Exposure to Photoperiod-Melatonin-Induced, Sexually-Activated Rams after Weaning Advances the Resumption of Sexual Activity in Post-Partum Mediterranean Ewes Lambing in January
Abstract
:1. Introduction
2. Material and Methods
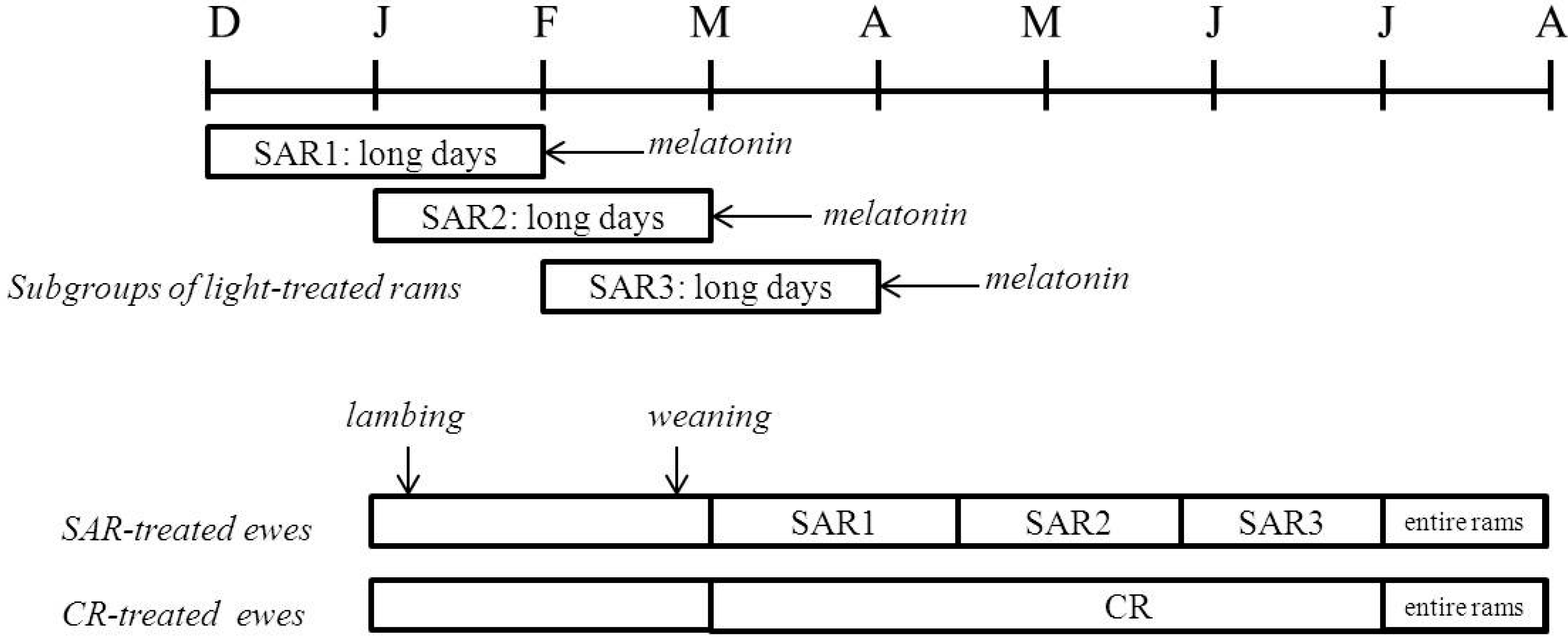
2.1. Experimental Procedure
2.2. Sexual Activation of Rams
2.3. Females
2.4. Measurements
2.5. Hormonal Assays
2.6. Statistical Analysis
2.7. Ethical Note
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yates, N.T. The breeding season of the sheep with particular reference to its modification by artificial means using light. J. Agric. Sci. 1949, 39, 1–43. [Google Scholar] [CrossRef]
- Bronson, F.H. Climate change and seasonal reproduction in mammals. Philos. Trans. R. Soc. Lond. B. 2009, 364, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Menassol, J.B.; Collet, A.; Chesneau, D.; Malpaux, B.; Scaramuzzi, R.J. The interaction between photoperiod and nutrition and its effects on seasonal rhythms of reproduction in the ewe. Biol. Reprod. 2012, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Helm, B.; Ben-Shlomo, R.; Sheriff, M.J.; Hut, R.A.; Foster, R.; Barnes, B.M.; Dominoni, D. Annual rhythms that underlie phenology: Biological time-keeping meets environmental change. Proc. R. Soc. Lond. B 2013, 280, 20130016. [Google Scholar] [CrossRef] [PubMed]
- Ortavant, R.; Pelletier, J.; Ravault, J.P.; Thimonier, J.; Volland-Nail, P. Photoperiod: main proximal and distal factor of the circannual cycle of reproduction in farm mammals. Oxf. Rev. Reprod. Biol. 1985, 7, 305–345. [Google Scholar] [PubMed]
- Martin, G.B.; Tjondronegoro, S.; Boukhliq, R.; Blackberry, M.A.; Briegel, J.R.; Blache, D.; Fisher, J.A.; Adams, N.R. Determinants of the annual pattern of reproduction in mature male Merino and Suffolk sheep: Modification of endogenous rhythms by photoperiod. Reprod. Fertil. Dev. 1999, 11, 355–366. [Google Scholar] [PubMed]
- Forcada, F.; Abecia, J.A.; Sierra, I. Seasonal changes in estrous activity and ovulation rate in Rasa Aragonesa ewes maintained at two different body condition levels. Small Rumin. Res. 1992, 8, 313–324. [Google Scholar] [CrossRef]
- Wheeler, A.G.; Land, R.B. Seasonal variation in oestrus and ovarian activity of Finnish Landrace, Tasmanian Merino and Scottish Blackface ewes. Anim. Prod. 1977, 24, 363–376. [Google Scholar] [CrossRef]
- Lindsay, D.R. Environment and reproductive behaviour. Anim. Reprod. Sci. 1996, 42, 1–12. [Google Scholar] [CrossRef]
- Quirke, J.F.; Stabenfeldt, G.H.; Bradford, G.E. Resumption of ovarian function in autumn lambing Dorset, Rambouillet and Finnish Landrace ewes. Theriogenology 1983, 19, 243–248. [Google Scholar] [CrossRef]
