A Novel Chimeric Fiber-C4/D11 Subunit Vaccine Induces Cross-Neutralizing Antibodies and Provides Better Protection Against Fowl Adenovirus (FAdV) Type 4 and Type 11 Than the Fiber-D11/C4 Subunit Vaccine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell, Viruses, Plasmids and Animals
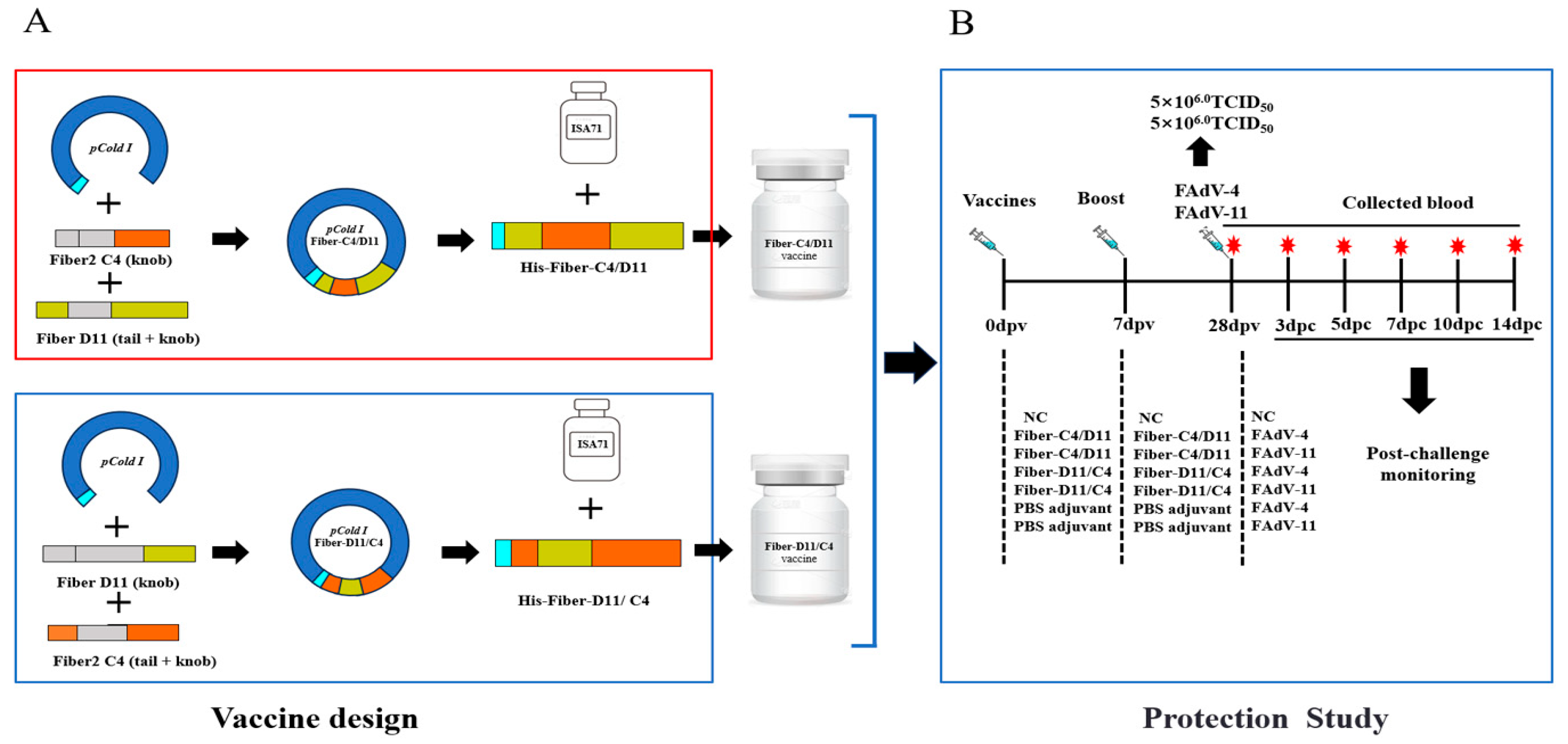
2.2. Expression and Purification of Recombinant Proteins
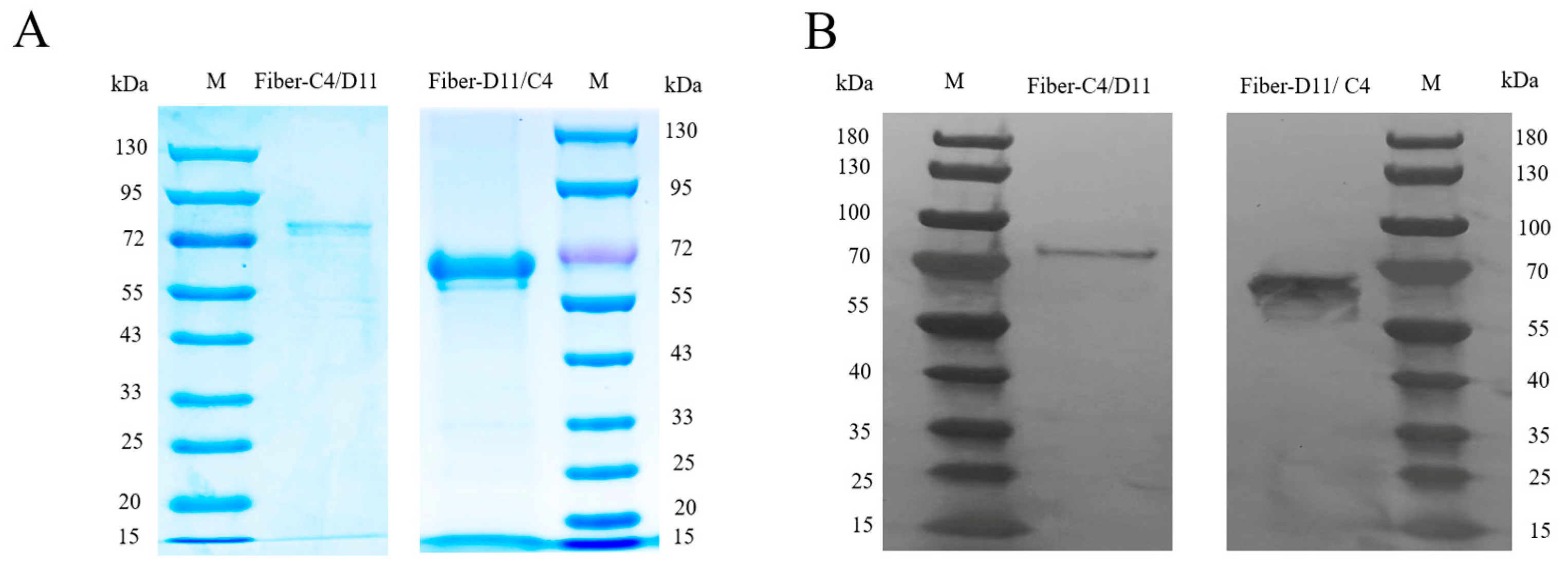
2.3. SDS-PAGE and Western Blot
2.4. Animal Experiment
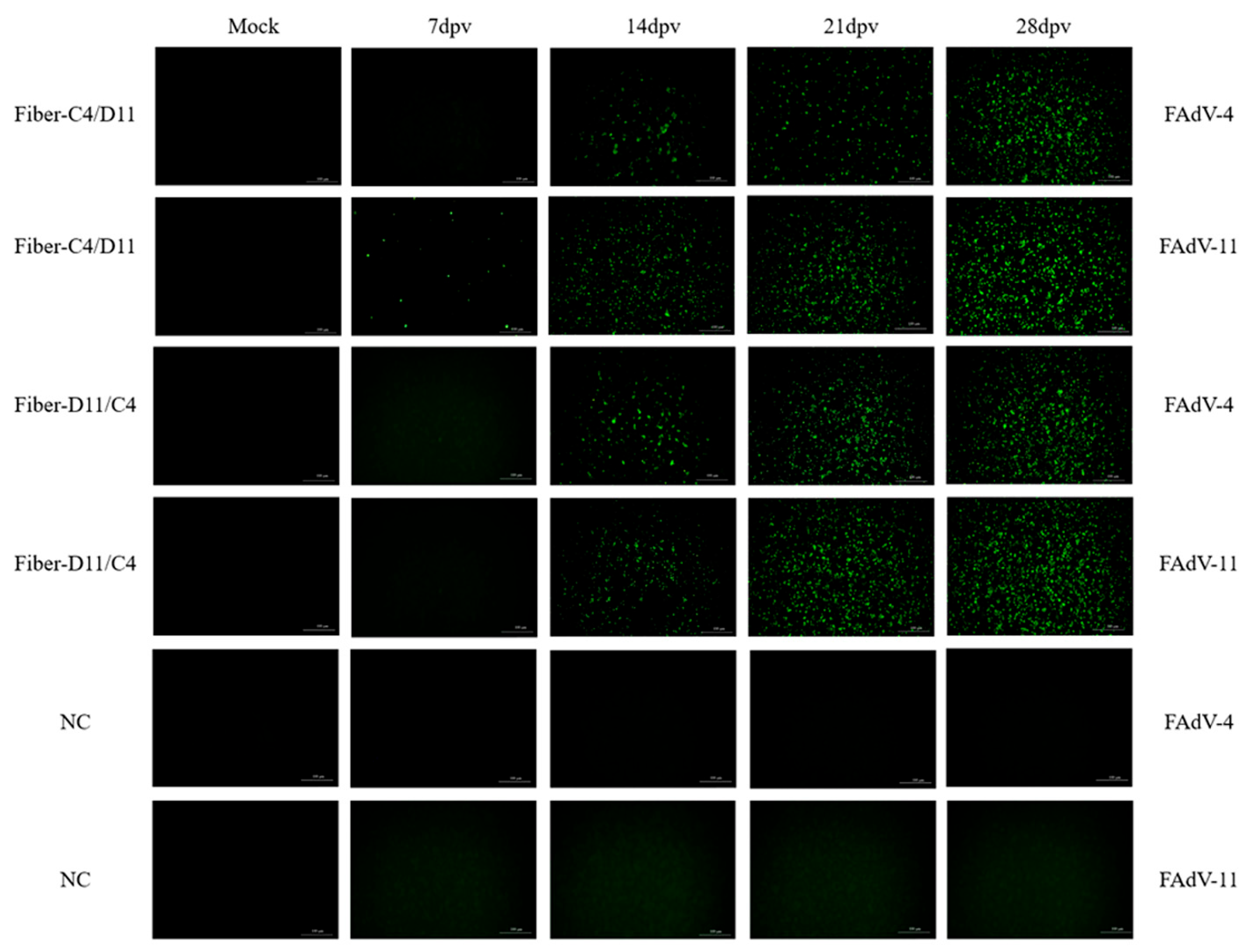
2.5. Indirect Immunofluorescence Assay
2.6. Enzyme-Linked Immunosorbent Assay and Serum Neutralization Test
2.7. Viral Titers in Target Tissues and Cloacal Swabs
2.8. Statistical Analysis
3. Results
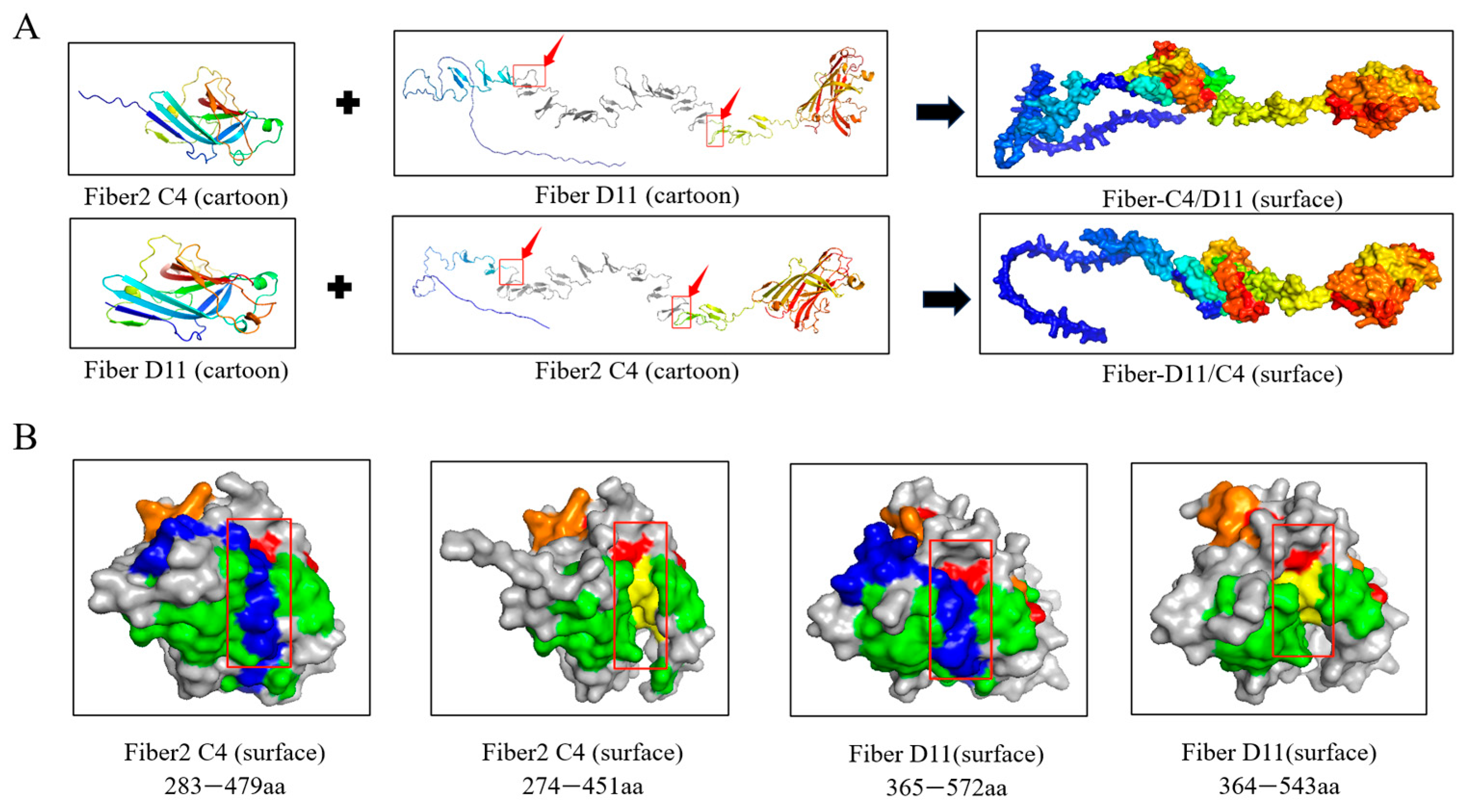
3.1. Construction, Expression and Purification of Recombinant Proteins
3.2. Indirect Immunofluorescence Assay (IFA)
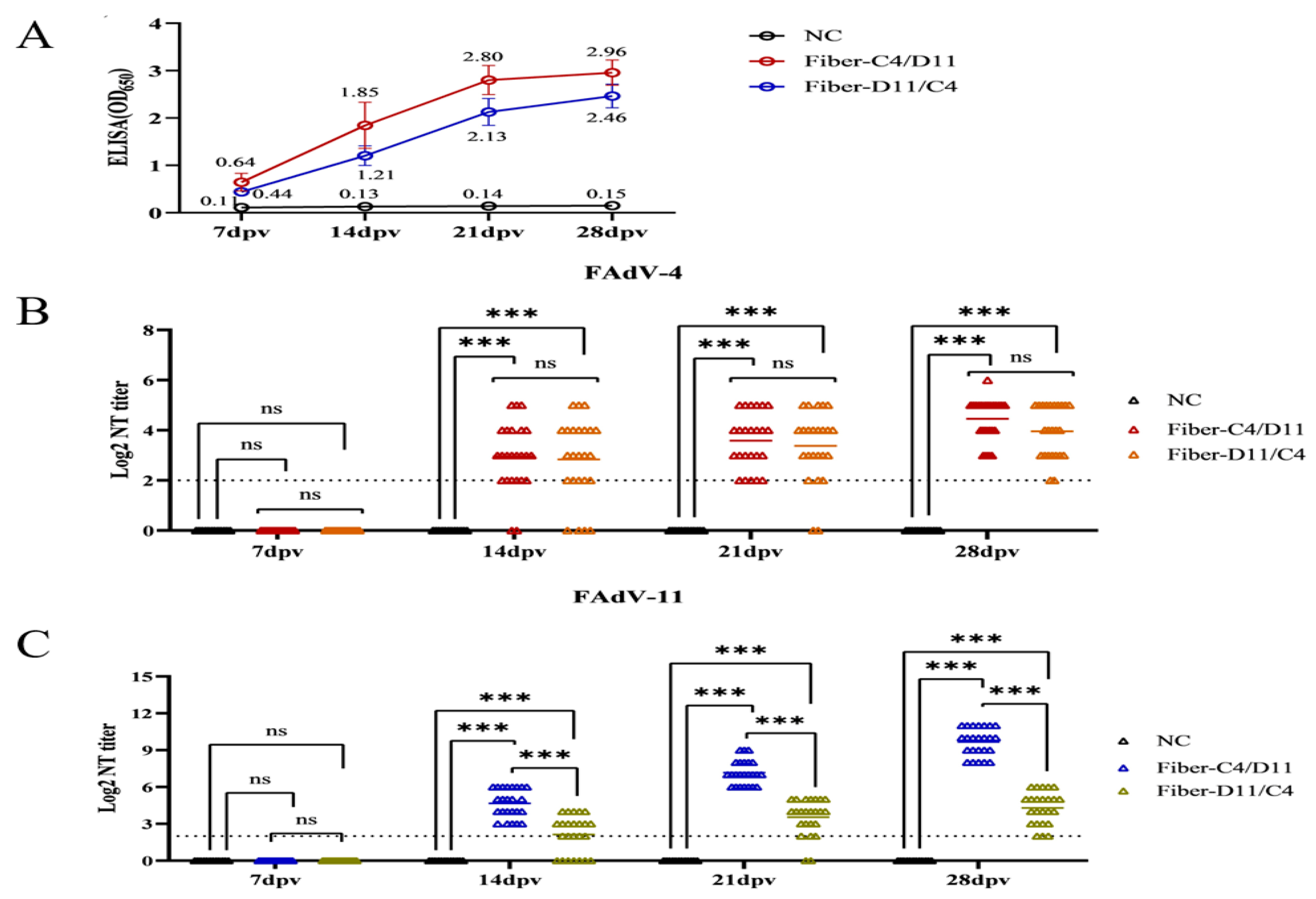
3.3. Enzyme-Linked Immunosorbent Assay (ELISA) and Serum Neutralization Test (SNT)
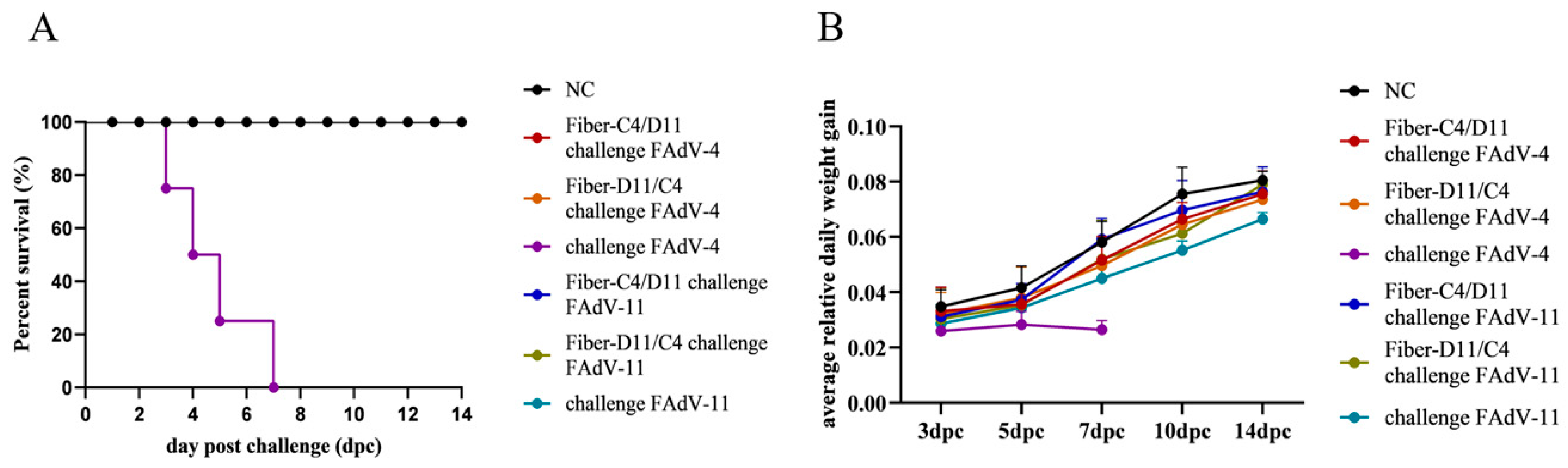
3.4. Clinical Protection
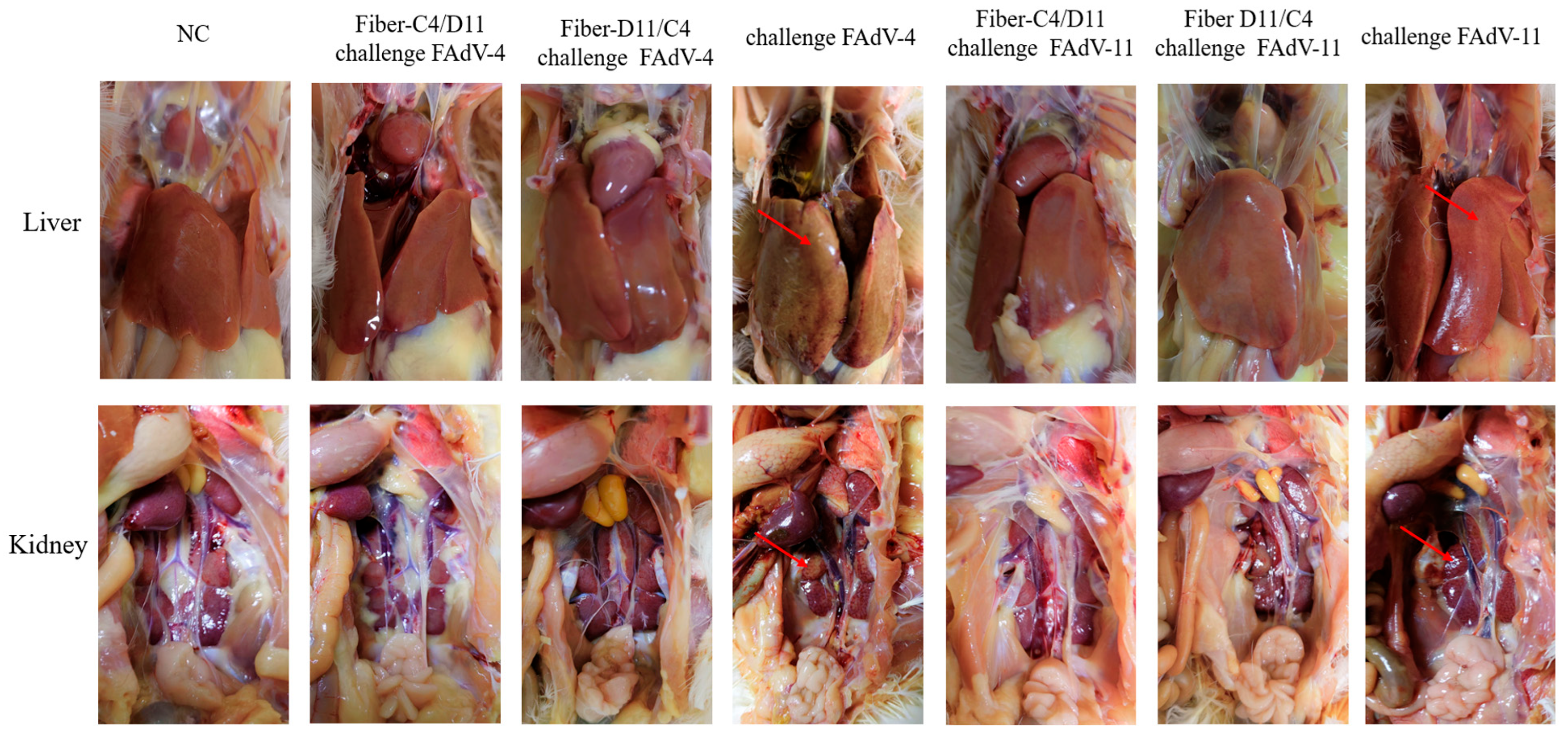
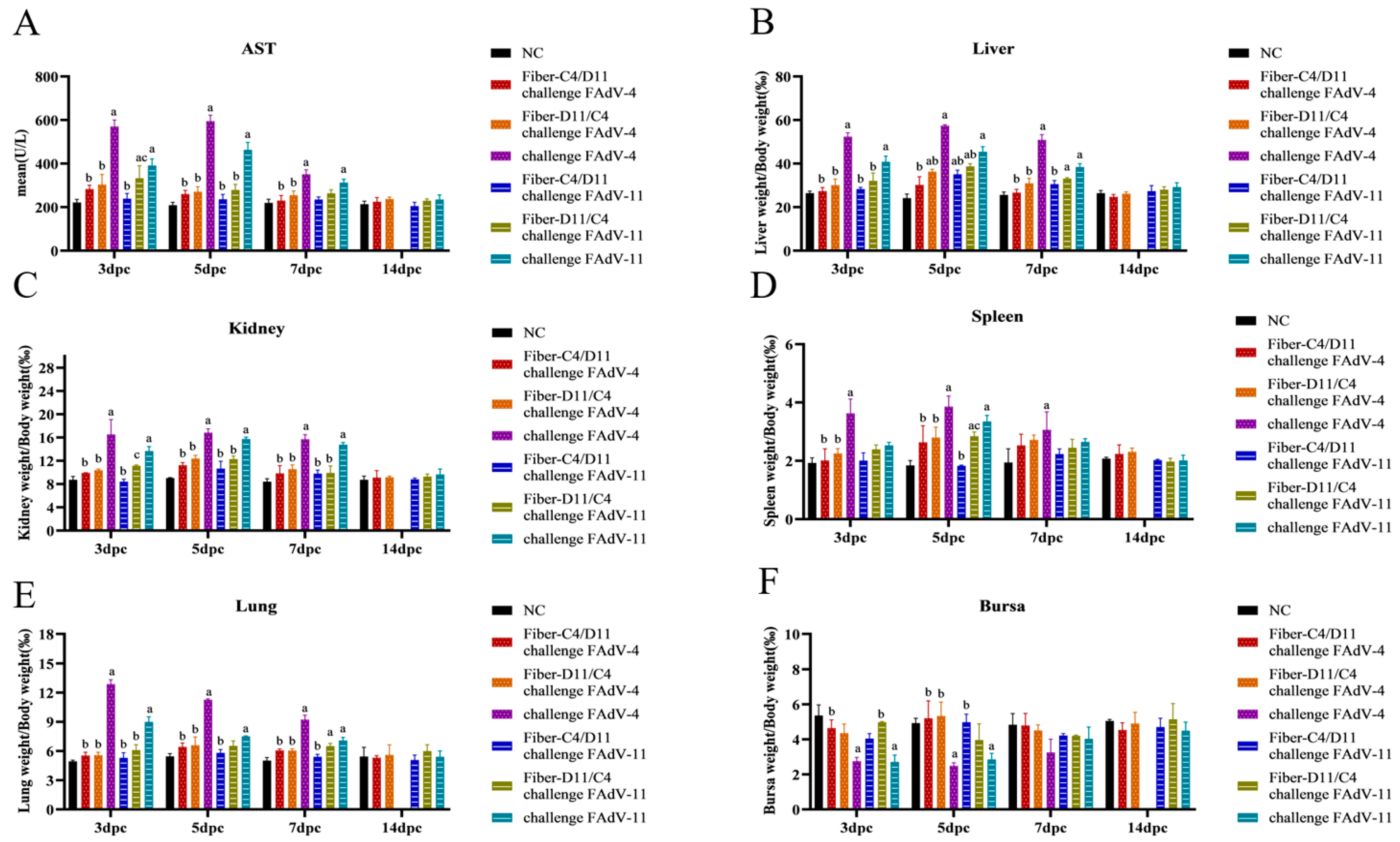
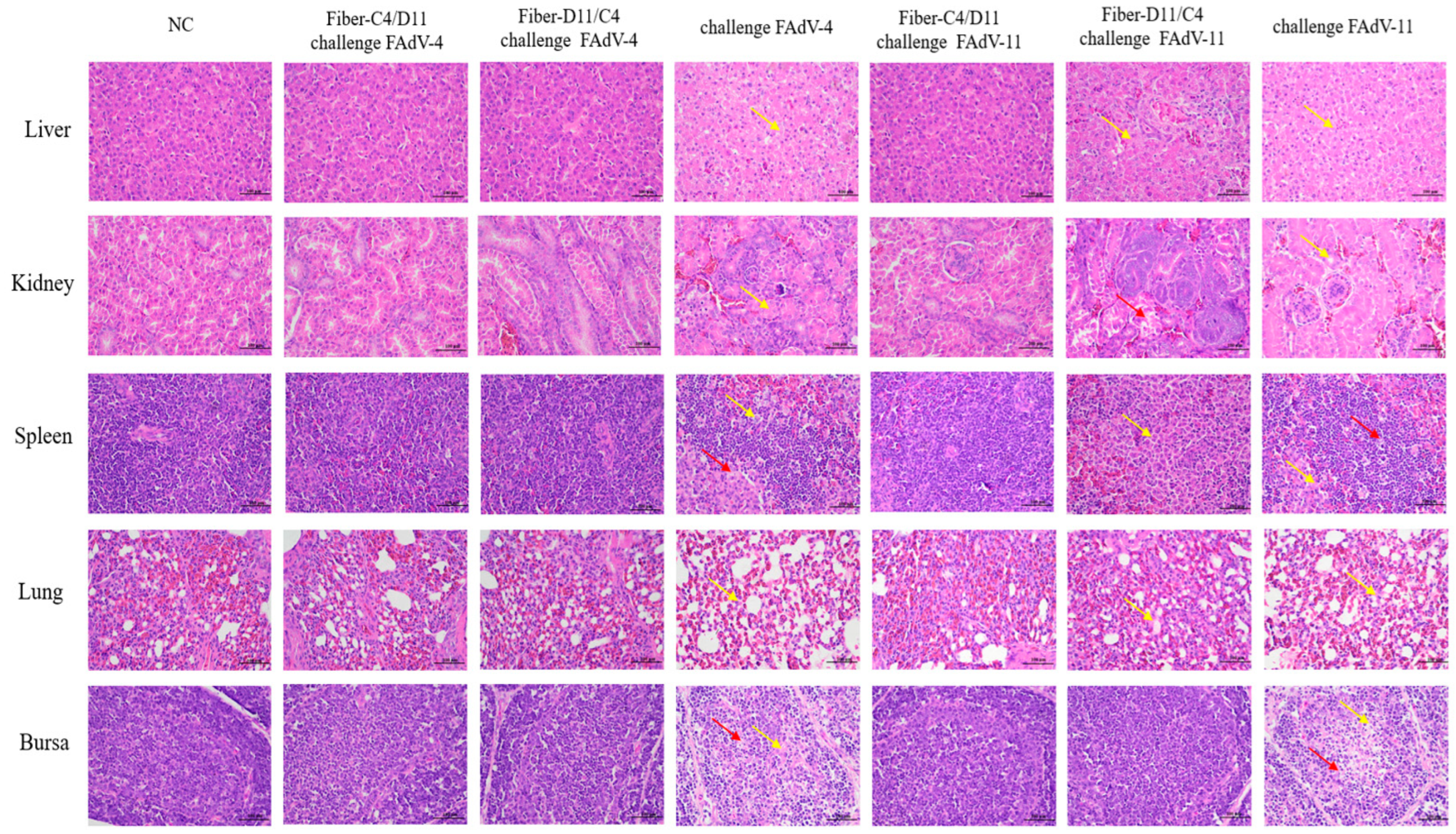
3.5. Histological Analysis
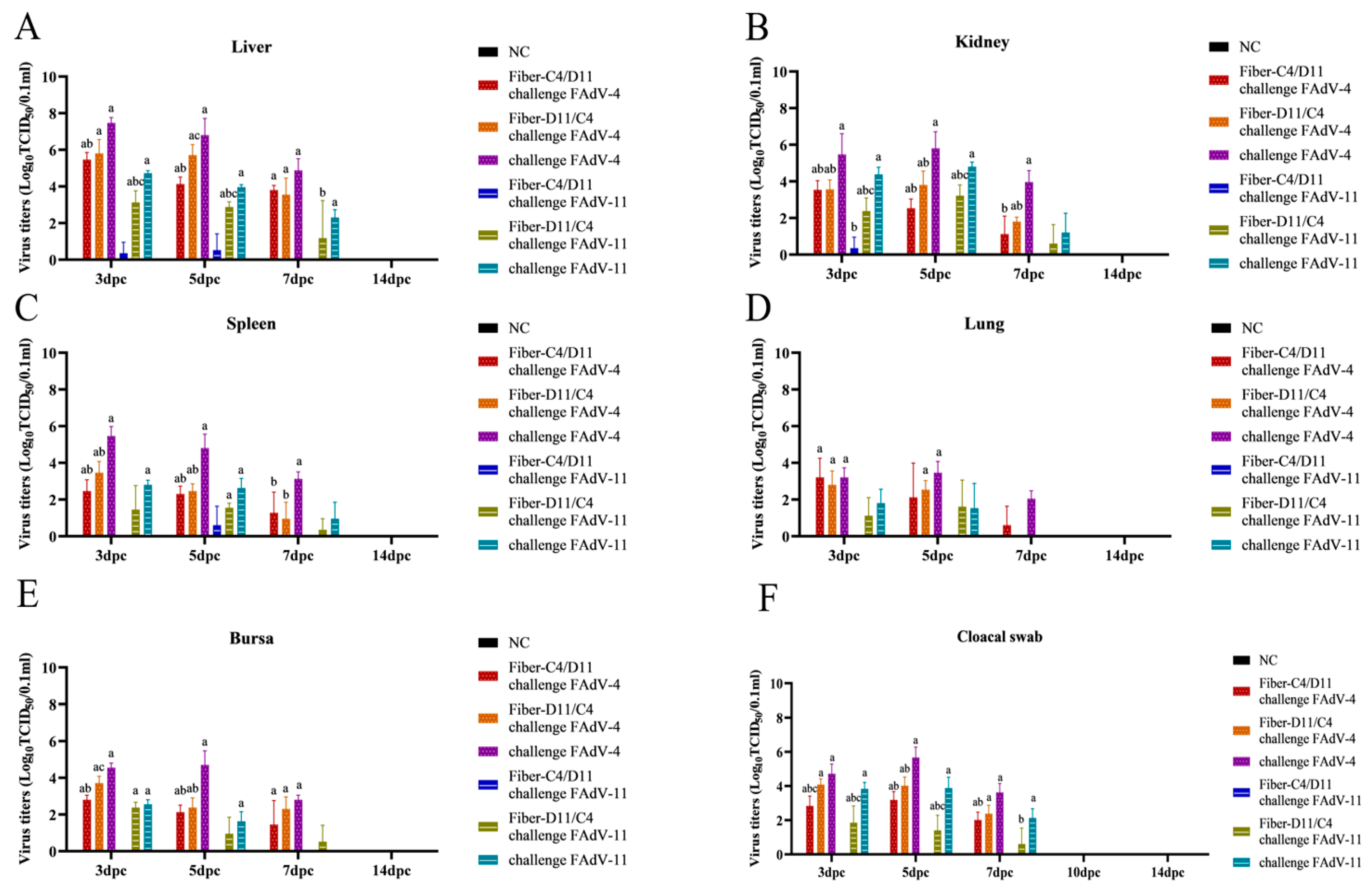
3.6. Viral Titers in Target Tissues and Cloacal Swabs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Liu, C.; Du, X.; Ma, Y.; Chen, L.; Cao, S.; Lu, J.; Li, Y.; Si, Z. Complete genome sequence and pathogenicity analysis of a highly pathogenic FAdV-4 strain. Res. Vet. Sci. 2023, 159, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Schachner, A.; Matos, M.; Grafl, B.; Hess, M. Fowl adenovirus-induced diseases and strategies for their control—A review on the current global situation. Avian Pathol. 2018, 47, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.D.; Sabri, M.A.; Iqbal, Z. Hydropericarditis syndrome in broiler chickens in Pakistan. Vet. Rec. 1989, 124, 247–248. [Google Scholar] [CrossRef]
