Compounding and Use of Human Medicinal Products in Small Animal Practice: What Are the Perspectives of Veterinarians?—A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Questionnaire Format and Distribution
2.3. Statistical Analysis
3. Results
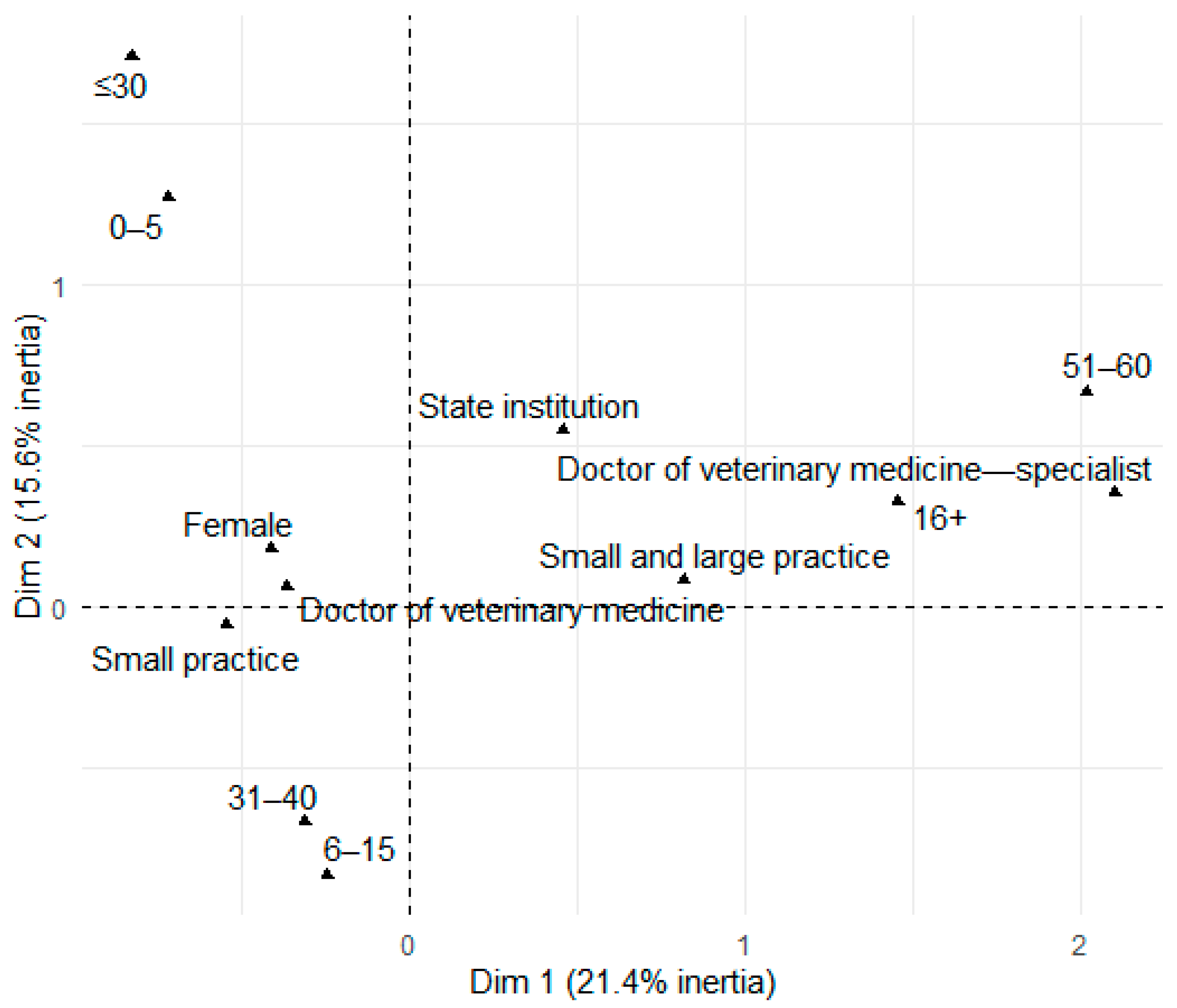
3.1. Sociodemographic Characteristics of the Respondents
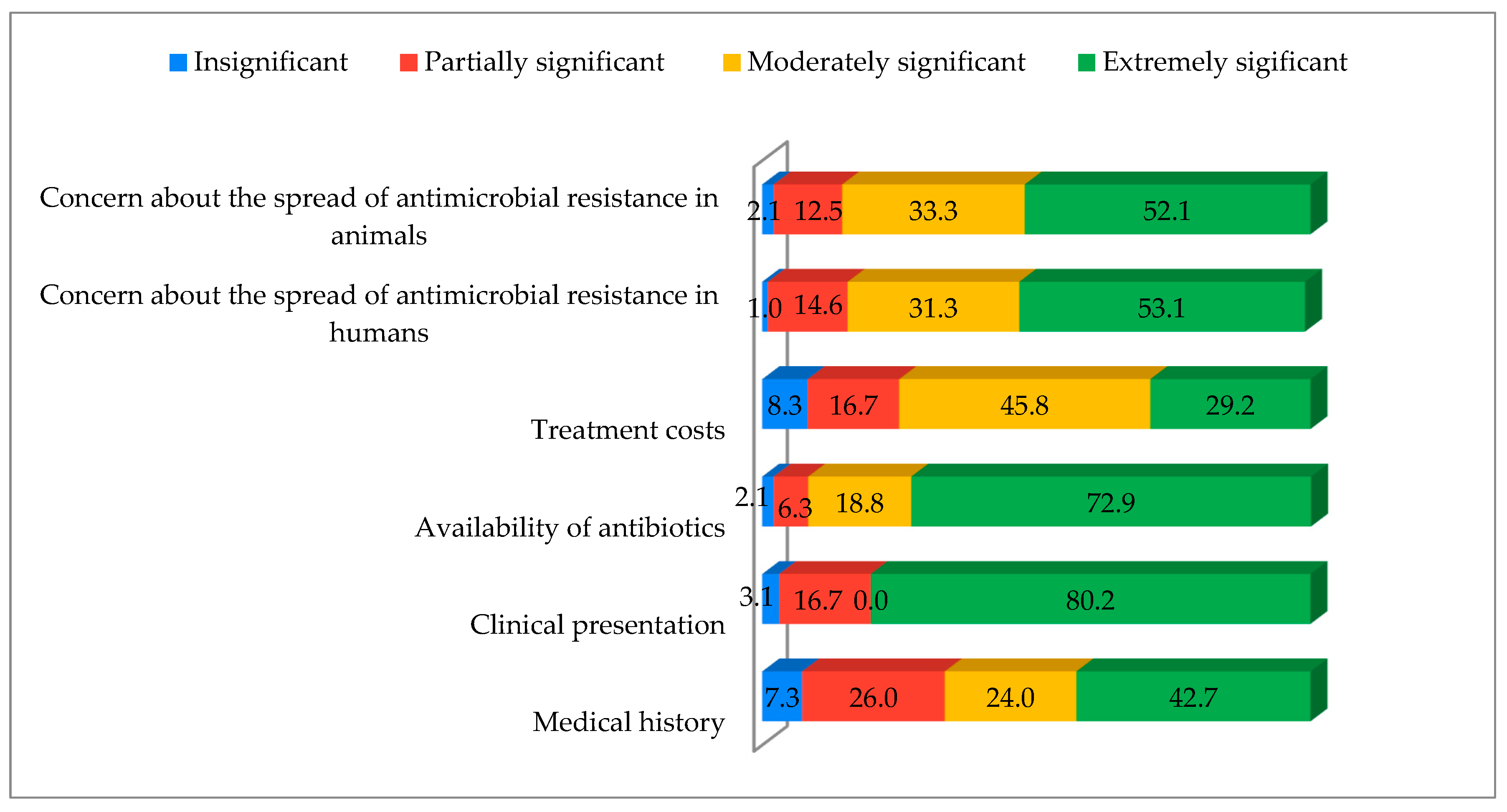
