Shape Variation in the Craniomandibular System and Prevalence of Dental Problems in Domestic Rabbits: A Case Study in Evolutionary Veterinary Science
Abstract
:1. Introduction
2. Materials and Methods
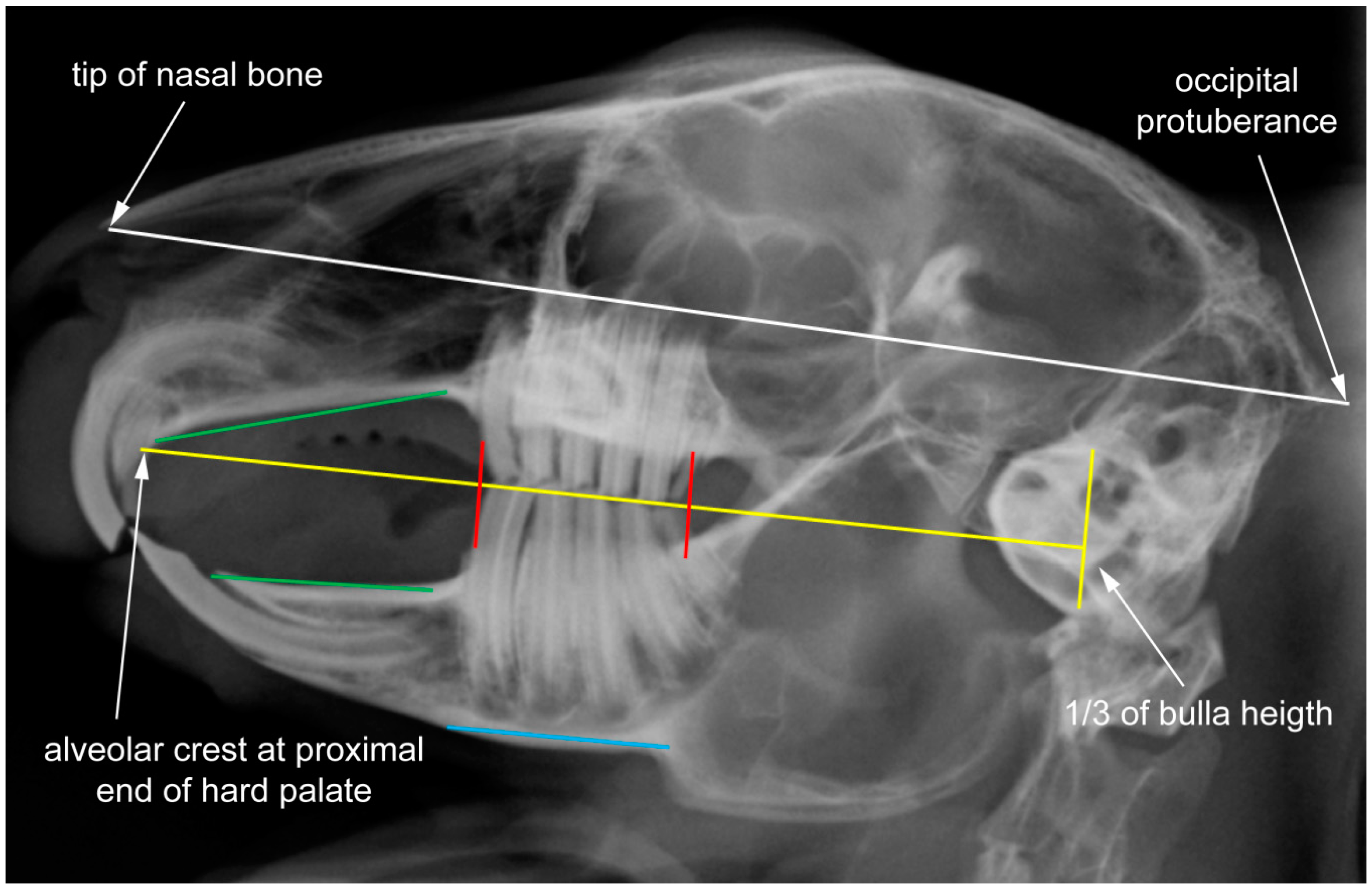
2.1. Data Set and Radiographic Screening
2.2. Shape Analysis
2.3. Integration and Modularity
3. Results
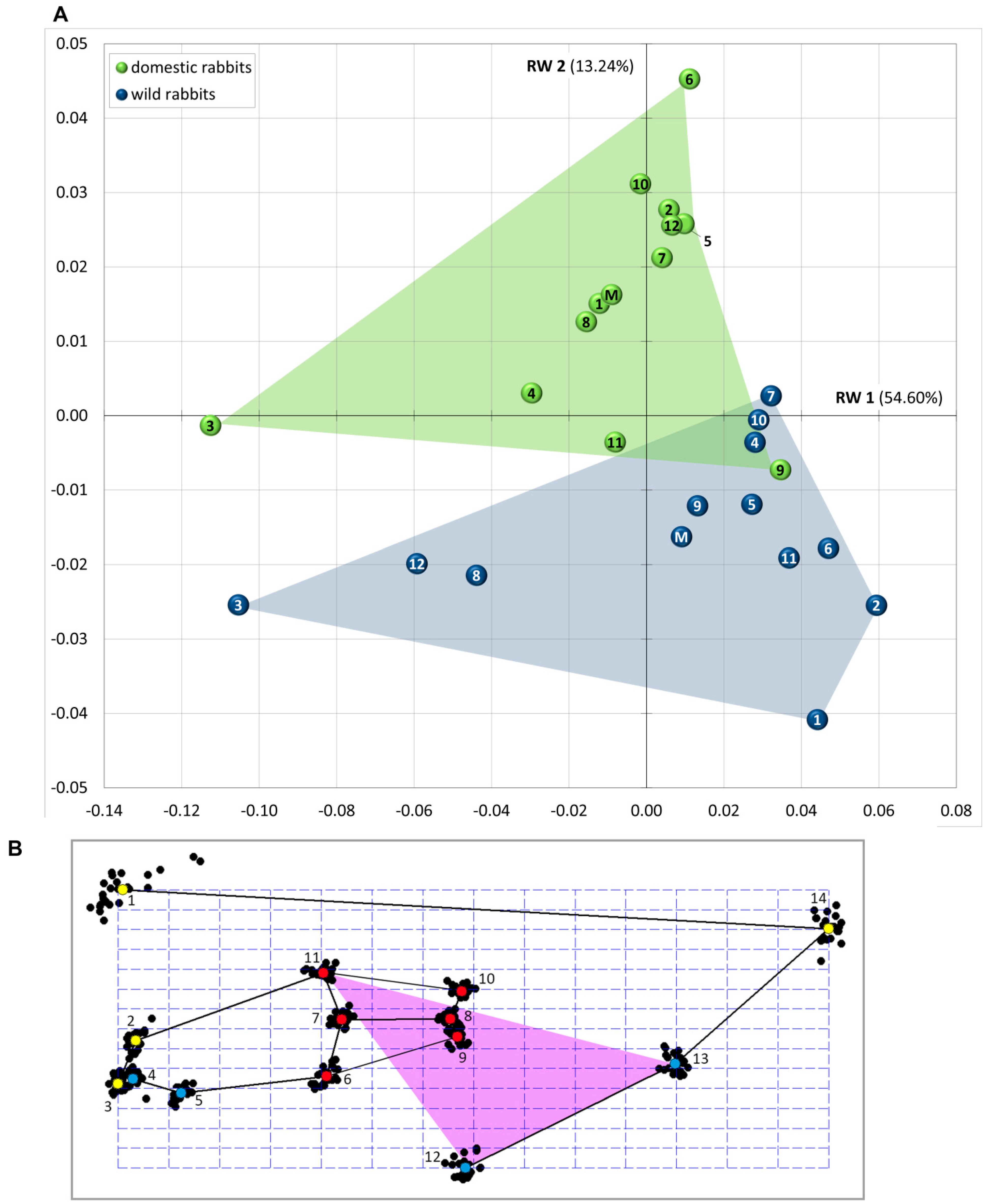
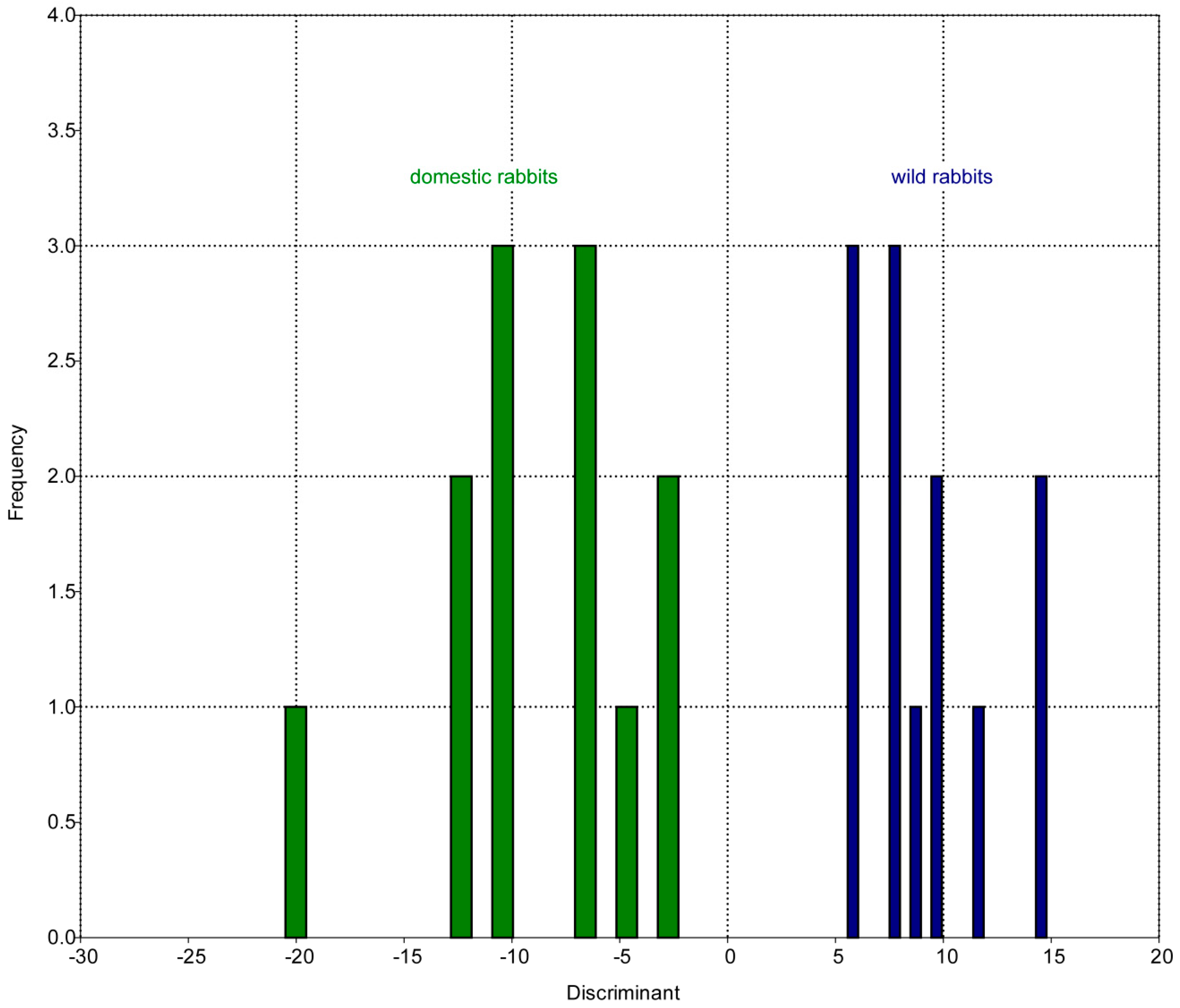
3.1. Patterns of Morphological Diversification
3.2. Allometry: Size and Shape
3.3. Landmark Variance
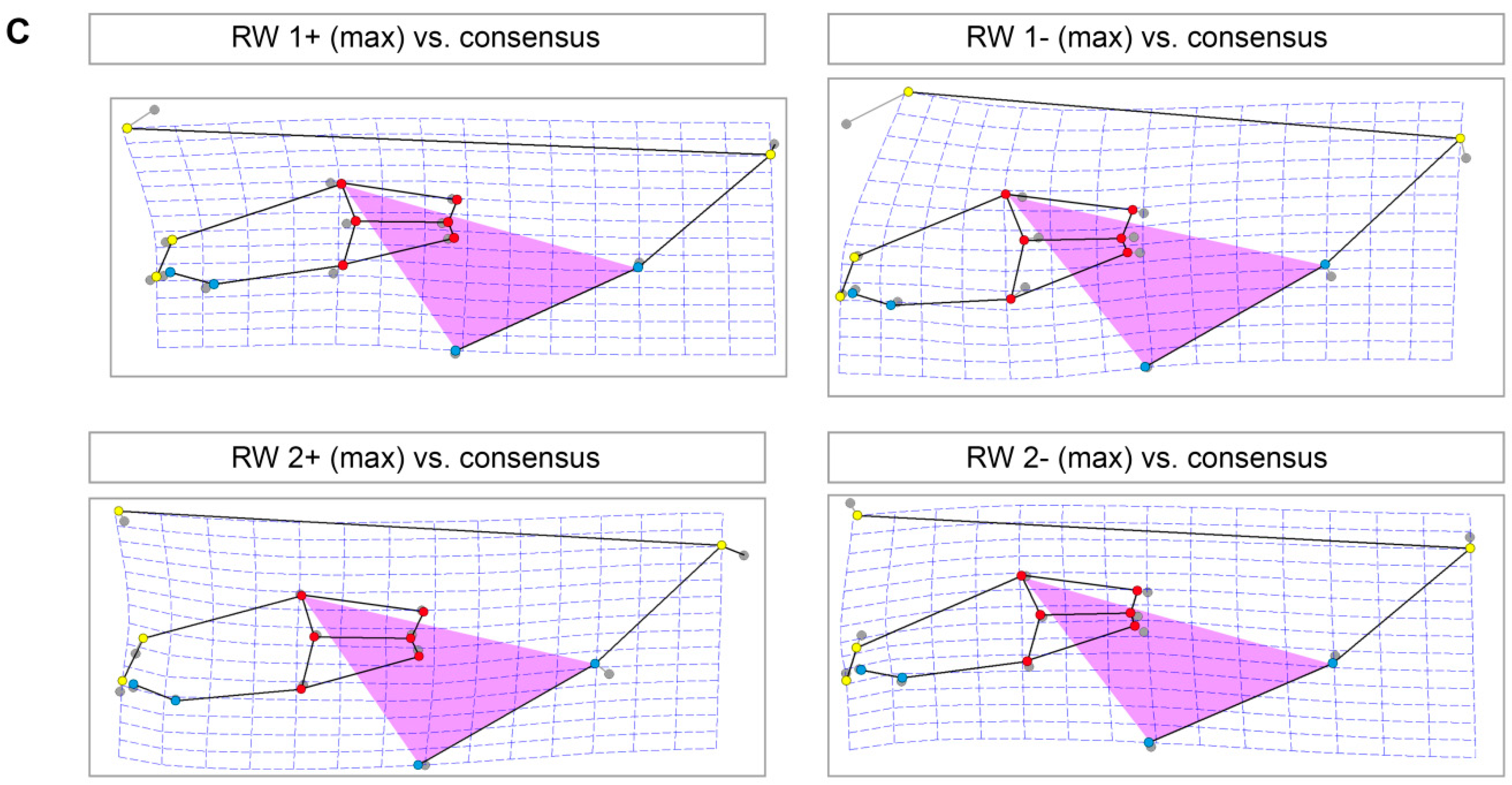
3.4. Skull Shape Variation (Thin-Plate Splines)
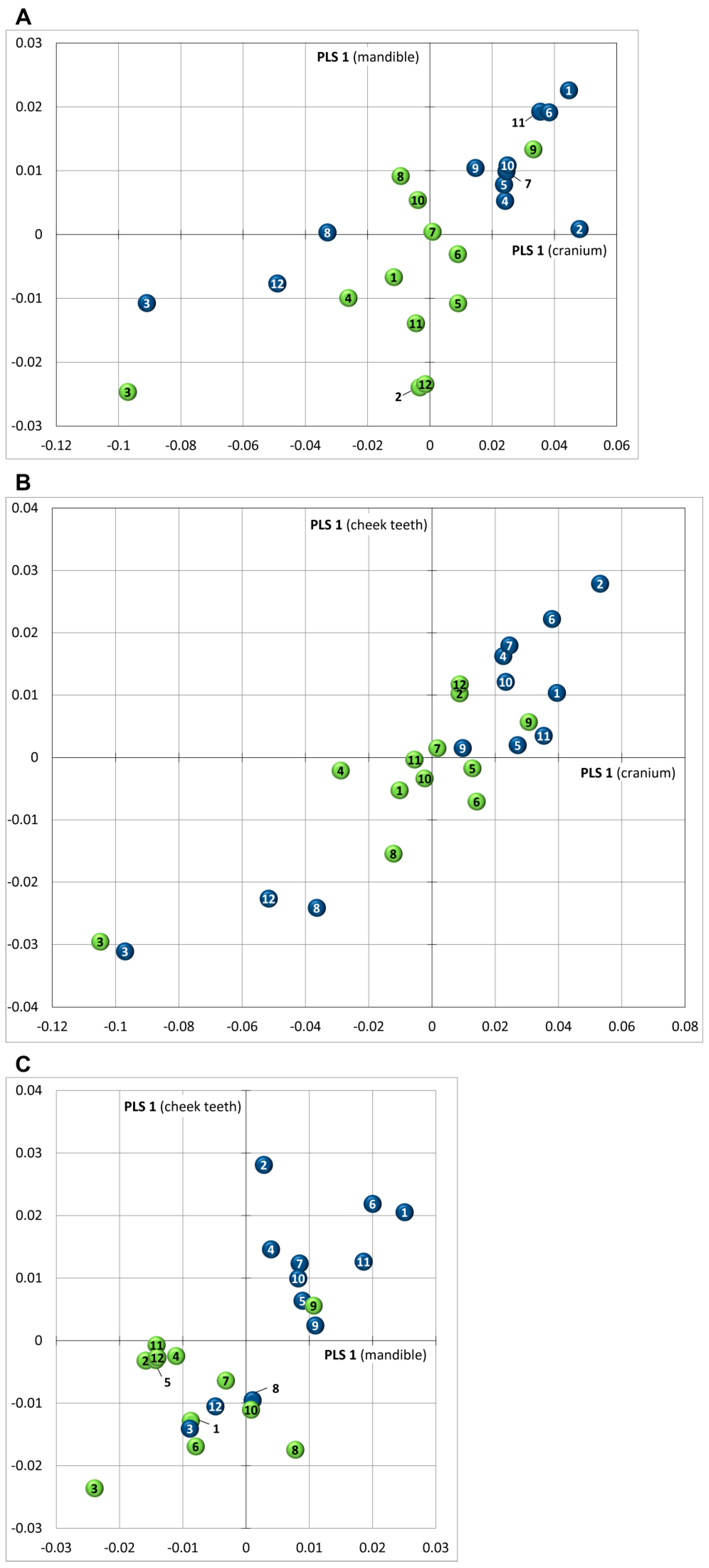
3.5. Covariance
4. Discussion
4.1. Morphological Diversification and Allometry
4.2. Variance and Covariance in Skull Shape: Implications for Diagnostic Analysis (Clinical Relevance)
4.3. Effect of Variation in Incisor Region
4.4. Constraints in Molar Region
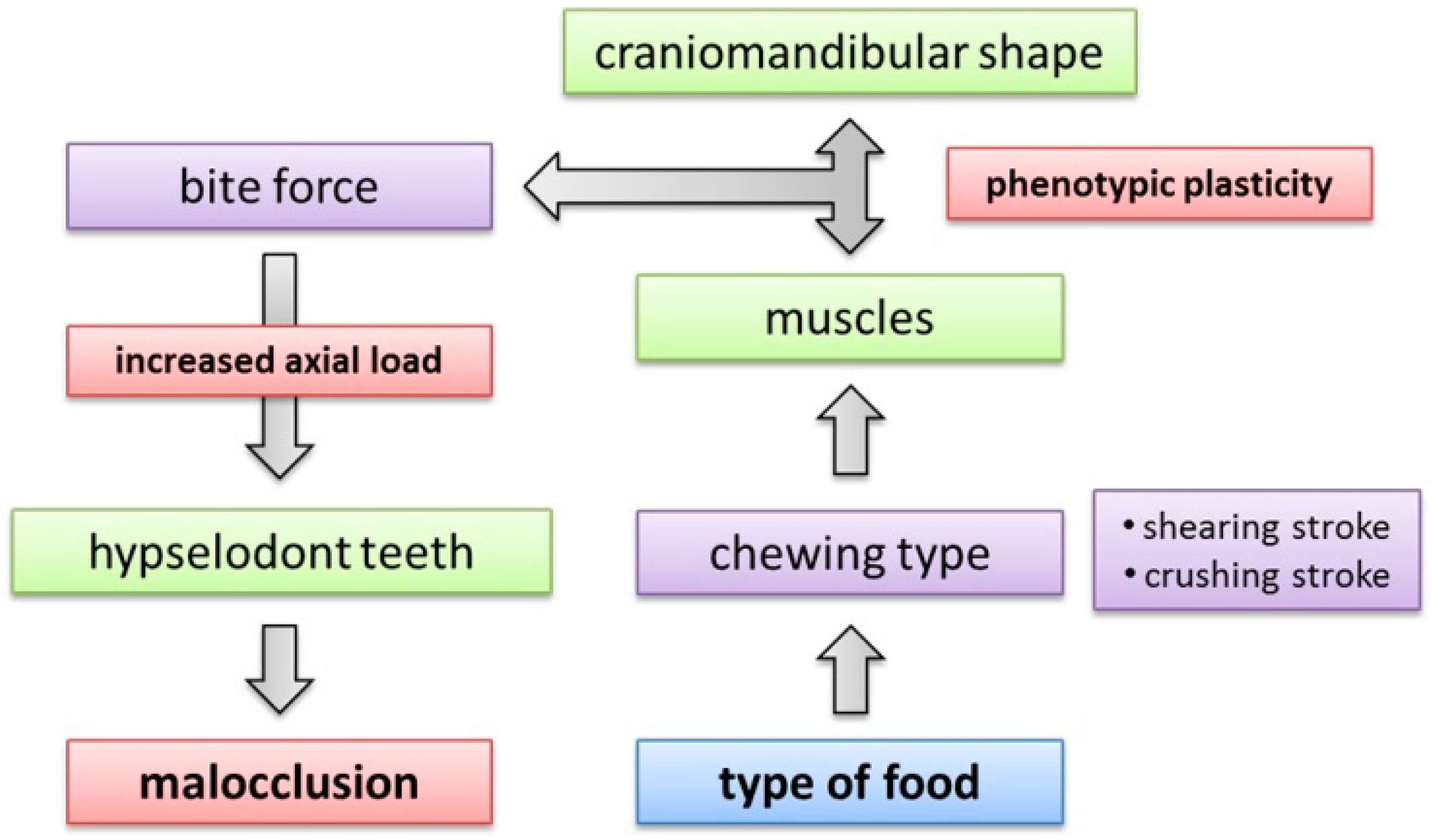
4.5. Implications of Craniomandibular Shape Variation for Masticatory Performance
4.6. Phenotypic Plasticity in the Mammalian Feeding Apparatus
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Böhmer, E. Dentistry in Rabbits and Rodents; Wiley Blackwell: Chichester, UK, 2015; p. 288. [Google Scholar]
- Jekl, V.; Hauptman, K.; Knotek, Z. Quantitative and qualitative assessments of intraoral lesions in 180 small herbivorous mammals. Vet. Rec. 2008, 162, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Crossley, D.A.; Penman, S. (Eds.) Manual of Small Animal Dentistry, 2nd ed.; British Small Animal Veterinary Association (BSAVA): Cheltenham, UK, 1995; p. 245.
- Böhmer, E.; Crossley, D. Objective interpretation of dental disease in rabbits, guinea pigs and chinchillas. Eur. J. Comp. Anim. Pract. 2011, 21, 47–56. [Google Scholar]
- Rooney, N.J.; Mullan, S.M.; Baker, P.E.; Hill, J.M.; Sealey, C.E.; Turner, M.J.; Held, S.D.E.; Blackwell, E.J.; Saunders, R. The current state of welfare, housing and husbandry of the English pet rabbit population. BMC Res. Notes 2014, 7, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Mullan, S.M.; Main, D.C. Survey of the husbandry, health and welfare of 102 pet rabbits. Vet. Rec. 2006, 159, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Harcourt-Brown, F.M. Diagnosis, treatment and prognosis of dental disease in pet rabbits. In Pract. 1997, 19, 407–427. [Google Scholar] [CrossRef]
- Crossley, D.A. Rodent and rabbit radiology. In An Atlas of Veterinary Dental Radiology; DeForge, D.H., Colmery, B.H., Eds.; Iowa State University Press: Ames, IA, USA, 2000; pp. 247–259. [Google Scholar]
- Gracis, M. Clinical technique: Normal dental radiography of rabbits, guinea pigs, and chinchillas. J. Exot. Pet. Med. 2008, 17, 78–86. [Google Scholar] [CrossRef]
- Crossley, D.A. Clinical aspects of rodent dental anatomy. J. Vet. Dent. 1995, 12, 131–135. [Google Scholar] [PubMed]
- Böhmer, E. Röntgendiagnostik bei Zahn-sowie Kiefererkrankungen der Hasenartigen und Nager. Teil 1: Tierartspezifische Zahn-und Kieferanatomie sowie Pathologie, Indikationen für die Röntgendiagnostik. Tierarzliche Praxis Kleintiere 2001, 29, 316–327. [Google Scholar]
- Darwin, C. The Variation of Animals and Plants under Domestication; John Murray: London, UK, 1868; p. 411. [Google Scholar]
- Clutton-Brock, J. A Natural History of Domesticated Mammals; Cambridge University Press: Cambridge, UK, 1999; p. 248. [Google Scholar]
- Larson, G.; Fuller, D.Q. The evolution of animal domestication. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 115–136. [Google Scholar] [CrossRef]
