Tick Paralysis: Solving an Enigma
Abstract
:1. Introduction
2. The Enigma: Arachnida as a Monophyletic Venomous Group: Implications for the Origins of Tick Toxins
2.1. Are Ticks Venomous Animals?
2.2. Do Arachnid and Tick Toxins Share a Common Ancestral History?
3. Toxins and Venomous Organisms
3.1. Significance of Paralysis Toxins for Ticks
3.2. What Is a Toxin?
- (1)
- Inhibition of ion, sodium, potassium, chloride, and/or calcium channels and synaptic vesicle release;
- (2)
- Receptor agonists and antagonists;
- (3)
- Cytoskeleton interference;
- (4)
- Calcium-mediated cytotoxicity or; and,
- (5)
- Neurotoxins with multiple effects.
3.3. Scorpion Toxins and Venoms
3.4. Spider Toxins and Venoms
3.5. Tick Toxins and Salivary Gland Proteins
4. Phenotypic Commonalities and Differences between Paralysis Ticks
5. Molecular Data on Tick Toxins
6. Strategies to Identify and Characterize Tick Paralysis Toxins
6.1. Purification of Paralysis Toxins Using Paralysis As Assay
6.2. Purification of Paralysis Toxins Based on Bioassays and Molecular Tools
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Howell, C.J.; Walker, J.B.; Nevill, E.M. Ticks, Mites and Insects of Domestic Animals of South Africa; Scientific Pamphlet, No. 393; Department of Agriculture-Technical Services, Republic of South Africa: Pretoria, South Africa, 1983.
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E. Order Ixodida. In A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 111–123. ISBN 978-0-89672-620-8. [Google Scholar]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 28, 1–28. [Google Scholar]
- Gregson, J.D. Tick Paralysis: An Appraisal of Natural and Experimental Data; Information Division, Canada Department of Agriculture: Ottawa, ON, Canada, 1973.
- Gothe, R.; Neitz, A.W.H. Tick Paralyses: Pathogenesis and etiology. In Advances in Disease and Vector Research; Harris, K.F., Ed.; Springer: New York, NY, USA, 1991; pp. 177–204. ISBN 978-1-4612-3110-3. [Google Scholar]
- Malik, R.; Farrow, B.R. Tick paralysis in North America and Australia. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 157–171. [Google Scholar] [CrossRef]
- Mans, B.J.; Gothe, R.; Neitz, A.W. Biochemical perspectives on paralysis and other forms of toxicoses caused by ticks. Parasitology 2004, 129, S95–S111. [Google Scholar] [CrossRef] [PubMed]
- Hall-Mendelin, S.; Craig, S.B.; Hall, R.A.; O’Donoghue, P.; Atwell, R.B.; Tulsiani, S.M.; Graham, G.C. Tick paralysis in Australia caused by Ixodes holocyclus Neumann. Ann. Trop. Med. Parasitol. 2011, 105, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Durden, L.A.; Mans, B.J. Tick paralysis: Some host and tick perspectives. In A Century of Parasitology: Discoveries, Ideas and Lessons Learned by Scientists Who Published in the Journal of Parasitology, 1914–2014, 1st ed.; Janovy, J., Esch, G.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 167–176. ISBN 978-1-118-88476-8. [Google Scholar]
- Gothe, R. Zecken Toxikosen, 1st ed.; Hieronyms: Münich, Germany, 1999; ISBN 3-89791-083-7. [Google Scholar]
- Neitz, W.O. The different forms of tick toxicosis: A review. In Proceedings of the Second Meeting of the FAO/OIE Expert Panel of Tick-Borne Diseases of Livestock, Cairo, UAR, 3–10 December 1962. [Google Scholar]
- Mans, B.J.; Steinman, C.M.L.; Venter, J.D.; Louw, A.I.; Neitz, A.W.H. Pathogenic mechanisms of sand tampan toxicosis induced by Ornithodoros savignyi. Toxicon 2002, 40, 1007–1016. [Google Scholar] [CrossRef]