- Whiteman, J.V.; Zollinger, W.A.; Thrift, F.A.; Gould, M.B. Postpartum mating performance of ewes involved in a twice-yearly lambing program. J. Agric. Sci. 1972, 35, 836–842. [Google Scholar] [CrossRef]
- Restall, B.J.; Kearins, R.D.; Starr, B.G. Studies of pituitary function in lactating ewes. J. Reprod. Ferti. 1977, 49, 291–296. [Google Scholar] [CrossRef]
- Amir, D.; Gacitua, H. Sexual activity of Assaf ewes after October and February lambings. Theriogenology 1987, 27, 377–382. [Google Scholar] [CrossRef]
- Monje, A.R.; Alberio, R.; Schiersmann, G.; Chedrese, J.; Caroú, N.; Callejas, S.S. Male effect on the post-partum sexual activity of cows maintained on two nutritional levels. Anim. Reprod. Sci. 1992, 29, 145–156. [Google Scholar] [CrossRef]
- Landaeta-Hernández, A.; Meléndez, P.; Bartolomé, J.; Rae, O.; Archbald, L.F. The effect of bull exposure on the early postpartum reproductive performance of suckling Angus cows. Rev. Cientif. FCV-LUZ 2008, 6, 682–691. [Google Scholar]
- Lassoued, N.; Naouali, M.; Khaldi, G.; Rekik, M. Influence of the permanent presence of rams on the resumption of sexual activity in postpartum Barbarine ewes. Small Rumin. Res. 2004, 54, 25–31. [Google Scholar] [CrossRef]
- Hamadeh, S.K.; Abi Said, M.; Tami, F.; Barbour, E.K. Weaning and the ram-effect on fertility, serum luteinizing hormone and prolactin levels in spring rebreeding of postpartum Awassi ewes. Small Rumin. Res. 2001, 41, 191–194. [Google Scholar] [CrossRef]
- Khaldi, G. Seasonal Variations of Ovarian Activity, Oestrus Behaviour and the Duration of Post-Partum Anoestrus of Ewes of the BARBARINE Breed: The Effects of the Level of Feeding and the Presence of Males. Ph.D. Thesis, Université des Sciences et Techniques du Languedoc, Montpellier, France, 1984. [Google Scholar]
- Abecia, J.A.; Chemineau, P.; Flores, J.A.; Keller, M.; Duarte, G.; Forcada, F.; Delgadillo, J.A. Continuous exposure to sexually active rams extends estrous activity in ewes in spring. Theriogenology 2015, 84, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; Chemineau, P.; Gómez, A.; Keller, M.; Forcada, F.; Delgadillo, J.A. Presence of photoperiod-melatonin-induced,sexually-activated rams in spring advances puberty inautumn-born ewe lambs. Anim. Reprod. Sci. 2016, 170, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Russel, A.J.F.; Doney, J.M.; Gunn, R.G. Subjective assessment of body fat in live sheep. J. Agric. Sci. Camb. 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Chemineau, P.; Malpaux, B.; Delgadillo, J.A.; Guérin, Y.; Ravault, J.P.; Thimonier, J.; Pelletier, J. Control of sheep and goat reproduction: Use of light and melatonin. Anim. Reprod. Sci. 1992, 30, 157–184. [Google Scholar] [CrossRef]