- Zeder, M.A. Core questions in domestication research. Proc. Natl. Acad. Sci. USA 2015, 112, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.; Rubin, C.J.; Di Palma, F.; Albert, F.W.; Alfoldi, J.; Barrio, A.M.; Pielberg, G.; Rafati, N.; Sayyab, S.; Turner-Maier, J.; et al. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science 2014, 345, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Albert, F.W.; Somel, M.; Carneiro, M.; Aximu-Petri, A.; Halbwax, M.; Thalmann, O.; Blanco-Aguiar, J.A.; Plyusnina, I.Z.; Trut, L.; Villafuerte, R.; et al. A comparison of brain gene expression levels in domesticated and wild animals. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Trut, L.; Oskina, I.; Kharlamova, A. Animal evolution during domestication: The domesticated fox as a model. Bioessays 2009, 31, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Frantz, L.A.; Schraiber, J.G.; Madsen, O.; Megens, H.J.; Cagan, A.; Bosse, M.; Paudel, Y.; Crooijmans, R.P.; Larson, G.; Groenen, M.A. Evidence of long-term gene flow and selection during domestication from analyses of Eurasian wild and domestic pig genomes. Nat. Genet. 2015, 47, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Piperno, D.R.; Allaby, R.G.; Purugganan, M.D.; Andersson, L.; Arroyo-Kalin, M.; Barton, L.; Vigueira, C.C.; Denham, T.; Dobney, K.; et al. Current perspectives and the future of domestication studies. Proc. Natl. Acad. Sci. USA 2014, 111, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Wiener, P.; Wilkinson, S. Deciphering the genetic basis of animal domestication. Proc. Biol. Sci. 2011, 278, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Wright, D. The genetic architecture of domestication in animals. Bioinform. Biol. Insights 2015, 9, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wayne, R.K. Cranial morphology of domestic and wild canids: The influence of development on morphological change. Evolution 1986, 40, 243–261. [Google Scholar] [CrossRef]
- Morey, D.F. Size, shape, and development in the evolution of the domestic dog. J. Archaeol. Sci. 1992, 19, 181–204. [Google Scholar] [CrossRef]
- Evin, A.; Dobney, K.; Schafberg, R.; Owen, J.; Vidarsdottir, U.S.; Larson, G.; Cucchi, T. Phenotype and animal domestication: A study of dental variation between domestic, wild, captive, hybrid and insular Sus scrofa. BMC Evol. Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Bannasch, D. Morphological variation in the dog. In The Dog and Its Genome; Ostrander, E.A., Giger, U., Lindblad-Toh, K., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2006; pp. 47–65. [Google Scholar]
- Drake, A.G.; Klingenberg, C.P. Large-scale diversification of skull shape in domestic dogs: Disparity and modularity. Am. Nat. 2010, 175, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.; Dobney, K.; Evin, A.; Cucchi, T.; Larson, G.; Vidarsdottir, U.S. The zooarchaeological application of quantifying cranial shape differences in wild boar and domestic pigs (Sus scrofa) using 3D geometric morphometrics. J. Archaeol. Sci. 2014, 43, 159–167. [Google Scholar] [CrossRef]
- Künzel, W.; Breit, S.; Oppel, M. Morphometric investigations of breed-specific features in feline skulls and considerations on their functional implications. Anat. Histol. Embryol. 2003, 32, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, M. Über die Variabilität der dorso-basalen Schädelknickungen bei Haushunden. Zool. Anz. 1982, 209, 1–32. [Google Scholar]
- Böhmer, E. Warum Leiden Hauskaninchen so Häufig an Gebiss-und Verdauungsproblemen? Ein Ratgeber für die Ernährung von Kaninchen; Curoxray: München, Germany, 2014; p. 248. [Google Scholar]
- Okuda, A.; Hori, Y.; Ichihara, N.; Asari, M.; Wiggs, R.B. Comparative observation of skeletal-dental abnormalities in wild, domestic, and laboratory rabbits. J. Vet. Dent. 2007, 24, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Ungar, P.S. Mammal. Teeth: Origin, Evolution, and Diversity; The Johns Hopkins University Press: Baltimore, MD, USA, 2010; p. 304. [Google Scholar]