- Neitz, W.O. Hyalomma transiens Schulze: A vector of sweating sickness. J. S. Afr. Vet. Med. Assoc. 1954, 25, 19–20. [Google Scholar]
- Neitz, W.O. Studies on the aetiology of sweating sickness. Onderstepoort J. Vet. Res. 1956, 27, 197–203. [Google Scholar]
- O’kelly, J.C.; Seifert, G.W. The effects of tick (Boophilus microplus) infestations on the blood composition of shorthorn X Hereford cattle on high and low planes of nutrition. Aust. J. Biol. Sci. 1970, 23, 681–690. [Google Scholar] [CrossRef]
- Constantin, T.; Paraschiv, I.; Ioniţă, M.; Mitrea, I.L. The efficacy of different acaricides against the hard tick Dermacentor marginatus on infested sheep. Sci. Works C Ser. Vet. Med. 2012, LVIII, 372–379. [Google Scholar]
- Thomas, A.D.; Neitz, W.O. Rhipicephaline tick toxicosis in cattle: It’s possible aggravating effects on certain diseases. J. S. Afr. Vet. Assoc. 1958, 29, 39–54. [Google Scholar]
- Kassis, I.; Offe-Uspensky, I.; Uspensky, I.; Mumcuoglu, K.Y. Human toxicosis caused by the tick Ixodes redikorzevi in Israel. Isr. J. Med. Sci. 1997, 33, 760–761. [Google Scholar] [PubMed]
- Roberts, F.H.S. A note on Argas (Ornithodoros) gurneyi Warburton. Aust. Vet. J. 1936, 12, 239–240. [Google Scholar] [CrossRef]
- Guernier, V.; Milinovich, G.J.; Bezerra Santos, M.A.; Haworth, M.; Coleman, G.; Soares Magalhaes, R.J. Use of big data in the surveillance of veterinary diseases: Early detection of tick paralysis in companion animals. Parasites Vectors 2016, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Binnington, K.C.; Kemp, D.H. Role of tick salivary glands in feeding and disease transmission. Adv. Parasitol. 1980, 18, 315–339. [Google Scholar] [PubMed]
- Mans, B.J.; Louw, A.I.; Neitz, A.W. The major tick salivary gland proteins and toxins from the soft tick, Ornithodoros savignyi, are part of the tick lipocalin family: Implications for the origins of tick toxicoses. Mol. Biol. Evol. 2003, 20, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Francischetti, I.M. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.S.; Sauer, J.R. Tick salivary glands: Function, physiology and future. Parasitology 2004, 129, S67–S81. [Google Scholar] [CrossRef] [PubMed]
- Hovius, J.W.R.; Levi, M.; Fikrig, E. Salivating for knowledge: Potential pharmacological agents in tick saliva. PLoS Med. 2008. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.; Sa-Nunes, A.; Mans, B.J.; Santos, I.M.; Ribeiro, J.M. The role of saliva in tick feeding. Front. Biosci. 2009, 14, 2051–2088. [Google Scholar] [CrossRef]
- Chmelar, J.; Calvo, E.; Pedra, J.H.; Francischetti, I.M.; Kotsyfakis, M. Tick salivary secretion as a source of antihemostatics. J. Proteom. 2012, 75, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Kazimírová, M.; Štibrániová, I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol. 2013, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.E. Antihemostatic strategies of blood-feeding arthropods. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Neitz, A.W. Adaptation of ticks to a blood-feeding environment: Evolution from a functional perspective. Insect Biochem. Mol. Biol. 2004, 34, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.; Doube, B.; Binnington, K.; Goodger, B. Toxins of the Australian paralysis tick Ixodes holocyclus. In Recent Advances in Acarology; Rodriguez, J.G., Ed.; Academic Press Inc.: London, UK, 1979; Volume 1, pp. 347–356. ISBN 978-0-12-592201-2. [Google Scholar]