3.2. Veterinarians’ Prescribing Practices Particularly Regarding Human-Approved Medications and Antibiotics
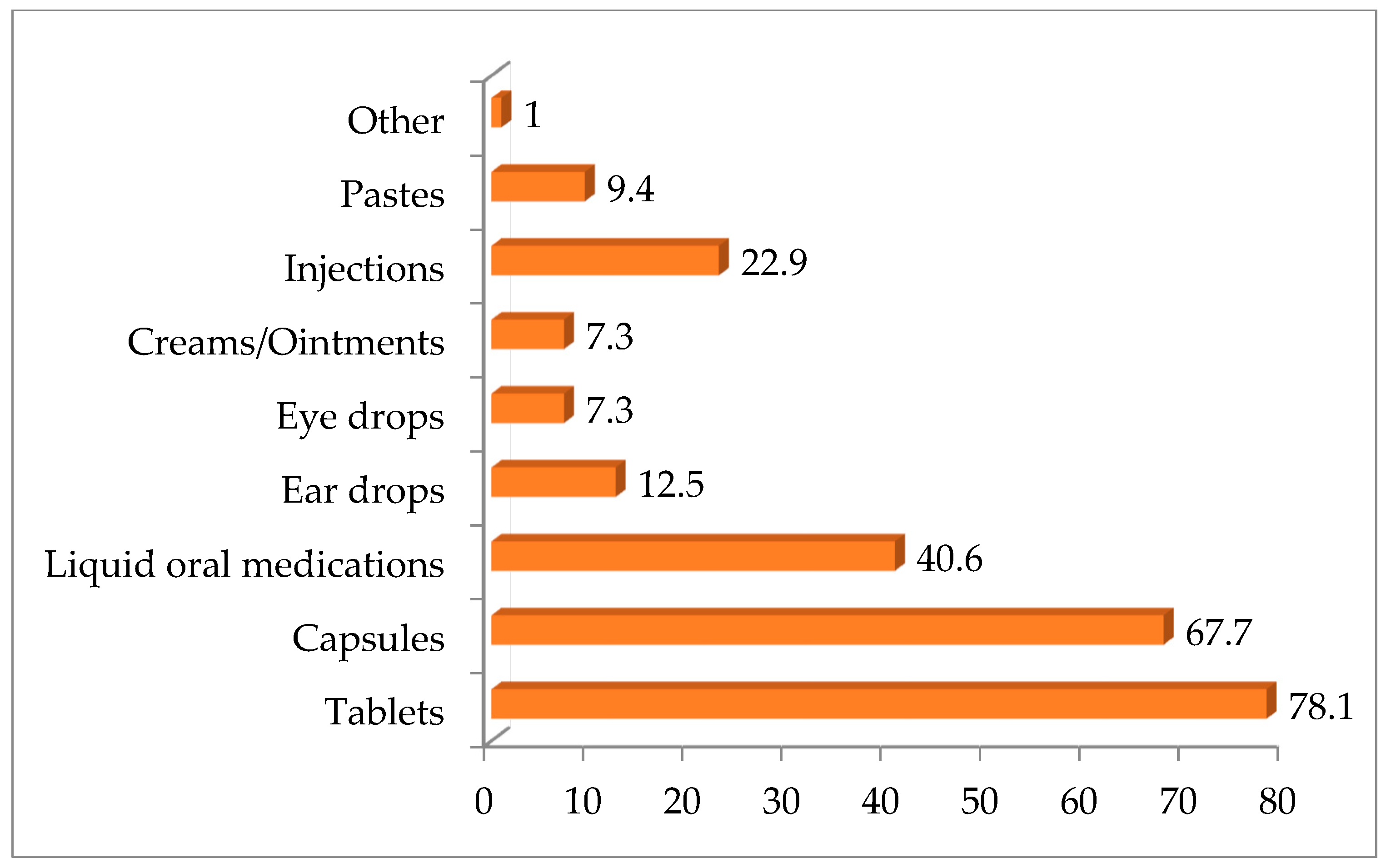
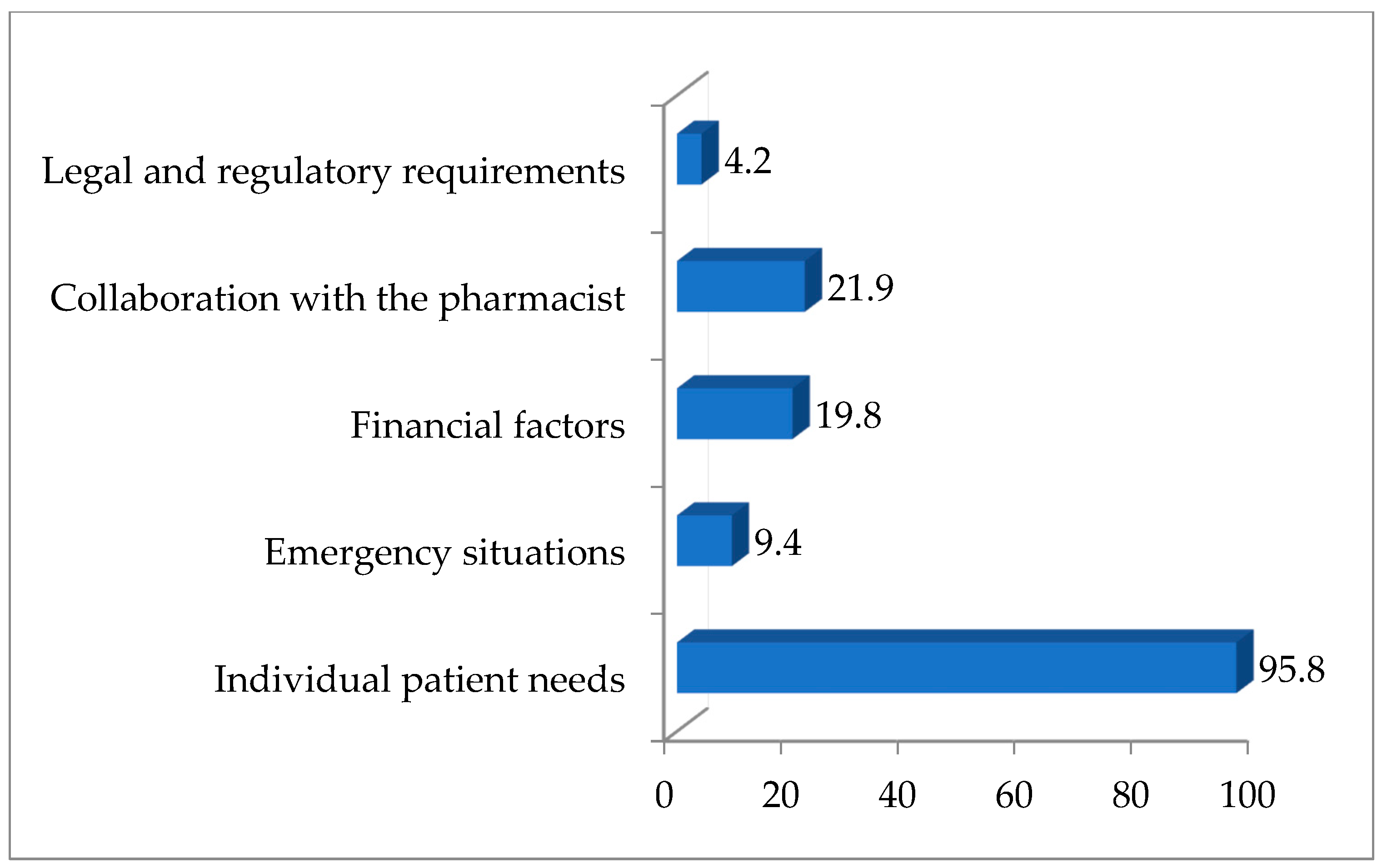
3.3. Veterinarians’ Attitudes and Experiences Regarding Compounded Medications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMs | Compounded medications |
| VMPs | Veterinary medicinal products |
| AMR | Antimicrobial resistance |
References
- Lust, E. Compounding for animal patients: Contemporary issues. J. Am. Pharm. Assoc. 2004, 44, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G. Compounding for Animals. In Pharmacotherapeutics for Veterinary Dispensing; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 43–74. [Google Scholar]
- Boothe, D.M. Veterinary compounding in small animals: A clinical pharmacologist’s perspective. Vet. Clin. Small Anim. Pract. 2006, 36, 1129–1173. [Google Scholar] [CrossRef] [PubMed]
- Sl. Glasnik RS 30/2010. Law on Medicines and Medical Devices of Serbia. Available online: https://www.paragraf.rs/propisi/zakon_o_lekovima_i_medicinskim_sredstvima.html (accessed on 20 March 2025).
