Absence of FGF4 Retrogene Insertion on Chromosome 18 Results in a Tall Phenotype in Grand Basset Griffon Vendéen Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
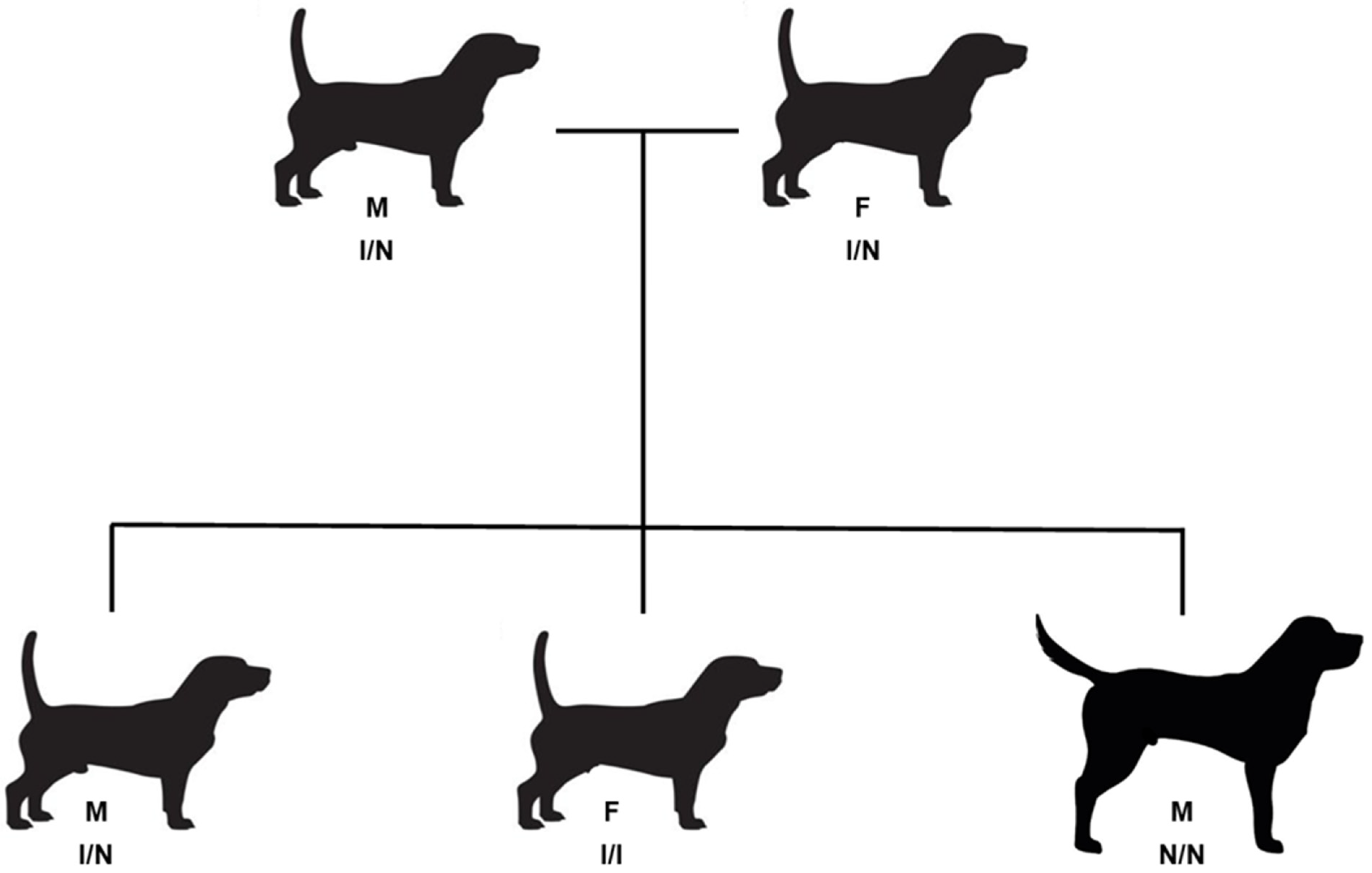
2.1. Subject Dogs
2.2. Molecular Genetics
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBGV | Grand Basset Griffon Vendéen |
| PBGV | Petit Basset Griffon Vendéen |
| EDTA | Ethylene diamine tetra acetic acid |
| PCR | Polymerase chain reaction |
| I | Insertion |
| N | Non-insertion |
| FGF4 | fibroblast growth factor 4 |
References
- Parker, H.G.; VonHoldt, B.M.; Quignon, P.; Margulies, E.H.; Shao, S.; Mosher, D.S.; Spady, T.C.; Elkahloun, A.; Cargill, M.; Jones, P.G.; et al. An Expressed Fgf4 Retrogene Is Associated with Breed-Defining Chondrodysplasia in Domestic Dogs. Science 2009, 325, 995–998. [Google Scholar] [CrossRef]
- Helmink, S.K.; Shanks, R.D.; Leighton, E.A. Investigation of Breeding Strategies to Increase the Probability That German Shepherd Dog and Labrador Retriever Dog Guides Would Attain Optimum Size. J. Anim. Sci. 2003, 81, 2950–2958. [Google Scholar] [CrossRef]
- Chen, F.L.; Zimmermann, M.; Hekman, J.P.; Lord, K.A.; Logan, B.; Russenberger, J.; Leighton, E.A.; Karlsson, E.K. Advancing Genetic Selection and Behavioral Genomics of Working Dogs Through Collaborative Science. Front. Vet. Sci. 2021, 8, 662429. [Google Scholar] [CrossRef]
- Hecht, E.E.; Smaers, J.B.; Dunn, W.D.; Kent, M.; Preuss, T.M.; Gutman, D.A. Significant Neuroanatomical Variation Among Domestic Dog Breeds. J. Neurosci. 2019, 39, 7748–7758. [Google Scholar] [CrossRef]
- Bannasch, D.; Batcher, K.; Leuthard, F.; Bannasch, M.; Hug, P.; Marcellin-Little, D.J.; Dickinson, P.J.; Drogemuller, M.; Drogemuller, C.; Leeb, T. The Effects of FGF4 Retrogenes on Canine Morphology. Genes 2022, 13, 325. [Google Scholar] [CrossRef]
- Sullivan, S.; Szeremeta, K.J.; Kutzler, M. Case Report: FGF4L1 Retrogene Insertion Is Lacking in the Tall Dachshund Phenotype. Front. Vet. Sci. 2024, 11, 1522745. [Google Scholar] [CrossRef]