- Vink, S.; Daly, N.L.; Steen, N.; Craik, D.J.; Alewood, P.F. Holocyclotoxin-1, a cystine knot toxin from Ixodes holocyclus. Toxicon 2014, 90, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chand, K.K.; Lee, K.M.; Lavidis, N.A.; Rodriguez-Valle, M.; Ijaz, H.; Koehbach, J.; Clark, R.J.; Lew-Tabor, A.; Noakes, P.G. Tick holocyclotoxins trigger host paralysis by presynaptic inhibition. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Valle, M.; Moolhuijzen, P.; Barrero, R.A.; Ong, C.T.; Busch, G.; Karbanowicz, T.; Booth, M.; Clark, R.; Koehbach, J.; Ijaz, H.; et al. Transcriptome and toxin family analysis of the paralysis tick, Ixodes holocyclus. Int. J. Parasitol. 2017, 48, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; de Castro, M.H.; Pienaar, R.; de Klerk, D.; Gaven, P.; Genu, S.; Latif, A.A. Ancestral reconstruction of tick lineages. Ticks Tick-Borne Dis. 2016, 7, 509–535. [Google Scholar] [CrossRef] [PubMed]
- Hauke, T.J.; Herzig, V. Dangerous arachnids—Fake news or reality? Toxicon 2017, 138, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, W.R. The evolution and distribution of noxious species of scorpions (Arachnida: Scorpiones). J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24. [Google Scholar] [CrossRef] [PubMed]
- Stampa, S. Tick paralysis in the Karoo areas of South Africa. Onderstepoort J. Vet. Res. 1959, 28, 169–227. [Google Scholar]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Valdés, J.J. Are ticks venomous animals? Front. Zool. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Klompen, J.S.H.; Black, W.C., IV; Keirans, J.E.; Norris, D.E. Systematics and biogeography of hard ticks, a total evidence approach. Cladistics 2000, 16, 79–102. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.N.; Fry, B.G. A tricky trait: Applying the fruits of the “function debate” in the philosophy of biology to the “venom debate” in the science of toxinology. Toxins 2016, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.; Ho, S.Y.; Hogg, P.J. Disulfide bond acquisition through eukaryotic protein evolution. Mol. Biol. Evol. 2011, 28, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J. Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J. Innate Immun. 2011, 3, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Moran, Y. The rise and fall of an evolutionary innovation: Contrasting strategies of venom evolution in ancient and young animals. PLoS Genet. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.E.; Proctor, H.C. Feeding behaviour and phylogeny: Observations on early derivative Acari. Exp. Appl. Acarol. 1998, 22, 39–50. [Google Scholar] [CrossRef]
- Foelix, R.; Erb, B. Mesothelae have venom glands. J. Arachnol. 2010, 38, 596–598. [Google Scholar] [CrossRef]
- Kuhn-Nentwig, L.; Stöcklin, R.; Nentwig, W. Venom composition and strategies in spiders: Is everything possible? Adv. Insect Phys. 2011, 40, 1–86. [Google Scholar]
- Wells, M.B.; Andrew, D.J. Salivary gland cellular architecture in the Asian malaria vector mosquito Anopheles stephensi. Parasites Vectors 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Yigit, N.; Benli, M. The venom gland of the scorpion species Euscorpius mingrelicus (Scorpiones: Euscorpiidae): Morphological and ultrastructural characterization. J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 466–480. [Google Scholar] [CrossRef]