- Mendelson, C.; Nothelfer, H.B.; Monreal, G. Identification and characterization of an avian adenovirus isolated from a ‘spiking mortality syndrome’ field outbreak in broilers on the Delmarva Peninsula, USA. Avian Pathol. 1995, 24, 693–706. [Google Scholar] [CrossRef]
- Mittal, D.; Jindal, N.; Tiwari, A.K.; Khokhar, R.S. Characterization of fowl adenoviruses associated with hydropericardium syndrome and inclusion body hepatitis in broiler chickens. Virusdisease 2014, 25, 114–119. [Google Scholar] [CrossRef]
- Hess, M.; Raue, R.; Prusas, C. Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathol. 1999, 28, 433–439. [Google Scholar] [CrossRef]
- Grgić, H.; Poljak, Z.; Sharif, S.; Nagy, É. Pathogenicity and cytokine gene expression pattern of a serotype 4 fowl adenovirus isolate. PLoS ONE 2013, 8, e77601. [Google Scholar] [CrossRef]
- Lai, V.D.; Min, K.; Lai, H.T.L.; Mo, J. Epidemiology of fowl adenovirus (FAdV) infections in South Korean chickens during 2013-2019 following introduction of FAdV-4 vaccines. Avian Pathol. 2021, 50, 182–189. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, L.; Jiang, S.; Li, X.; Lu, C.; Zhang, L.; Ren, W.; Li, C.; Tian, X.; Li, F.; et al. Isolation, identification and genetic characterization analysis of a fowl aviadenovirus serotype 4 strain from Tianjin, China. Infect. Genet. Evol. 2021, 96, 105078. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, Q.; Zhang, G.; Sun, W.; Liu, X.; Xiao, Y.; Shang, Y.; Liu, S. Epidemiological investigation of outbreaks of fowl adenovirus infections in commercial chickens in China. Transbound. Emerg. Dis. 2018, 65, e121–e126. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xu, Y.; Zhou, Z.; Xu, Q.; Wang, J.; Xiao, Y.; Li, Z.; Bi, D. Pathogenicity and Molecular Typing of Fowl Adenovirus-Associated With Hepatitis/Hydropericardium Syndrome in Central China (2015–2018). Front. Vet. Sci. 2020, 7, 190. [Google Scholar] [CrossRef]