- Schmerold, I.; van Geijlswijk, I.; Gehring, R. European regulations on the use of antibiotics in veterinary medicine. Eur. J. Pharm. Sci. 2023, 189, 106473. [Google Scholar] [CrossRef]
- Lhermie, G.; La Ragione, R.M.; Weese, J.S.; Olsen, J.E.; Christensen, J.P.; Guardabassi, L. Indications for the use of highest priority critically important antimicrobials in the veterinary sector. J. Antimicrob. Chemother. 2020, 75, 1671–1680. [Google Scholar] [CrossRef]
- Tesin, N.; Stojanovic, D.; Stancic, L.; Kladar, N.; Ružic, Z.; Spasojevic, J.; Tomanic, D.; Kovacevic, Z. Prevalence of the microbiological causes of canine otitis externa and the antibiotic susceptibility of the isolated bacterial strains. Pol. J. Vet. Sci. 2023, 26, 449–459. [Google Scholar] [CrossRef]
- Bennett, S.A.; Ruisinger, J.F.; Prohaska, E.S.; Steele, K.M.; Melton, B.L. Assessing pet owner and veterinarian perceptions of need for veterinary compounding services in a community pharmacy setting. Pharm. Pract. 2018, 16, 1224. [Google Scholar] [CrossRef]
- Ekakoro, J.E.; Okafor, C.C. Antimicrobial use practices of veterinary clinicians at a veterinary teaching hospital in the United States. Vet. Anim. Sci. 2019, 7, 100038. [Google Scholar] [CrossRef]
- MacFarland, T.W.; Yates, J.M.; MacFarland, T.W.; Yates, J.M. Mann–whitney u test. In Introduction to Nonparametric Statistics for the Biological Sciences Using R; Springer: Cham, Switzerland, 2016; pp. 103–132. [Google Scholar]
- Ostertagova, E.; Ostertag, O.; Kováč, J. Methodology and application of the Kruskal-Wallis test. Appl. Mech. Mater. 2014, 611, 115–120. [Google Scholar] [CrossRef]
- Rached, R.Z.; Pose, R.A.; Caetano, É.L.A.; Magalhães, J.G.; Grotto, D. Veterinary Prescriptions of Antibiotics Approved for Human Use: A Five-Year Analysis of Companion Animal Use and Regulatory Gaps in Brazil. Vet. Sci. 2025, 12, 652. [Google Scholar] [CrossRef]
- Suwanpakdee, S.; Chantong, B.; Wiratsudakul, A.; Tangcharoensathien, V.; Lekagul, A.; Sakcamduang, W. Antibiotic use in companion animals in veterinary teaching hospitals in Thailand. PLoS ONE 2025, 20, e0330750. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G. The compounding controversy: What veterinarians should know to protect themselves and their patients. J. Am. Anim. Hosp. Assoc. 2003, 39, 13–17. [Google Scholar] [CrossRef]
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. Available online: https://eur-lex.europa.eu/eli/dir/2001/83/oj/eng (accessed on 25 June 2025).
- The European Parliament and the Council of the Europe. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Off. J. Eur. Union 2019, L4, 43–167. [Google Scholar]
- EMA/CVMP. Reflection Paper on Off-Label Use of Antimicrobials in Veterinary Medicine in The European Union. Available online: https://www.ema.europa.eu/en (accessed on 25 June 2025).
- Hölsö, K.; Rantala, M.; Lillas, A.; Eerikäinen, S.; Huovinen, P.; Kaartinen, L. Prescribing antimicrobial agents for dogs and cats via university pharmacies in Finland–patterns and quality of information. Acta Vet. Scand. 2005, 46, 87. [Google Scholar] [CrossRef]
- Odensvik, K.; Grave, K.; Greko, C. Antibacterial drugs prescribed for dogs and cats in Sweden and Norway 1990–1998. Acta Vet. Scand. 2001, 42, 189. [Google Scholar] [CrossRef]
- Tanaka, N.; Takizawa, T.; Miyamoto, N.; Funayama, S.; Tanaka, R.; Okano, S.; Iwasaki, T. Real world data of a veterinary teaching hospital in Japan: A pilot survey of prescribed medicines. Vet. Rec. Open 2017, 4, e000218. [Google Scholar] [CrossRef] [PubMed]
- Escher, M.; Vanni, M.; Intorre, L.; Caprioli, A.; Tognetti, R.; Scavia, G. Use of antimicrobials in companion animal practice: A retrospective study in a veterinary teaching hospital in Italy. J. Antimicrob. Chemother. 2011, 66, 920–927. [Google Scholar] [CrossRef]
- Gómez-Poveda, B.; Moreno, M.A. Antimicrobial prescriptions for dogs in the capital of Spain. Front. Vet. Sci. 2018, 5, 309. [Google Scholar] [CrossRef]
- Lloyd, D.H. Reservoirs of antimicrobial resistance in pet animals. Clin. Infect. Dis. 2007, 45, S148–S152. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO’s List of Medically Important Antimicrobials: A risk management tool for mitigating antimicrobial resistance due to non-human use. Available online: https://cdn.who.int/media/docs/default-source/gcp/who-mia-list-2024-lv.pdf (accessed on 1 June 2025).