- Mans, B.J. Glandular matrices and secretions: Blood-feeding arthropods. In Extracellular Composite Matrices in Arthropods, 1st ed.; Cohen, E., Moussian, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 625–688. ISBN 978-3-319-40738-8. [Google Scholar]
- Berkov, A.; Rodríguez, N.; Centeno, P. Convergent evolution in the antennae of a cerambycid beetle, Onychocerus albitarsis, and the sting of a scorpion. Naturwissenschaften 2008, 95, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Krenn, H.W.; Aspöck, H. Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod Struct. Dev. 2012, 41, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Why do we study animal toxins? Zool. Res. 2015, 36, 183–222. [Google Scholar] [PubMed]
- Stone, B.F.; Binnington, K.C.; Gauci, M.; Aylward, J.H. Tick/host interactions for Ixodes holocyclus: Role, effects, biosynthesis and nature of its toxic and allergenic oral secretions. Exp. Appl. Acarol. 1989, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Crause, J.C.; Verschoor, J.A.; Burger, D.B.; Neitz, A.W.H. Preparation of monoclonal antibodies against salivary gland immunogens of female Rhipicephalus evertsi evertsi. Exp. Appl. Acarol. 1991, 13, 75–80. [Google Scholar] [CrossRef]
- Crause, J.C.; Verschoor, J.A.; Coetzee, J.; Hoppe, H.C.; Taljaard, J.N.; Gothe, R.; Neitz, A.W. The localization of a paralysis toxin in granules and nuclei of pre-fed female Rhipicephalus evertsi evertsi tick salivary gland cells. Exp. Appl. Acarol. 1993, 17, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Gothe, R.; Gold, Y.; Bezuidenhout, J.D. Investigations into the paralysis-inducing ability of Rhipicephalus evertsi mimeticus and that of hybrids between this subspecies and Rhipicephalus evertsi evertsi. Onderstepoort J. Vet. Res. 1986, 53, 25–29. [Google Scholar] [PubMed]
- Kordiš, D.; Gubenšek, F. Adaptive evolution of animal toxin multigene families. Gene 2000, 261, 43–52. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Labruna, M.B.; Mans, B.J.; Maruyama, S.R.; Francischetti, I.M.; Barizon, G.C.; de Miranda Santos, I.K. The sialotranscriptome of Antricola delacruzi female ticks is compatible with non-hematophagous behavior and an alternative source of food. Insect Biochem. Mol. Biol. 2012, 42, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.B.; Pham, V.M.; Mans, B.J.; Andersen, J.F.; Mather, T.N.; Lane, R.S.; Ribeiro, J.M.C. The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae). Insect Biochem. Mol. Biol. 2005, 35, 1142–1161. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N.M.; Neitz, A.W. Biochemical studies on the eggs of Amblyomma hebraeum. Onderstepoort J. Vet. Res. 1987, 54, 451–459. [Google Scholar] [PubMed]
- Sunagar, K.; Undheim, E.A.B.; Chan, A.H.C.; Koludarov, I.; Muñoz-Gómez, S.A.; Antunes, A.; Fry, B.G. Evolution stings: The origin and diversification of scorpion toxin peptide scaffolds. Toxins 2013, 5, 2456–2487. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Norman, J.A. Tentacles of venom: Toxic protein convergence in the kingdom Animalia. J. Mol. Evol. 2009, 68, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Undheim, E.A.; Mobli, M.; King, G.F. Toxin structures as evolutionary tools: Using conserved 3D folds to study the evolution of rapidly evolving peptides. Bioessays 2016, 38, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Alarcon-Chaidez, F.; Francischetti, I.M.; Mans, B.J.; Mather, T.N.; Valenzuela, J.G.; Wikel, S.K. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 2006, 36, 111–129. [Google Scholar] [CrossRef] [PubMed]