- Brown, E.A.; Dickinson, P.J.; Mansour, T.; Sturges, B.K.; Aguilar, M.; Young, A.E.; Korff, C.; Lind, J.; Ettinger, C.L.; Varon, S.; et al. FGF4 Retrogene on CFA12 Is Responsible for Chondrodystrophy and Intervertebral Disc Disease in Dogs. Proc. Natl. Acad. Sci. USA 2017, 114, 11476–11481. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Khan, S.; Awano, T.; Shahid, S.A.; Siakotos, A.N.; Johnson, G.S. A Mutation in the CLN8 Gene in English Setter Dogs with Neuronal Ceroid-Lipofuscinosis. Biochem. Biophys. Res. Commun. 2005, 327, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Coates, J.R.; Johnson, G.C.; Hansen, L.; Awano, T.; Kolicheski, A.; Ivansson, E.; Perloski, M.; Lindblad-Toh, K.; O’Brien, D.P.; et al. Breed Distribution of SOD1 Alleles Previously Associated with Canine Degenerative Myelopathy. J. Vet. Intern. Med. 2014, 28, 515–521. [Google Scholar] [CrossRef]
- Thalmann, O.; Shapiro, B.; Cui, P.; Schuenemann, V.J.; Sawyer, S.K.; Greenfield, D.L.; Germonpre, M.B.; Sablin, M.V.; Lopez-Giraldez, F.; Domingo-Roura, X.; et al. Complete Mitochondrial Genomes of Ancient Canids Suggest a European Origin of Domestic Dogs. Science 2013, 342, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Ni Leathlobhair, M.; Perri, A.R.; Irving-Pease, E.K.; Witt, K.E.; Linderholm, A.; Haile, J.; Lebrasseur, O.; Ameen, C.; Blick, J.; Boyko, A.R.; et al. The Evolutionary History of Dogs in the Americas. Science 2018, 361, 81–85. [Google Scholar] [CrossRef]
- Sinding, M.-H.S.; Gopalakrishnan, S.; Ramos-Madrigal, J.; de Manuel, M.; Pitulko, V.V.; Kuderna, L.; Feuerborn, T.R.; Frantz, L.A.F.; Vieira, F.G.; Niemann, J.; et al. Arctic-Adapted Dogs Emerged at the Pleistocene-Holocene Transition. Science 2020, 368, 1495–1499. [Google Scholar] [CrossRef]
- Batcher, K.; Dickinson, P.; Giuffrida, M.; Sturges, B.; Vernau, K.; Knipe, M.; Rasouliha, S.H.; Drögemüller, C.; Leeb, T.; Maciejczyk, K.; et al. Phenotypic Effects of FGF4 Retrogenes on Intervertebral Disc Disease in Dogs. Genes 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, V.L.J.; Jokinen, T.S.; Lilja-Maula, L.; Hytonen, M.K.; Lappalainen, A.K. Allelic Frequency of 12-FGF4RG and the Association between the Genotype with Number of Calcified Intervertebral Discs Visible on Radiographs in Coton de Tulear and French Bulldog Breeds. BMC Vet. Res. 2025, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.K.; Blacksmith, M.S.; Kidd, J.M. Duplications and Retrogenes Are Numerous and Widespread in Modern Canine Genomic Assemblies. Genome Biol. Evol. 2024, 16, evae142. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, Y.; Adetula, A.A.; Wu, Y.; Chen, H. Analysis of New Retrogenes Provides Insight into Dog Adaptive Evolution. Ecol. Evol. 2019, 9, 11185–11197. [Google Scholar] [CrossRef]