- Koenigswald, W.V. Diversity of hypsodont teeth in mammalian dentitions—Construction and classification. Palaeontogr. Abt. A 2011, 294, 63–94. [Google Scholar]
- Damuth, J.; Janis, C.M. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol. Rev. Camb. Philos. Soc. 2011, 86, 733–758. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.H.; Kay, R.F. A comparative test of adaptive explanations for hypsodonty in ungulates and rodents. J. Mamm. Evol. 2001, 8, 207–229. [Google Scholar] [CrossRef]
- Rose, K.D.; DeLeon, V.B.; Missiaen, P.; Rana, R.S.; Sahni, A.; Singh, L.; Smith, T. Early Eocene lagomorph (Mammalia) from Western India and the early diversification of Lagomorpha. Proc. Biol. Sci. 2008, 275, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Wen, Z.; Xia, L.; Zhang, Z.; Erbajeva, M.; Huang, C.; Yang, Q. Evolutionary history of lagomorphs in response to global environmental change. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ni, X.; Bi, S.; Wu, W.; Ye, J.; Meng, J.; Windley, B.F. Synchronous turnover of flora, fauna, and climate at the Eocene-Oligocene Boundary in Asia. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.C.; Ferrand, N.; Hackländer, K. (Eds.) Lagomorph Biology. Evolution, Ecology, and Conservation; Springer: Berlin, Germany, 2008; p. 413.
- Herre, W.; Röhrs, M. Haustiere—Zoologisch Gesehen; Springer: Berlin, Germany, 2013; p. 412. [Google Scholar]
- Carneiro, M.; Afonso, S.; Geraldes, A.; Garreau, H.; Bolet, G.; Boucher, S.; Ferrand, N. The genetic structure of domestic rabbits. Mol. Biol. Evol. 2011, 28, 1801–1816. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.; Sanson, G. The association of tooth wear with sociality of free-ranging male koalas (Phascolarctos cinereus Goldfuss). Aust. J. Zool. 2002, 50, 621–626. [Google Scholar] [CrossRef]
- Cox, P.G.; Hautier, L. Evolution of the Rodents: Advances in Phylogeny, Functional Morphology and Development; Cambridge University Press: Cambridge, UK, 2015; p. 624. [Google Scholar]
- Schmidt-Kittler, N. Feeding specializations in rodents. Senckenberg. Lethaea 2002, 82, 141–152. [Google Scholar] [CrossRef]
- Müller, J.; Clauss, M.; Codron, D.; Schulz, E.; Hummel, J.; Fortelius, M.; Hatt, J.M. Growth and wear of incisor and cheek teeth in domestic rabbits (Oryctolagus cuniculus) fed diets of different abrasiveness. J. Exp. Zool. A Ecol. Genet. Physiol. 2014, 321, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Kamphues, J. Probleme der art-und bedarfsgerechten Ernährung kleiner Nager als Heimtiere. Prakt Tierarzt 1995, 88, 1088–1092. [Google Scholar]
- Wolf, P.; Kamphues, J. Untersuchungen zu Fütterungseinflüssen auf die Entwicklung der Incisivi bei Kaninchen, Chinchilla und Ratte. Kleintierpraxis 1996, 10, 723–732. [Google Scholar]
- Rohlf, F.J. tpsDig2. 2.17. Stony Brook, New York: Department of Ecology and Evolution, State University of New York at Stony Brook, 2013. Available online: http://life.bio.sunysb.ed/morph/ (accessed on 4 October 2012).
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991; p. 435. [Google Scholar]
- O'Higgins, P.; Jones, N. Morphologika2. 2.5: Hull York Medical School, 2006. Available online: http://sites.google.com/site/hymsfme/downloadmorphologica (accessed on 13 October 2012).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Palaeontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometrics for Biologists: A Primer; Elsevier Academic Press: New York, NY, USA, 2004; p. 443. [Google Scholar]
- Foote, M. Contributions of individual taxa to overall morphological disparity. Paleobiology 1993, 19, 403–419. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Corti, M. Use of two-block partial least-squares to study covariation in shape. Syst. Biol. 2000, 49, 740–753. [Google Scholar] [PubMed]