- Su, Q.; Meng, F.; Li, Y.; Zhang, Y.; Zhang, Z.; Cui, Z.; Chang, S.; Zhao, P. Chicken infectious anemia virus helps fowl adenovirus break the protection of maternal antibody and cause inclusion body hepatitis-hydropericardium syndrome in layers after using co-contaminated Newcastle disease virus-attenuated vaccine. Poult. Sci. 2019, 98, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, H.; Li, Y.; Yang, Z.; Ye, J.; Chen, H. Characterization and pathogenicity of fowl adenovirus serotype 4 isolated from eastern China. BMC Vet. Res. 2019, 15, 373. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, Q.; Zhu, M.; Zhao, J.; Zhang, G.; Liu, X.; Xiao, Y.; Liu, S. Molecular epidemiology and phylogenetic analysis of fowl adenoviruses caused hydropericardium outbreak in China during 2015. Poult. Sci. 2018, 97, 803–811. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, Y.; Cui, H.; Wang, X.; Gao, Y.; Pan, Q. Advances in Vaccine Development of the Emerging Novel Genotype Fowl Adenovirus 4. Front. Immunol. 2022, 13, 916290. [Google Scholar] [CrossRef]
- Meng, K.; Yuan, X.; Yu, J.; Zhang, Y.; Ai, W.; Wang, Y. Identification, Pathogenicity of Novel Fowl Adenovirus Serotype 4 SDJN0105 in Shandong, China and Immunoprotective Evaluation of the Newly Developed Inactivated Oil-emulsion FAdV-4 Vaccine. Viruses 2019, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, A.; Cui, H.; Qi, X.; Liu, C.; Zhang, Y.; Li, K.; Gao, L.; Wang, X.; Pan, Q.; et al. An inactivated vaccine based on artificial non-pathogenic fowl adenovirus 4 protects chickens against hepatitis-hydropericardium syndrome. Vet. Microbiol. 2022, 264, 109285. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xie, Q.; Zhang, W.; Zhang, J.; Wang, W.; Lian, M.; Zhao, Z.; Ren, D.; Xie, S.; Lin, Y.; et al. A Novel Recombinant FAdV-4 Virus with Fiber of FAdV-8b Provides Efficient Protection against Both FAdV-4 and FAdV-8b. Viruses 2022, 14, 376. [Google Scholar] [CrossRef]
