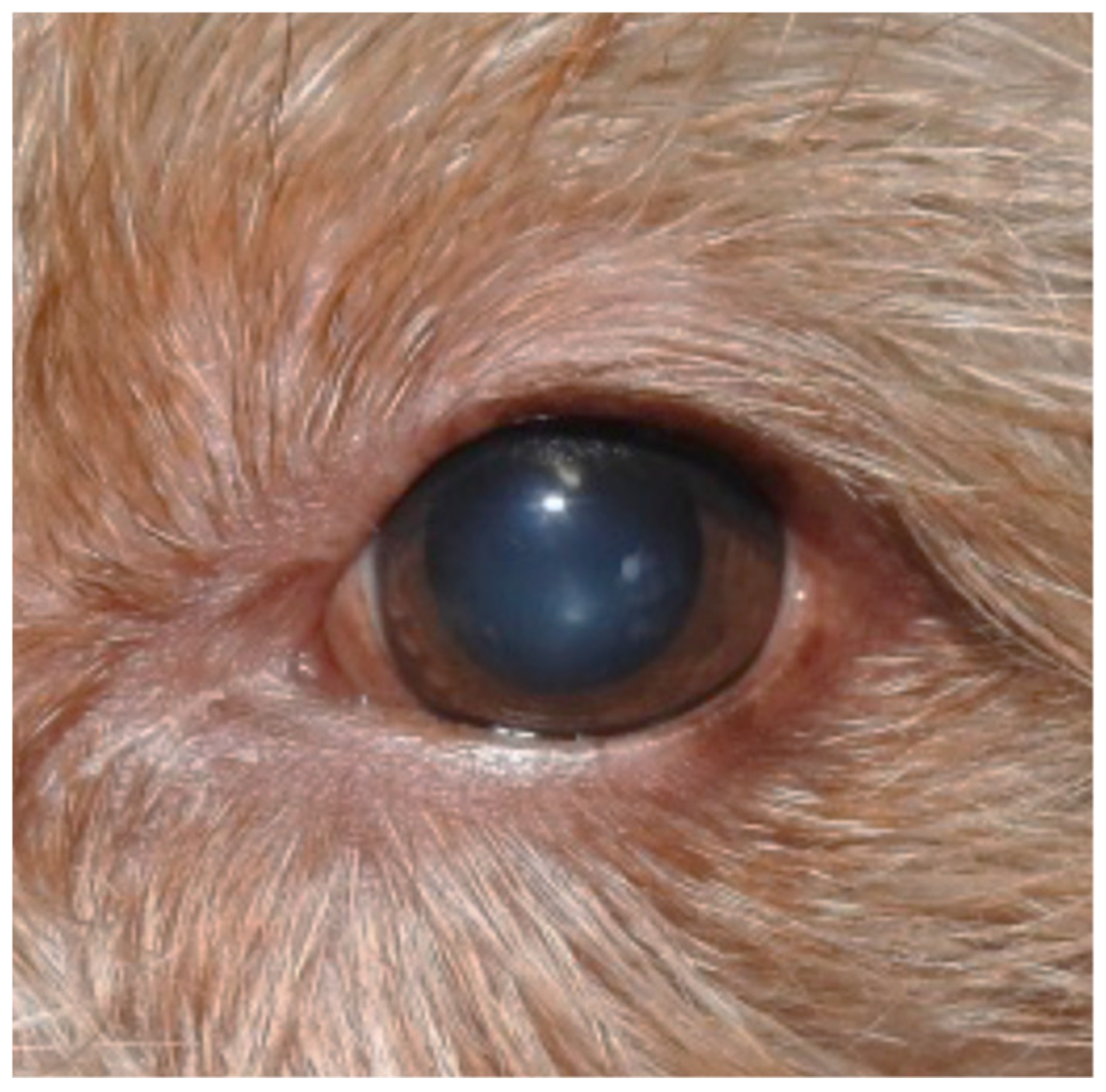
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knop, E.; Knop, N.; Millar, K.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L. Granulomatous sebaceous adenitis. In Muller & Kirk’s Small Animal Dermatology, 7th ed.; Elsevier: St. Louis, MO, USA, 2013; pp. 695–699. [Google Scholar]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The International workshop on Meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Vinas, M.; Maggio, F.; D’Anna, N.; Rabozzi, R.; Peruccio, C. Meibomian gland dysfunction (MGD), as diagnosed by non-contact infrared Meibography, in dogs with ocular surface disorders (OSD): A retrospective study. BMC Vet. Res. 2019, 15, 443–450. [Google Scholar] [CrossRef] [PubMed]
- McCulley, J.P.; Shine, W.E. Meibomian gland function and the tear lipid layer. Ocul. Surf. 2003, 1, 97–106. [Google Scholar] [CrossRef]
- McCulley, J.P.; Shine, W.E. The lipid layer of tears: Dependent on meibomian gland function. Exp. Eye Res. 2004, 78, 361–365. [Google Scholar] [CrossRef]
- Lortz, J.; Favrot, C.; Mecklenburg, L.; Nett, C.; Rüfenacht, S.; Seewald, W.; Linek, M. A multicentre placebo-controlled clinical trial on the efficacy of oral ciclosporin A in the treatment of canine idiopathic sebaceous adenitis in comparison with conventional topical treatment. Vet. Dermatol. 2010, 21, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Geerling, G.; Tauber, J.; Baudouin, C.; Goto, E.; Matsumoto, Y.; O’Brien, T.; Rolando, M.; Tsubota, K.; Nichols, K.K. The International workshop on Meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2050–2064. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartori, R.; Peruccio, C. A Case of Sebaceous Adenitis and Concurrent Meibomian Gland Dysfunction in a Dog. Vet. Sci. 2020, 7, 37. https://doi.org/10.3390/vetsci7020037
Sartori R, Peruccio C. A Case of Sebaceous Adenitis and Concurrent Meibomian Gland Dysfunction in a Dog. Veterinary Sciences. 2020; 7(2):37. https://doi.org/10.3390/vetsci7020037
Chicago/Turabian StyleSartori, Roberta, and Claudio Peruccio. 2020. "A Case of Sebaceous Adenitis and Concurrent Meibomian Gland Dysfunction in a Dog" Veterinary Sciences 7, no. 2: 37. https://doi.org/10.3390/vetsci7020037
APA StyleSartori, R., & Peruccio, C. (2020). A Case of Sebaceous Adenitis and Concurrent Meibomian Gland Dysfunction in a Dog. Veterinary Sciences, 7(2), 37. https://doi.org/10.3390/vetsci7020037