- Ge, D.; Yao, L.; Xia, L.; Zhang, Z.; Yang, Q. Geometric morphometric analysis of skull morphology reveals loss of phylogenetic signal at the generic level in extant lagomorphs (Mammalia: Lagomorpha). Contrib. Zool. 2015, 84, 267–284. [Google Scholar]
- Kraatz, B.P.; Sherratt, E.; Bumacod, N.; Wedel, M.J. Ecological correlates to cranial morphology in Leporids (Mammalia, Lagomorpha). PeerJ 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Koenigswald, W.v.; Anders, U.; Engels, S.; Schultz, J.A.; Ruf, I. Tooth morphology in fossil and extant Lagomorpha (Mammalia) reflects different mastication patterns. J. Mamm. Evol. 2010, 17, 275–299. [Google Scholar] [CrossRef]
- Watson, P.J.; Groning, F.; Curtis, N.; Fitton, L.C.; Herrel, A.; McCormack, S.W.; Fagan, M.J. Masticatory biomechanics in the rabbit: A multi-body dynamics analysis. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Shadle, A.R. The attrition and extrusive growth of the four major incisor teeth of domestic rabbits. J. Mammal. 1936, 17, 15–21. [Google Scholar] [CrossRef]
- Hamidur Rahman, A.S.M.; Al-Mahmud, K.A.; Nashiru-Islam, K.M. Dental malocclusion in New Zealand white rabbit. Bangladesh Vet. J. 1983, 16, 85–88. [Google Scholar]
- Keil, A. Über die Frage des natürlichen Vorkommens und der experimentellen Erzeugung echter Karies bei Tieren (Discussion of the natural occurrence of dental caries and it sexperimental production of true caries in animals. Deutsche Zahnärztl. Ztschr. 1949, 4, 694–704. [Google Scholar]
- Harcourt-Brown, F.M. Metabolic Bone Disease as a Possible Cause of Dental Disease in Pet Rabbits. Postgraduate Fellowship Thesis, The Royal College of Veterinary Surgeons (RCVS), London, UK, 2006. [Google Scholar]
- Ardran, G.M.; Kemp, F.H. A radiographic analysis of mastication and swallowing in the domestic rabbit: Oryctolagus cuniculus. Proc. Zool. Soc. Lond. 1958, 130, 257–274. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamamura, K. Possible factors which may affect phase durations in the natural chewing rhythm. Brain Res. 1996, 706, 237–242. [Google Scholar] [CrossRef]
- Weijs, W.A.; Dantuma, R. Functional anatomy of the masticatory apparatus in the rabbit (Oryctolagus cuniculus L.). Neth. J. Zool. 1981, 31, 99–147. [Google Scholar] [CrossRef]
- Crossley, D.A.; Miguelez, M.M. Skull size and cheek-tooth length in wild-caught and captive-bred chinchillas. Arch. Oral Biol. 2001, 46, 919–928. [Google Scholar] [CrossRef]
- Cornette, R.; Tresset, A.; Herrel, A. The shrew tamed by Wolff’s law: Do functional constraints shape the skull through muscle and bone covariation? J. Morphol. 2015, 276, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Smuts, G.L. Age determination of the African lion (Panthera leo). J. Zool. 1978, 185, 365–373. [Google Scholar] [CrossRef]
- Ravosa, M.J.; Scott, J.E.; McAbee, K.R.; Veit, A.J.; Fling, A.L. Chewed out: An experimental link between food material properties and repetitive loading of the masticatory apparatus in mammals. PeerJ 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Hartstone-Rose, A.; Selvey, H.; Villari, J.R.; Atwell, M.; Schmidt, T. The three-dimensional morphological effects of captivity. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Saragusty, J.; Shavit-Meyrav, A.; Yamaguchi, N.; Nadler, R.; Bdolah-Abram, T.; Gibeon, L.; Shamir, M.H. Comparative skull analysis suggests species-specific captivity-related malformation in lions (Panthera leo). PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, P.; Wróblewska, M.; Nowak, P.; Pezinska, K. Biometry of the skull of wild and farm long-tailed Chinchilla (Chinchilla laniger, Molina, 1782). Int. J. Morphol. 2013, 31, 1003–1011. [Google Scholar] [CrossRef]