- King, G.F.; Hardy, M.C. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Klint, J.K.; Senff, S.; Rupasinghe, D.B.; Er, S.Y.; Herzig, V.; Nicholson, G.M.; King, G.F. Spider-venom peptides that target voltage-gated sodium channels: Pharmacological tools and potential therapeutic leads. Toxicon 2012, 60, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Andersen, J.F.; Francischetti, I.M.; Valenzuela, J.G.; Schwan, T.G.; Pham, V.M.; Garfield, M.K.; Hammer, C.H.; Ribeiro, J.M. Comparative sialomics between hard and soft ticks: Implications for the evolution of blood-feeding behavior. Insect Biochem. Mol. Biol. 2008, 38, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Featherston, J.; de Castro, M.H.; Pienaar, R. Gene duplication and protein evolution in tick-host interactions. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.S.; Grimaldi, D.A. Primitive new ants in cretaceous amber from Myanmar, New Jersey, and Canada (Hymenoptera: Formicidae). Am. Mus. Novit. 2005, 23, 1–24. [Google Scholar] [CrossRef]
- Hwang, W.S.; Weirauch, C. Evolutionary history of assassin bugs (Insecta: Hemiptera: Reduviidae): insights from divergence dating and ancestral state reconstruction. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.A.; Weirauch, C.; Fry, B.G.; King, G.F. Venoms of Heteropteran insects: A treasure trove of diverse pharmacological toolkits. Toxins 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.A.; Madio, B.; Jin, J.; Undheim, E.A.; Fry, B.G.; King, G.F. Melt with this kiss: Paralyzing and liquefying venom of the assassin bug Pristhesancus plagipennis (Hemiptera: Reduviidae). Mol. Cell. Proteom. 2017, 16, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.A.; Mayhew, M.L.; Jin, J.; Herzig, V.; Undheim, E.A.B.; Sombke, A.; Fry, B.G.; Merrit, D.J.; King, G.F. The assassin bug Pristhesancus plagipennis produces two distinct venoms in separate gland lumens. Nat. Commun. 2018, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.A.; Hernández-Vargas, M.J.; Corzo, G.; Fry, B.G.; King, G.F. Giant fish-killing water bug reveals ancient and dynamic venom evolution in Heteroptera. Cell. Mol. Life Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gothe, R.; Lämmler, M. Sensitivity of laboratory animals to Rhipicephalus evertsi evertsi paralysis. Zentralbl. Veterinarmed. B 1982, 29, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mans, B.J. Tick-induced paralysis and toxicoses. In Biology of Ticks, 2nd ed.; Sonenshine, D.E., Roe, M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 2, pp. 313–332. ISBN 978-0-19-974406-0. [Google Scholar]
- Masina, S.; Broady, K.W. Tick paralysis: Development of a vaccine. Int. J. Parasitol. 1999, 29, 535–541. [Google Scholar] [CrossRef]
- Spickett, A.M.; Elliott, E.G.; Heyne, H.; Neser, J.A. Paralysis of laboratory rabbits by nymphae of Ixodes rubicundus, Neumann 1904 (Acarina: Ixodidae) and some effects on the life-cycle following feeding under different temperature conditions. Onderstepoort J. Vet. Res. 1989, 56, 59–62. [Google Scholar] [PubMed]