- Ciomborowska-Basheer, J.; Staszak, K.; Kubiak, M.R.; Makalowska, I. Not So Dead Genes-Retrocopies as Regulators of Their Disease-Related Progenitors and Hosts. Cells 2021, 10, 912. [Google Scholar] [CrossRef]
- Vinckenbosch, N.; Dupanloup, I.; Kaessmann, H. Evolutionary Fate of Retroposed Gene Copies in the Human Genome. Proc. Natl. Acad. Sci. USA 2006, 103, 3220–3225. [Google Scholar] [CrossRef]
- Boulet, A.M.; Capecchi, M.R. Signaling by FGF4 and FGF8 Is Required for Axial Elongation of the Mouse Embryo. Dev. Biol. 2012, 371, 235–245. [Google Scholar] [CrossRef]
- Batcher, K.; Varney, S.; Affolter, V.K.; Friedenberg, S.G.; Bannasch, D. An SNN Retrocopy Insertion Upstream of GPR22 Is Associated with Dark Red Coat Color in Poodles. G3 2022, 12, jkac227. [Google Scholar] [CrossRef]
- Machado, J.P.; Antunes, A. The Genomic Context of Retrocopies Increases Their Chance of Functional Relevancy in Mammals. Genomics 2020, 112, 2410–2417. [Google Scholar] [CrossRef] [PubMed]

| 18-FGF4RG Insertion | Short | Tall |
|---|---|---|
| Insertion/Insertion | 14 | 0 |
| Insertion/No Insertion | 27 | 0 |
| No Insertion/No Insertion | 0 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhlanga-Mutangadura, T.; Hansen, L.; Katz, M.L. Absence of FGF4 Retrogene Insertion on Chromosome 18 Results in a Tall Phenotype in Grand Basset Griffon Vendéen Dogs. Vet. Sci. 2025, 12, 916. https://doi.org/10.3390/vetsci12090916
Mhlanga-Mutangadura T, Hansen L, Katz ML. Absence of FGF4 Retrogene Insertion on Chromosome 18 Results in a Tall Phenotype in Grand Basset Griffon Vendéen Dogs. Veterinary Sciences. 2025; 12(9):916. https://doi.org/10.3390/vetsci12090916
Chicago/Turabian StyleMhlanga-Mutangadura, Tendai, Liz Hansen, and Martin L. Katz. 2025. "Absence of FGF4 Retrogene Insertion on Chromosome 18 Results in a Tall Phenotype in Grand Basset Griffon Vendéen Dogs" Veterinary Sciences 12, no. 9: 916. https://doi.org/10.3390/vetsci12090916
APA StyleMhlanga-Mutangadura, T., Hansen, L., & Katz, M. L. (2025). Absence of FGF4 Retrogene Insertion on Chromosome 18 Results in a Tall Phenotype in Grand Basset Griffon Vendéen Dogs. Veterinary Sciences, 12(9), 916. https://doi.org/10.3390/vetsci12090916