- Menegaz, R.A.; Sublett, S.V.; Figueroa, S.D.; Hoffman, T.J.; Ravosa, M.J. Phenotypic plasticity and function of the hard palate in growing rabbits. Anat. Rec. 2009, 292, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Menegaz, R.A.; Sublett, S.V.; Figueroa, S.D.; Hoffman, T.J.; Ravosa, M.J.; Aldridge, K. Evidence for the influence of diet on cranial form and robusticity. Anat. Rec. 2010, 293, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Hylander, W.L. The functional significance of primate mandibular form. J. Morphol. 1979, 160, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Hylander, W.L. Mandibular function in Galago crassicaudatus and Macaca fascicularis: An in vivo approach to stress analysis of the mandible. J. Morphol. 1979, 159, 253–296. [Google Scholar] [CrossRef] [PubMed]
- Zuccarelli, M.D. Comparative morphometric analysis of captive vs. wild African lion (Panthera leo) skulls. Bios 2004, 75, 131–138. [Google Scholar] [CrossRef]
- O’Regan, H.J.; Kitchener, A.C. The effects of captivity on the morphology of captive, domesticated and feral mammals. Mammal. Rev. 2005, 35, 215–230. [Google Scholar] [CrossRef]
- Weijs, W.A.; Brugman, P.; Grimbergen, C.A. Jaw movements and muscle activity during mastication in growing rabbits. Anat. Rec. 1989, 224, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Unhold, G.; Bouvier, M. The effect of diet texture on healing following temporomandibular joint discectomy in rabbits. J. Oral Maxillofac. Surg. 1988, 46, 580–588. [Google Scholar] [CrossRef]
- Ravosa, M.J.; Kunwar, R.; Stock, S.R.; Stack, M.S. Pushing the limit: Masticatory stress and adaptive plasticity in mammalian craniomandibular joints. J. Exp. Biol. 2007, 210, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Beecher, R.M.; Corruccini, R.S. Effects of dietary consistency on craniofacial and occlusal development in the rat. Angle Orthod. 1981, 51, 61–69. [Google Scholar] [PubMed]
- He, T.; Kiliaridis, S. Effects of masticatory muscle function on craniofacial morphology in growing ferrets (Mustela putorius furo). Eur. J. Oral Sci. 2003, 111, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Avis, V. The significance of the angle of the mandible: An experimental and comparative study. Am. J. Phys. Anthropol. 1961, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Herring, S.W. The ontogeny of mammalian mastication. Am. Zool. 1985, 25, 339–349. [Google Scholar] [CrossRef]
- Renaud, S.; Auffray, J.C.; de la Porte, S. Epigenetic effects on the mouse mandible: Common features and discrepancies in remodeling due to muscular dystrophy and response to food consistency. BMC Evol. Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.S.; Renaud, S.; Rayfield, E.J. Adaptive plasticity in the mouse mandible. BMC Evol. Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.B.; Jones, K.E.; Kunwar, R.; Ravosa, M.J. Dietary consistency and plasticity of masseter fiber architecture in postweaning rabbits. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006, 288, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, M.; Zimny, M.L. Effects of mechanical loads on surface morphology of the condylar cartilage of the mandible in rats. Acta Anat. 1987, 129, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ciochon, R.L.; Nisbett, R.A.; Corruccini, R.S. Dietary consistency and craniofacial development related to masticatory function in minipigs. J. Craniofac. Genet. Dev. Biol. 1997, 17, 96–102. [Google Scholar] [PubMed]
- Gotthard, K.; Nylin, S. Adaptive plasticity and plasticity as an adaptation: A selective review of plasticity in animal morphology and life history. Oikos 1995, 74, 3–17. [Google Scholar] [CrossRef]