- Jessup, D.A. Tick paralysis in a grey fox. J. Wildl. Dis. 1979, 15, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E. Dermacentor rhinocerinus (Denny 1843) (Acari: Ixodida: ixodidae): Re-description of the male, female and nymph and first description of the larva. Onderstepoort J. Vet. Res. 1993, 60, 59–68. [Google Scholar] [PubMed]
- Lysyk, T.J.; Majak, W. Increasing the paralyzing ability of a laboratory colony of Dermacentor andersoni Stiles. J. Med. Entomol. 2003, 40, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Gregson, J.D. The enigma of tick paralysis. Proc. J. Entomol. Soc. B. C. 1943, 40, 19–23. [Google Scholar]
- Ilkiw, J.E.; Turner, D.M.; Howlett, C.R. Infestation in the dog by the paralysis tick Ixodes holocyclus. 1. Clinical and histological findings. Aust. Vet. J. 1987, 64, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, G.J.; van Wyngaardt, S.; Gothe, R.; Visser, L.; Bezuidenhout, J.D.; Neitz, A.W. The detection and isolation of a paralysis toxin present in Argas (Persicargas) walkerae. Onderstepoort J. Vet. Res. 1990, 57, 163–168. [Google Scholar] [PubMed]
- Gothe, R. Tick paralysis in chickens caused by Argas (Persicargas) persicus-larvae—I. Influence of state of engorgement and infestation rate on clinical symptoms. Z. Parasitenkd. 1971, 35, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Gothe, R.; Kunze, K. Stimulus conduction of efferent and afferent peripheral nerve fibers in fowl tick paralysis caused by Argas (Persicargas) persicus larvae. Z. Tropenmed. Parasitol. 1971, 22, 292–296. [Google Scholar] [PubMed]
- Maritz, C.; Louw, A.I.; Gothe, R.; Neitz, A.W.H. Neuropathogenic properties of Argas (Persicargas) walkerae larval homogenates. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 233–239. [Google Scholar] [CrossRef]
- Cooper, B.J.; Spence, I. Temperature dependent inhibition of evoked acetylcholine release in tick paralysis. Nature 1976, 263, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Zamponi, G.W. Block of voltage-gated calcium channels by peptide toxins. Neuropharmacology 2017, 127, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Thurn, M.J.; Gooley, A.; Broady, K.W. Identification of the neurotoxin from the Australian paralysis tick, Ixodes holocyclus. In Recent Advances in Toxicology Research; Gopalakrishnakone, P., Tan, C.K.Ž., Eds.; National University of Singapore Venom and Toxin Research Group: Singapore, 1992; Volume 2, pp. 243–256. [Google Scholar]
- Maritz, C.; Louw, A.I.; Gothe, R.; Neitz, A.W.H. Detection and micro-scale isolation of a low molecular mass paralysis toxin from the tick, Argas (Persicargas) walkerae. Exp. Appl. Acarol. 2000, 24, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, G.J.; Bezuidenhout, J.D.; Oberem, P.T.; Vermeulen, N.M.; Visser, L.; Gothe, R.; Neitz, A.W. Isolation of a neurotoxin from the salivary glands of female Rhipicephalus evertsi evertsi. J. Parasitol. 1986, 72, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Crause, J.C.; van Wyngaardt, S.; Gothe, R.; Neitz, A.W.H. A shared epitope found in the major paralysis inducing tick species of Africa. Exp. Appl. Acarol. 1994, 18, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, S. Tick paralysis in sheep. Farming S. Afr. 1928, 2, 661–662. [Google Scholar]