- Popowich, S.; Gupta, A.; Chow-Lockerbie, B.; Ayalew, L.; Ambrose, N.; Ojkic, D.; Gunawardana, T.; Kurukulasuriya, S.; Willson, P.; Tikoo, S.K.; et al. Broad spectrum protection of broiler chickens against inclusion body hepatitis by immunizing their broiler breeder parents with a bivalent live fowl adenovirus vaccine. Res. Vet. Sci. 2018, 118, 262–269. [Google Scholar] [CrossRef]
- Gupta, A.; Popowich, S.; Ojkic, D.; Kurukulasuriya, S.; Chow-Lockerbie, B.; Gunawardana, T.; Goonewardene, K.; Karunarathna, R.; Ayalew, L.E.; Ahmed, K.A.; et al. Inactivated and live bivalent fowl adenovirus (FAdV8b + FAdV11) breeder vaccines provide broad-spectrum protection in chicks against inclusion body hepatitis (IBH). Vaccine 2018, 36, 744–750. [Google Scholar] [CrossRef]
- Steer-Cope, P.A.; Sandy, J.R.; O’Rourke, D.; Scott, P.C.; Browning, G.F.; Noormohammadi, A.H. Vaccination with FAdV-8a induces protection against inclusion body hepatitis caused by homologous and heterologous strains. Avian Pathol. 2019, 48, 396–405. [Google Scholar] [CrossRef]
- Ruan, S.; Zhao, J.; Yin, X.; He, Z.; Zhang, G. A subunit vaccine based on fiber-2 protein provides full protection against fowl adenovirus serotype 4 and induces quicker and stronger immune responses than an inactivated oil-emulsion vaccine. Infect. Genet. Evol. 2018, 61, 145–150. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, W. A novel recombinant chimeric Fiber-8a/8b-AD subunit vaccine provides complete protection against both FAdV-8a and FAdV-8b. Vet. Microbiol. 2025, 303, 110425. [Google Scholar] [CrossRef]