- Katsaros, C.; Berg, R.; Kiliaridis, S. Influence of masticatory muscle function on transverse skull dimensions in the growing rat. J. Orofac. Orthop. 2002, 63, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kiliaridis, S.; Engstrom, C.; Thilander, B. The relationship between masticatory function and craniofacial morphology. I. A cephalometric longitudinal analysis in the growing rat fed a soft diet. Eur. J. Orthod. 1985, 7, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Park, J.C.; Kang, D.W.; Kim, B.O.; Yoon, J.H.; Cho, S.I.; Bae, C.S. Correlation of immunohistochemical characteristics of the craniomandibular joint with the degree of mandibular lengthening in rabbits. J. Oral Maxillofac. Surg. 2003, 61, 1189–1197. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Bresin, A.; Kiliaridis, S. Morphometric analysis of the mandible in growing rats with different masticatory functional demands: Adaptation to an upper posterior bite block. Eur. J. Oral Sci. 2004, 112, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, A.; Ammann, P.; Bresin, A.; Kiliaridis, S. Masticatory demands induce region-specific changes in mandibular bone density in growing rats. Angle Orthod. 2005, 75, 625–630. [Google Scholar] [PubMed]
- Bouvier, M.; Hylander, W.L. The effect of dietary consistency on gross and histologic morphology in the craniofacial region of young rats. Am. J. Anat. 1984, 170, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, G.; van de Pavert, S.; Savalle, W.; Korfage, H.; van Eijden, T. Influence of food consistency on the rabbit masseter muscle fibres. Eur. J. Oral. Sci. 2003, 111, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, G.E.; Weijs, W.A.; Koolstra, J.H. Biomechanical changes in the rabbit masticatory system during postnatal development. Anat. Rec. 1991, 230, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Jasarevic, E.; Ning, J.; Daniel, A.N.; Menegaz, R.A.; Johnson, J.J.; Stack, M.S.; Ravosa, M.J. Masticatory loading, function, and plasticity: A microanatomical analysis of mammalian circumorbital soft-tissue structures. Anat. Rec. 2010, 293, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Greaves, W.S. Mammalian Jaw. A Mechanical Analysis; Cambridge University Press: Cambridge, UK, 2012; p. 114. [Google Scholar]
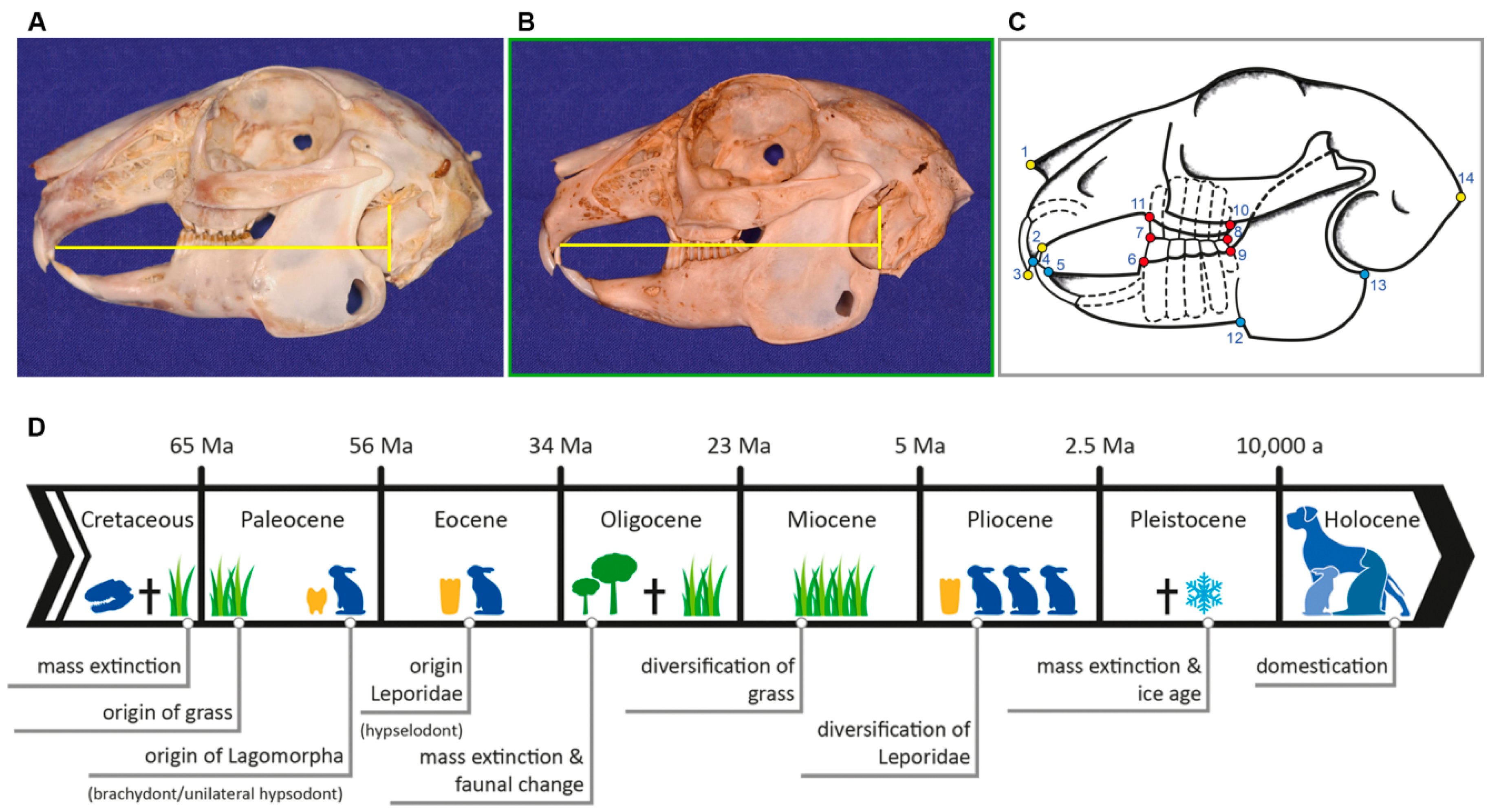

| LM | Type | Definition |
|---|---|---|
| 1 | II | most anterior point of nasal bone |
| 2 | I | intersection between second maxillary incisor (I2) (peg tooth) and maxillary bone |
| 3 | II | most anterior tip of first maxillary incisor (I1) |
| 4 | II | most anterior tip of first mandibular incisor (i1) |
| 5 | I | intersection between first mandibular incisor (i1) and mandibular bone |
| 6 | I | anterior intersection between mandible and first mandibular cheek tooth (p2) |
| 7 | II | most anterior point of occlusal plane between maxillary and mandibular first cheek tooth (P2, p2) |
| 8 | II | most posterior point of occlusal plane between maxillary and mandibular last molar (M3, m3) |
| 9 | I | posterior intersection between mandible and last mandibular molar (m3) |
| 10 | I | posterior intersection between maxillary bone and last maxillary molar (M3) |
| 11 | I | anterior intersection between maxillary bone and first maxillary cheek tooth (P2) |
| 12 | II | antegonial notch of mandible |
| 13 | II | most posterior dorsal point of angular process |
| 14 | II | most posterior point of occipital protuberance |
| RW | Variance (%) | Cumulative Variance (%) |
|---|---|---|
| RW 1 | 54.60 | 54.60 |
| RW 2 | 13.24 | 67.83 |
| RW 3 | 9.91 | 77.74 |
| RW 4 | 5.25 | 83.00 |
| RW 5 | 3.72 | 86.72 |
| RW 6 | 2.80 | 89.52 |
| RW 7 | 2.67 | 92.19 |
| RW 8 | 2.14 | 94.33 |
| RW 9 | 1.40 | 95.73 |
| Log Centroid Size | ||
|---|---|---|
| r² | p-Value | |
| RW 1 | 0.035066 | 0.38092 |
| RW 2 | 0.168757 | 0.046143 * |
| RW 3 | 0.010572 | 0.63258 |
| RW 4 | 0.510982 | 8.67 × 10 −5 * |
| RW 5 | 0.023031 | 0.47901 |
| RW 6 | 0.017082 | 0.54269 |
| RW 7 | 0.046522 | 0.31144 |
| RW 8 | 0.001987 | 0.83617 |
| RW 9 | 0.004693 | 0.75044 |
| LM | Variance (s²) |
|---|---|