- Stone, B.F.; Neish, A.L.; Wright, I.G. Tick (Ixodes holocyclus) paralysis in the dog-quantitative studies on immunity following artificial infestation with the tick. Aust. Vet. J. 1983, 60, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.C. An experimental study of tick paralysis in Australia. Parasitology 1927, 18, 310–329. [Google Scholar] [CrossRef]
- Ross, I.C. Tick paralysis: A fatal disease of dogs and other animals in eastern Australia. J. Counc. Sci. Ind. Res. Aust. 1935, 8, 8–13. [Google Scholar]
- Kaire, G.H. Isolation of tick paralysis toxin from Ixodes holocyclus. Toxicon 1966, 4, 91–97. [Google Scholar] [CrossRef]
- Stone, B.F.; Binnington, K.C. The paralyzing toxin and other immunogens of the tick Ixodes holocyclus and the role of the salivary gland in their biosynthesis. In Morphology, Physiology and Behavioral Biology of Ticks; Sauer, J.R., Hair, J.A., Eds.; Ellis Horwood Ltd.: Herts, UK, 1986; pp. 75–99. ISBN 0853128413. [Google Scholar]
- Stone, B.F.; Aylward, J.H. Tick toxicoses and the causal toxins: Tick paralysis. In Progress in Venom and Toxin Research, Proceedings of the First Asia–Pacific Congress on Animal, Plant and Microbial Toxins; Gopalakrishnakone, P., Tan, C.K., Eds.; National University of Singapore Press: Singapore, 1987; pp. 594–602. ISBN 9971621525. [Google Scholar]
- Kraiss, A.; Gothe, R. Efficacy of the paraimmunity inducer PIND-AVI in Rhipicephalus evertsi evertsi infestations in sheep: Current perspectives in tick control and the prevention of tick paralysis. Zentralbl. Veterinarmed. B 1984, 31, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Paesen, G.C.; Siebold, C.; Dallas, M.L.; Peers, C.; Harlos, K.; Nuttall, P.A.; Nunn, M.A.; Stuart, D.I.; Esnouf, R.M. An Ion-channel Modulator from the saliva of the Brown Ear tick has a highly modified Kunitz/BPTI Structure. J. Mol. Biol. 2009, 389, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Liu, J.; Zhang, M.; Wang, G.; Zhao, G.; Zhang, Y.; Hu, K.; Lai, R. A sodium channel inhibitor ISTX-I with a novel structure provides a new hint at the evolutionary link between two toxin folds. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C.; Makoul, G.T.; Levine, J.; Robinson, D.R.; Spielman, A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J. Exp. Med. 1985, 161, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Mather, T.N. Ixodes scapularis: Salivary kininase activity is a metallo dipeptidyl carboxypeptidase. Exp. Parasitol. 1998, 89, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Marti-Renom, M.A.; Stuart, A.C.; Fiser, A.; Sanchez, R.; Melo, F.; Sali, A. Comparative protein structure modelling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 291–325. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.B.; Meng, Z.; Mans, B.J.; Gadderra, N.; Hall, M.; Veenstra, T.D.; Pham, V.M.; Kotsyfakis, M.; Ribeiro, J.M. An insight into the salivary transcriptome and proteome of the soft tick and vector of epizootic bovine abortion, Ornithodoros coriaceus. J. Proteom. 2008, 71, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Li, T.; Wang, S.; Lv, Y.; Zuo, Y.; Yang, L. Prediction of presynaptic and postsynaptic neurotoxins by combining various Chou’s pseudo components. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Sleator, R.D.; Walsh, P. An overview of in silico protein function prediction. Arch. Microbiol. 