- De Luca, C.; Schachner, A.; Heidl, S.; Hess, M. Vaccination with a fowl adenovirus chimeric fiber protein (crecFib-4/11) simultaneously protects chickens against hepatitis-hydropericardium syndrome (HHS) and inclusion body hepatitis (IBH). Vaccine 2022, 40, 1837–1845. [Google Scholar] [CrossRef]
- Chandra, R.; Shukla, S.K.; Kumar, M.; Garg, S.K. Electron microscopic demonstration of an adenovirus in the hepatocytes of birds experimentally infected with hydropericardium syndrome. Vet. Rec. 1997, 140, 70–71. [Google Scholar] [CrossRef]
- Chandra, R.; Shukla, S.K.; Kumar, M. The hydropericardium syndrome and inclusion body hepatitis in domestic fowl. Trop. Anim. Health Prod. 2000, 32, 99–111. [Google Scholar] [CrossRef]
- Hess, M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000, 29, 195–206. [Google Scholar] [CrossRef]
- Marek, A.; Nolte, V.; Schachner, A.; Berger, E.; Schlötterer, C.; Hess, M. Two fiber genes of nearly equal lengths are a common and distinctive feature of Fowl adenovirus C members. Vet. Microbiol. 2012, 156, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, J.; Wang, W.; Li, T.; Liang, G.; Shao, H.; Gao, W.; Qin, A.; Ye, J. A novel monoclonal antibody efficiently blocks the infection of serotype 4 fowl adenovirus by targeting fiber-2. Vet. Res. 2018, 49, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Q.; Li, T.; Geng, T.; Chen, H.; Xie, Q.; Shao, H.; Wan, Z.; Qin, A.; Ye, J. Fiber-1, Not Fiber-2, Directly Mediates the Infection of the Pathogenic Serotype 4 Fowl Adenovirus via Its Shaft and Knob Domains. J. Virol. 2020, 94, e00954-20. [Google Scholar] [CrossRef] [PubMed]
- Feichtner, F.; Schachner, A.; Berger, E.; Hess, M. Fiber-based fluorescent microsphere immunoassay (FMIA) as a novel multiplex serodiagnostic tool for simultaneous detection and differentiation of all clinically relevant fowl adenovirus (FAdV) serotypes. J. Immunol. Methods 2018, 458, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Molinier-Frenkel, V.; Gahery-Segard, H.; Mehtali, M.; Le Boulaire, C.; Ribault, S.; Boulanger, P.; Tursz, T.; Guillet, J.G.; Farace, F. Immune response to recombinant adenovirus in humans: Capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J. Virol. 