| 1 | 1.6041 × 10 −3 |
| 14 | 0.32197 × 10 −3 |
| 12 | 0.23093 × 10 −3 |
| 6 | 0.20758 × 10 −3 |
| 13 | 0.1961 × 10 −3 |
| 2 | 0.1612 × 10 −3 |
| 11 | 0.13185 × 10 −3 |
| 4 | 0.12278 × 10 −3 |
| 5 | 0.10884 × 10 −3 |
| 7 | 0.10744 × 10 −3 |
| 9 | 0.10476 × 10 −3 |
| 10 | 0.09878 × 10 −3 |
| 8 | 0.08468 × 10 −3 |
| 3 | 0.07512 × 10 −3 |
| PLS | Covariance (%) | Cumulative Covariance (%) |
|---|---|---|
| module 1 vs. 2 | ||
| PLS 1 | 82.08 | 82.08 |
| PLS 2 | 12.35 | 94.43 |
| module 1 vs. 3 | ||
| PLS 1 | 90.53 | 90.53 |
| PLS 2 | 8.51 | 99.04 |
| module 2 vs. 3 | ||
| PLS 1 | 59.55 | 59.55 |
| PLS 2 | 22.60 | 82.15 |
| Module | Log Centroid Size | |
|---|---|---|
| r² | p-Value | |
| module 1 vs. 2 (wild rabbits) | 0.66167 | 0.0012895 * |
| module 1 vs. 2 (domestic rabbits) | 0.27903 | 0.077491 * |
| module 1 vs. 3 (wild rabbits) | 0.85269 | 1.82 × 10 −5 * |
| module 1 vs. 3 (domestic rabbits) | 0.64699 | 0.0016086 * |
| module 2 vs. 3 (wild rabbits) | 0.46661 | 0.014344 * |
| module 2 vs. 3 (domestic rabbits) | 0.034312 | 0.56437 |
| Species | Ref. | Diet Fed | Feeding Period | Background | Results (Morphology, Anatomy) |
|---|---|---|---|---|---|
| laboratory mice (3 weeks old) | [88] | rodent pellets vs. ground pellets mixed with jelly | about 5 months | food consistency significantly influenced bone remodeling (shape of the mandible) as hard food generates greater stress in the jaw (bone remodeling) | mice fed on hard food displayed mandibles functionally more efficient for hard-food processing (higher mechanical advantage values), extended coronoid and angular processes, ventrally expanded incisor and molar zones; all functional modules except the molar zone showed shape differences. Mice fed on soft food showed jaw elongations (reduced mechanical advantage values) |
| mice (after weaning), healthy animals and mice with muscle dystrophy (pathological muscular defect) | [87] | hard pellets vs. pellets under the form of jelly | 30 weeks | remodeling of the mandible as response to food consistency and muscular dystrophy | significant changes in mandible size whereby some parts of the mandible were more prone to remodeling (such as the angular process which is less robust when fed soft diet) |
| rats | [83] | hard diet vs. soft diet | about 4 months | in particular, the mandible depends on muscular function to grow to its normal size, maxillary growth seems to be under closer genetic programming | soft-diet animals had smaller jaw muscles and smaller jaws |
| farm-reared long-tailed chinchillas vs. museum skulls | [73] | granular feed (pellets) vs. natural diet | life-long | under natural habitat conditions, fiber constitutes almost 66% of the chinchilla diet, whereas under conditions of farm and domestic keeping granular feed with the fiber ranging from 12% to 18% is the main food; this does not require such hard work of the masticatory apparatus | crania and mandibles of farm-reared chinchillas were significantly larger than the museum specimens; only the frontal length did not show any significant differences between both groups; the length of the maxillary cheek-tooth row was larger in the museum crania |
| domestic (captive-bred) long-tailed chinchillas vs. wild-caught chinchillas and zoo specimens | [67] | granular feed (pellets) vs. natural diet | lifelong | captive bred animals with a normocclusion had longer cheek teeth (7.4 mm) than wild-caught chinchillas (5.9 mm) due to prolonged chewing of the naturally abrasive diet, zoo specimens lay in between (6.6 mm) | skulls of captive-bred chinchillas were on average 16% longer and slightly higher than the others (assumed to unrestricted food intake) |