2010, 192, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lobb, B.; Doxey, A.C. Novel function discovery through sequence and structural data mining. Curr. Opin. Struct. Biol. 2016, 38, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Valdés, J.J.; Moal, I.H. Prediction of Kunitz ion channel effectors and protease inhibitors from the Ixodes ricinus sialome. Ticks Tick-Borne Dis. 2014, 5, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M., Jr.; Kato, I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980, 49, 593–626. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Louw, A.I.; Neitz, A.W.H. Savignygrin, a platelet aggregation inhibitor from the soft tick Ornithodoros savignyi, presents the RGD integrin recognition motif on the Kunitz-BPTI fold. J. Biol. Chem. 2002, 277, 21371–21378. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.X.; Zhang, A.D.; Huang, J.F. Evolution, expansion and expression of the Kunitz/BPTI gene family associated with long-term blood feeding in Ixodes scapularis. BMC Evol. Biol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Weisel-Eichler, A.; Libersat, F. Venom effects on monoaminergic systems. J. Comp. Physiol. A 2004, 190, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Ribeiro, J.M.; Andersen, J.F. Structure, function, and evolution of biogenic amine-binding proteins in soft ticks. J. Biol. Chem. 2008, 283. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M. Blood-feeding arthropods: Live syringes or invertebrate pharmacologists? Infect. Agents Dis. 1995, 4, 143–152. [Google Scholar] [PubMed]

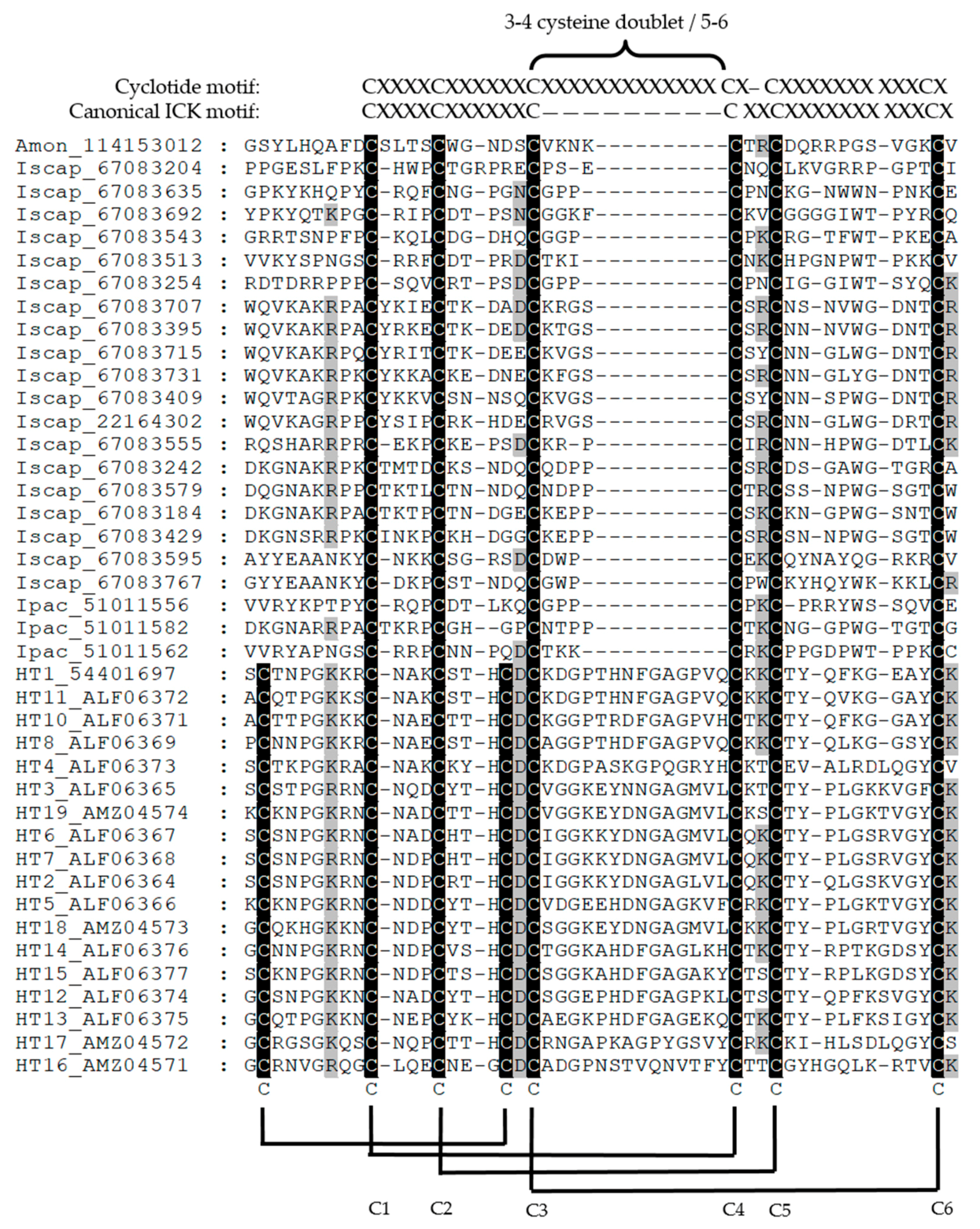
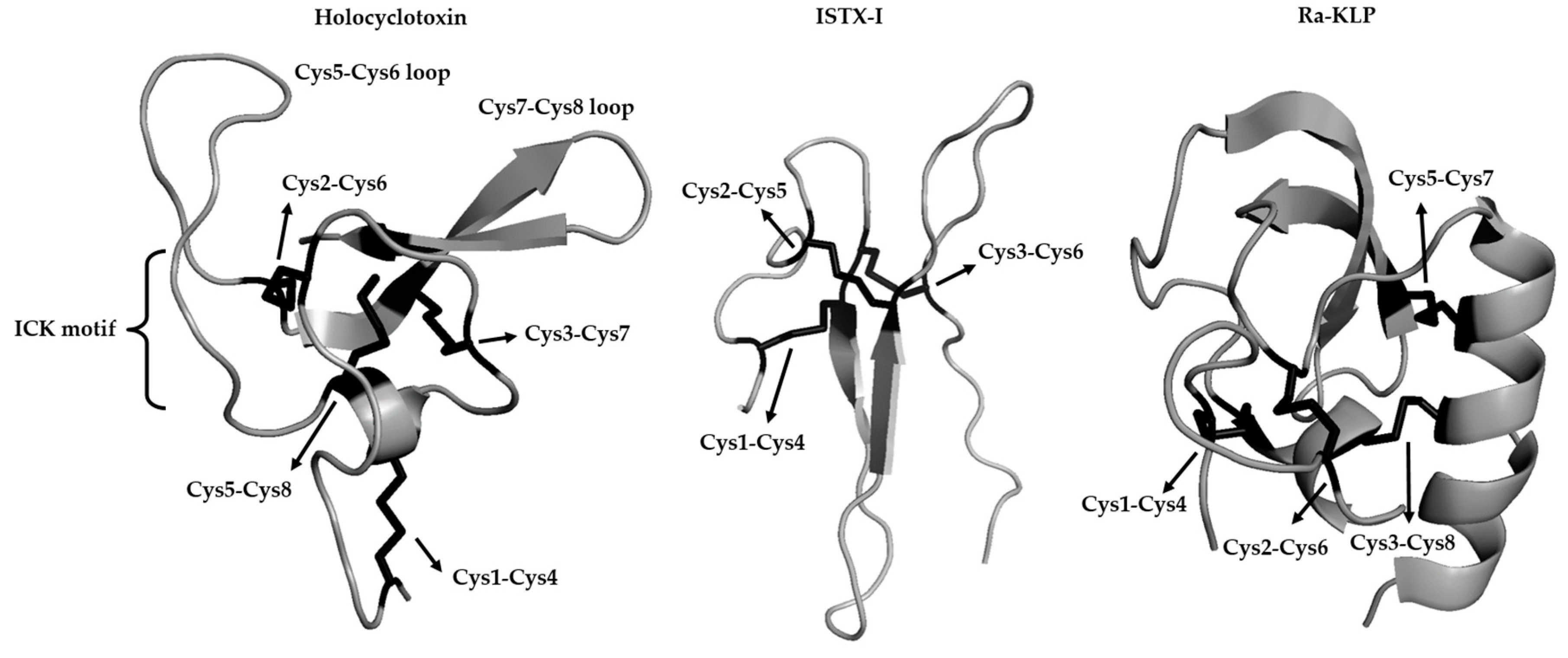
| Characteristic | I. holocyclus | R. evertsi evertsi | A. walkerae | D. andersoni |
|---|---|---|---|---|
| Life stage that cause paralysis | Nymphs and adults | Adults | Larvae | Adults |
| Mechanism of toxin | Inhibits synaptic vesicle (acetylcholine) release when binding to the synaptosomes at neuromuscular junction | Impair the conduction of impulses along the peripheral nerve fibers (nodes of Ranvier) | Inhibits Ca2+ dependent synaptic vesicle release and desensitizing its receptor | Motor polyneuropahty with limited participation of the afferent pathways |
| Size | 40–80 kDa; HT-1 = 5kDa | 68–70 kDa 74 kDa | 11 kDa/range of 11–115 kDa 80–100 kDa/32 and 60 kDa | 37–43 kDa |
| Recovery after tick removal | Prolonged (Days to weeks) with initial deterioration of host’s condition | Within hours to two days | Within hours | Within hours |
| Antiserum therapy | Useful in early stage of paralysis | None available | None available | None available |
| Immunity | Full | Limited | Partial | Dose dependent immunity |
| Isoelectric point | 8.86 4.5–5 | 6 | 4.5 | Unknown |
| Protease digestion | Resistant | Inactivate toxin | Unknown | Unknown |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pienaar, R.; Neitz, A.W.H.; Mans, B.J. Tick Paralysis: Solving an Enigma. Vet. Sci. 2018, 5, 53. https://doi.org/10.3390/vetsci5020053
Pienaar R, Neitz AWH, Mans BJ. Tick Paralysis: Solving an Enigma. Veterinary Sciences. 2018; 5(2):53. https://doi.org/10.3390/vetsci5020053
Chicago/Turabian StylePienaar, Ronel, Albert W. H. Neitz, and Ben J. Mans. 2018. "Tick Paralysis: Solving an Enigma" Veterinary Sciences 5, no. 2: 53. https://doi.org/10.3390/vetsci5020053
APA StylePienaar, R., Neitz, A. W. H., & Mans, B. J. (2018). Tick Paralysis: Solving an Enigma. Veterinary Sciences, 5(2), 53. https://doi.org/10.3390/vetsci5020053