2000, 74, 7678–7682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, R.; Tian, K.; Wang, Z.; Yang, X.; Gao, D.; Zhang, Y.; Fu, J.; Wang, H.; Zhao, J. Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerg. Microbes Infect. 2018, 7, 199. [Google Scholar] [CrossRef]
- Chen, L.; Yin, L.; Zhou, Q.; Li, Q.; Luo, Y.; Xu, Z.; Zhang, Y.; Xue, C.; Cao, Y. Immunogenicity and protective efficacy of recombinant fiber-2 protein in protecting SPF chickens against fowl adenovirus 4. Vaccine 2018, 36, 1203–1208. [Google Scholar] [CrossRef]
- Schachner, A.; De Luca, C.; Heidl, S.; Hess, M. Recombinantly Expressed Chimeric Fibers Demonstrate Discrete Type-Specific Neutralizing Epitopes in the Fowl Aviadenovirus E (FAdV-E) Fiber, Promoting the Optimization of FAdV Fiber Subunit Vaccines towards Cross-Protection in vivo. Microbiol. Spectr. 2022, 10, e0212321. [Google Scholar] [CrossRef]
- Liu, S.; Dong, X.; Lei, B.; Zhang, W.; Wang, X.; Yuan, W.; Zhao, K. A novel subunit vaccine based on Fiber1/2 knob domain provides full protection against fowl adenovirus serotype 4 and induces stronger immune responses than a Fiber2 subunit vaccine. Poult. Sci. 2024, 103, 103888. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z.; Liu, L.; Li, Y.; Gao, W.; Song, X.; Li, X. Knob domain of Fiber 2 protein provides full protection against fowl adenovirus serotype 4. Virus Res. 2023, 330, 199113. [Google Scholar] [CrossRef]