- Agricultural and Food Research Council. Energy and Protein Requirements of Ruminants by the AFRC Technical Committee; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Cardozo, J.A.; Fernández-Juan, M.; Forcada, F.; Abecia, J.A.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Monthly variations in ovine seminal plasma proteins analyzed by two-dimensional polyacrylamide gel electrophoresis. Theriogenology 2006, 66, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Vega, S.; Palacín, I.; Pérez-Pe, R.; Laviña, A.; Quintín, F.J.; Sevilla, E.; Abecia, J.A.; Cebrián-Pérez, J.A.; Forcada, F.; et al. Effects of melatonin implants during non-breeding season on sperm motility and reproductive parameters in Rasa Aragonesa rams. Reprod. Domest. Anim. 2010, 45, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. The effect of exogenous melatonin during the non-reproductive season on the seminal plasma hormonal profile and the antioxidant defense system of Rasa Aragonesa rams. Anim. Reprod. Sci. 2013, 138, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Andueza, D.; Alabart, J.L.; Lahoz, B.; Muñoz, F.; Folch, J. Early pregnancy diagnosis in sheep using near-infrared spectroscopy on blood plasma. Theriogenology 2014, 81, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; Forcada, F.; Zarazaga, L. A note on the effect of level of nutrition after weaning on the resumption of reproductive activity by ewes of two Spanish breeds lambing in spring. Anim. Prod. 1993, 56, 273–276. [Google Scholar] [CrossRef]
- Forcada, F.; Zarazaga, L.; Abecia, J.A. Effect of exogenous melatonin and plane of nutrition after weaning on estrous activity, endocrine status and ovulation rate in Salz ewes lambing in the seasonal anoestrus. Theriogenology 1995, 43, 1179–1193. [Google Scholar] [CrossRef]
- Abecia, J.A.; Forcada, F.; Zarazaga, L.; Lozano, J.M. Effect of plane of protein after weaning on resumption of reproductive activity in Rasa Aragonesa ewes lambing in late spring. Theriogenology 1993, 39, 353–560. [Google Scholar] [CrossRef]
- Delgadillo, J.A.; Flores, J.A.; Hernández, H.; Poindron, P.; Keller, M.; Fitz-Rodríguez, G.; Duarte, G.; Vielma, J.; Fernández, I.G.; Chemineau, P. Sexually active males prevent the display of seasonal anestrus in female goats. Horm. Behav. 2015, 69, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I. Controlled Reproduction in Sheep and Goats; CABI Publishing: Wallingford, UK, 1997. [Google Scholar]