| suckling rabbits | [100] | small food particles vs. milk | about 4 weeks | postnatal development of the masticatory apparatus due to change in function from suckling to chewing (shift of muscle activity) | the facial skull becomes higher and longer, increase in mandibular height and development of an angular process, anterior part of the superficial masseter attains a more vertical position, displacement of the mandibular angle in a ventroposterior direction, stronger jaw closing muscles and increased bite-force |
| juvenile rabbits | [99] | hard pellets vs. soft pellets (soaked in water) | 87 days | influence of food consistency on the rabbit masseter muscle fibers (plasticity) | rabbits adjusted to altered foods within days resulting in changes in the masseter muscle; hard-diet animals increased the occlusal forces (larger fiber cross-sectional area); soft-diet animals decreased the occlusal forces (small fiber cross-sectional area) |
| rabbits (weanlings) | [74] | soft and hard/tough diet | 15 weeks | influence of masticatory stresses on the development and structure of the hard palate (phenotypic plasticity) | rabbits subjected to elevated masticatory loading developed hard palates with significantly greater bone area, greater cortical bone thickness and thicker anterior plates |
| rabbits (weanlings) | [89] | ground rabbit pellets vs. intact pellets and hay blocks | 105 days | phenotypic plasticity of the superficial masseter fiber architecture as dietary consistency influences its fiber type composition | tough diet causes an increase in physiological cross-sectional areas of the masseter muscle (increased muscle mass) |
| New Zealand rabbits (weanlings) | [101] | powdered pellets, intact pellets, intact pellets and hay blocks | 26 weeks | diet-induced variations in masticatory stresses influence postorbital soft tissues (fibrocartilage) | more degraded organization of collagen fibers in the postorbital region due to increased masticatory forces (pellets and hay) |
| New Zealand white rabbits (4-week-old weanlings) | [82] | ground pellets vs. intact pellets with hay blocks | 15 weeks | diet-related variation in masticatory stress affects structural properties and extracellular matrix composition of the TMJ and the symphysis (histology and immunohistochemistry of articular cartilage revealed a diminished articular cartilage viscoelasticity) | elevated masticatory loads result in an increase of the masseter muscle mass and a partial skull bone enlargement (mandibular corpus, condyle, symphysis) with a greater local bone density |
| ferrets (5 weeks old) | [84] | hard pellets vs. soft pellets (soaked in water) | 6 months | effect of masticatory muscle function on craniofacial morphology | less tension on the periosteal membrane of the cranial bones, resulting in less periosteal bone apposition in the inserting areas |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhmer, C.; Böhmer, E. Shape Variation in the Craniomandibular System and Prevalence of Dental Problems in Domestic Rabbits: A Case Study in Evolutionary Veterinary Science. Vet. Sci. 2017, 4, 5. https://doi.org/10.3390/vetsci4010005
Böhmer C, Böhmer E. Shape Variation in the Craniomandibular System and Prevalence of Dental Problems in Domestic Rabbits: A Case Study in Evolutionary Veterinary Science. Veterinary Sciences. 2017; 4(1):5. https://doi.org/10.3390/vetsci4010005
Chicago/Turabian StyleBöhmer, Christine, and Estella Böhmer. 2017. "Shape Variation in the Craniomandibular System and Prevalence of Dental Problems in Domestic Rabbits: A Case Study in Evolutionary Veterinary Science" Veterinary Sciences 4, no. 1: 5. https://doi.org/10.3390/vetsci4010005
APA StyleBöhmer, C., & Böhmer, E. (2017). Shape Variation in the Craniomandibular System and Prevalence of Dental Problems in Domestic Rabbits: A Case Study in Evolutionary Veterinary Science. Veterinary Sciences, 4(1), 5. https://doi.org/10.3390/vetsci4010005