- He, Z.R.; Ruan, S.F.; Zhao, J.; Yang, H.M.; Zhang, G.Z. Recombinant Fiber-2 Protein-Based Indirect ELISA for Antibody Detection of Fowl Adenovirus Serotype 4. Avian Dis. 2018, 62, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, X.; Lei, B.; Han, Y.; An, T.; Zhang, W.; Zhang, H.; Li, B.; Yuan, W. Recombinant porcine Interferon-α and Interleukin-2 fusion protein (rPoIFNα+IL-2) shows potent anti-pseudorabies virus activity in vitro and in vivo. Vet. Microbiol. 2023, 279, 109678. [Google Scholar] [CrossRef]
- De Luca, C.; Schachner, A.; Mitra, T.; Heidl, S.; Liebhart, D.; Hess, M. Fowl adenovirus (FAdV) fiber-based vaccine against inclusion body hepatitis (IBH) provides type-specific protection guided by humoral immunity and regulation of B and T cell response. Vet. Res. 2020, 51, 143. [Google Scholar]
- Grafl, B.; Berger, E.; Wernsdorf, P.; Hess, M. Successful reproduction of adenoviral gizzard erosion in 20-week-old SPF layer-type chickens and efficacious prophylaxis due to live vaccination with an apathogenic fowl adenovirus serotype 1 strain (CELO). Vaccine 2020, 38, 143–149. [Google Scholar] [CrossRef]
- Wu, E.; Pache, L.; Von Seggern, D.J.; Mullen, T.M.; Mikyas, Y.; Stewart, P.L.; Nemerow, G.R. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 2003, 77, 7225–7235. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhao, Q.; Zhao, Y.; Li, Y.; Pan, S.; Li, Y.; Liu, Y.; Shi, W. Evaluation of the Immune Effect of a Trivalent Fowl Adenovirus Inactivated Vaccine Against FAdV-4/8a/8b. Vet. Sci. 2025, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Schachner, A.; Gonzalez, G.; Endler, L.; Ito, K.; Hess, M. Fowl Adenovirus (FAdV) Recombination with Intertypic Crossovers in Genomes of FAdV-D and FAdV-E, Displaying Hybrid Serological Phenotypes. Viruses 2019, 11, 1094. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, X.; Huang, X.; Huang, Y.; Yang, S.; Zhang, L.; Cui, N.; Xu, C. Pathogenicity and complete genome sequence of a fowl adenovirus serotype 8b isolate from China. Poult. Sci. 2019, 98, 573–580. [Google Scholar] [CrossRef]