- Ungerfeld, R.; Forsberg, M.; Rubianes, E. Overview of the response of anoestrous ewes to the ram effect. Reprod. Fertil. Dev. 2004, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.A.; Clarke, I.J. Refractoriness to a static melatonin signal develops in the pituitary gland for the control of prolactin secretion in the ram. Biol. Reprod. 1997, 57, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Tchamitchian, L.; Ricordeau, G.; Lefevre, C.; Desvignes, A. Observations sur l’anestrus post-partum des brebis Romanov apres un agnelage en saison sexuelle. Ann. Zootec. 1973, 22, 295–301. [Google Scholar] [CrossRef]
| SAR-Treated | CR-Treated | |
|---|---|---|
| n = 18 | n = 18 | |
| Lambing date | January 5 ± 4 | January 6 ± 6 |
| Weaning | February 25 | February 25 |
| Duration lactation (days) | 51 ± 4 | 52 ± 6 |
| First ovulation | March 21 ± 13 | March 23 ± 26 |
| Interval weaning-ovulation (days) | 26 ± 13 | 24 ± 26 |
| First estrus | April 26 ± 17 a | June 6 ± 47 b |
| Interval weaning-estrus (days) | 61 ± 17 a | 102 ± 47 b |
| Number of ovulations before 1st estrus | 2.2 ± 1.5 a | 4.5 ± 3.1 b |
| March | April | May | June | |||||
|---|---|---|---|---|---|---|---|---|
| SAR | CR | SAR | CR | SAR | CR | SAR | CR | |
| Ewes ovulating | 14/18 | 13/18 | 18/18 a | 14/18 b | 18/18 a | 14/18 b | 18/18 | 18/18 |
| 78% | 72% | 100% | 78% | 100% | 78% | 100% | 100% | |
| Ewes in estrus | 0/18 | 2/18 | 13/18 c | 3/18 d | 16/18 c | 8/18 d | 18/18 c | 11/18 d |
| 0% | 11% | 72% | 17% | 89% | 44% | 100% | 61% | |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abecia, J.A.; Chemineau, P.; Gómez, A.; Palacios, C.; Keller, M.; Delgadillo, J.A. Exposure to Photoperiod-Melatonin-Induced, Sexually-Activated Rams after Weaning Advances the Resumption of Sexual Activity in Post-Partum Mediterranean Ewes Lambing in January. Vet. Sci. 2017, 4, 4. https://doi.org/10.3390/vetsci4010004
Abecia JA, Chemineau P, Gómez A, Palacios C, Keller M, Delgadillo JA. Exposure to Photoperiod-Melatonin-Induced, Sexually-Activated Rams after Weaning Advances the Resumption of Sexual Activity in Post-Partum Mediterranean Ewes Lambing in January. Veterinary Sciences. 2017; 4(1):4. https://doi.org/10.3390/vetsci4010004
Chicago/Turabian StyleAbecia, José A., Philippe Chemineau, Andrea Gómez, Carlos Palacios, Matthieu Keller, and José A. Delgadillo. 2017. "Exposure to Photoperiod-Melatonin-Induced, Sexually-Activated Rams after Weaning Advances the Resumption of Sexual Activity in Post-Partum Mediterranean Ewes Lambing in January" Veterinary Sciences 4, no. 1: 4. https://doi.org/10.3390/vetsci4010004
APA StyleAbecia, J. A., Chemineau, P., Gómez, A., Palacios, C., Keller, M., & Delgadillo, J. A. (2017). Exposure to Photoperiod-Melatonin-Induced, Sexually-Activated Rams after Weaning Advances the Resumption of Sexual Activity in Post-Partum Mediterranean Ewes Lambing in January. Veterinary Sciences, 4(1), 4. https://doi.org/10.3390/vetsci4010004