- Steer, P.A.; Sandy, J.R.; O’Rourke, D.; Scott, P.C.; Browning, G.F.; Noormohammadi, A.H. Chronological analysis of gross and histological lesions induced by field strains of fowl adenovirus serotypes 1, 8b and 11 in one-day-old chickens. Avian Pathol. 2015, 44, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Niczyporuk, J.S.; Czekaj, H. A comparative pathogenicity analysis of two adenovirus strains, 1/A and 8a/E, isolated from poultry in Poland. Arch. Virol. 2018, 163, 3005–3013. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, K.; Lei, B.; Jiang, W.; Qiao, Y.; Yuan, W. A Novel Chimeric Fiber-C4/D11 Subunit Vaccine Induces Cross-Neutralizing Antibodies and Provides Better Protection Against Fowl Adenovirus (FAdV) Type 4 and Type 11 Than the Fiber-D11/C4 Subunit Vaccine. Vet. Sci. 2025, 12, 920. https://doi.org/10.3390/vetsci12090920
Wang X, Zhao K, Lei B, Jiang W, Qiao Y, Yuan W. A Novel Chimeric Fiber-C4/D11 Subunit Vaccine Induces Cross-Neutralizing Antibodies and Provides Better Protection Against Fowl Adenovirus (FAdV) Type 4 and Type 11 Than the Fiber-D11/C4 Subunit Vaccine. Veterinary Sciences. 2025; 12(9):920. https://doi.org/10.3390/vetsci12090920
Chicago/Turabian StyleWang, Xiangqin, Kuan Zhao, Baishi Lei, Wenming Jiang, Yanliang Qiao, and Wanzhe Yuan. 2025. "A Novel Chimeric Fiber-C4/D11 Subunit Vaccine Induces Cross-Neutralizing Antibodies and Provides Better Protection Against Fowl Adenovirus (FAdV) Type 4 and Type 11 Than the Fiber-D11/C4 Subunit Vaccine" Veterinary Sciences 12, no. 9: 920. https://doi.org/10.3390/vetsci12090920
APA StyleWang, X., Zhao, K., Lei, B., Jiang, W., Qiao, Y., & Yuan, W. (2025). A Novel Chimeric Fiber-C4/D11 Subunit Vaccine Induces Cross-Neutralizing Antibodies and Provides Better Protection Against Fowl Adenovirus (FAdV) Type 4 and Type 11 Than the Fiber-D11/C4 Subunit Vaccine. Veterinary Sciences, 12(9), 920. https://doi.org/10.3390/vetsci12090920