- Shikoska, I.; Cvetkovikj, A.; Nikolovski, M.; Cvetkovikj, I. Understanding Antimicrobial Prescription Practices: Insights from Small Animal Veterinarians in North Macedonia. Maced. Vet. Rev. 2024, 47, 103–114. [Google Scholar] [CrossRef]
- Tomanic, D.; Stojanovic, D.; Belić, B.; Davidov, I.; Novakov, N.; Radinovic, M.; Kladar, N.; Kovacevic, Z. Off label use of human approved drugs in treatment of dogs in the Republic of Serbia. Pol. J. Vet. Sci. 2021, 24, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Servia-Dopazo, M.; Taracido-Trunk, M.; Figueiras, A. Non-clinical factors determining the prescription of antibiotics by veterinarians: A systematic review. Antibiotics 2021, 10, 133. [Google Scholar] [CrossRef]
- Etienne, J.; Chirico, S.; Gunabalasingham, T.; Dautzenberg, S.; Gysen, S. EU Insights–Perceptions on the human health impact of antimicrobial resistance (AMR) and antibiotics use in animals across the EU. EFSA Support. Publ. 2017, 14, 1183E. [Google Scholar] [CrossRef]
- Hopman, N.E.; Hulscher, M.E.; Graveland, H.; Speksnijder, D.C.; Wagenaar, J.A.; Broens, E.M. Factors influencing antimicrobial prescribing by Dutch companion animal veterinarians: A qualitative study. Prev. Vet. Med. 2018, 158, 106–113. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, J.; Meena, H. Assessment of awareness about antibiotic resistance and practices followed by veterinarians for judicious prescription of antibiotics: An exploratory study in eastern Haryana region of India. Trop. Anim. Health Prod. 2019, 51, 677–687. [Google Scholar] [CrossRef]
- Smith, M.; King, C.; Davis, M.; Dickson, A.; Park, J.; Smith, F.; Currie, K.; Flowers, P. Pet owner and vet interactions: Exploring the drivers of AMR. Antimicrob. Resist. Infect. Control 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.O.; Smed, S.; Klit, K.J.; Olsen, J.E. Factors influencing Danish veterinarians’ choice of antimicrobials prescribed for intestinal diseases in weaner pigs. Vet. Rec. 2019, 184, 798. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Zhuo, A.; Govendir, M.; Rowbotham, S.J.; Labbate, M.; Degeling, C.; Gilbert, G.L.; Dominey-Howes, D.; Ward, M.P. Factors influencing the behaviour and perceptions of Australian veterinarians towards antibiotic use and antimicrobial resistance. PLoS ONE 2019, 14, e0223534. [Google Scholar]
- Ceresia, M.L.; Fasser, C.E.; Rush, J.E.; Scheife, R.T.; Orcutt, C.J.; Michalski, D.L.; Mazan, M.R.; Dorsey, M.T.; Bernardi, S.P. The role and education of the veterinary pharmacist. Am. J. Pharm. Educ. 2009, 73, 16. [Google Scholar] [CrossRef]
- Gargiulo, D.; Chemal, C.; Joda, L.; Lee, Y.; Pilkington, M.; Haywood, A.; Garg, S. Extemporaneous compounding in veterinary practice: A New Zealand perspective. N. Z. Vet. J. 2013, 61, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G. Veterinary compounding: Regulation, challenges, and resources. Pharmaceutics 2017, 9, 5. [Google Scholar] [CrossRef]
- Agelová, J.; Macesková, B. Analysis of drugs used in out-patient practice of veterinary medicine. Ceska A Slov. Farm. 2005, 54, 34–38. [Google Scholar]
- Spenser, E.L. Compounding, extralabel drug use, and other pharmaceutical quagmires in avian and exotics practice. Semin. Avian Exot. Pet Med. 2004, 13, 16–24. [Google Scholar] [CrossRef]
- Sjöholm, E.; Mathiyalagan, R.; Wang, X.; Sandler, N. Compounding tailored veterinary chewable tablets close to the point-of-care by means of 3D printing. Pharmaceutics 2022, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Khor, K. Oral drug formulation and compliance in veterinary medicine. J. Vet. Malays. 2012, 24, 18–22. [Google Scholar]
- Kukanich, B.; Papich, M. Fluoroquinolone stability in vehicles for oral administration. In Proceedings of the 21st ACVIM Forum Proceedings, Proceedings of the American College of Veterinary Internal Medicine Annual Forum, Charlotte, NC, USA, 4–7 June 2003; American College of Veterinary Internal Medicine: Greenwood Village, CO, USA, 2003. [Google Scholar]
- Nieto, J.E.; Spier, S.; Pipers, F.S.; Stanley, S.; Aleman, M.R.; Smith, D.C.; Snyder, J.R. Comparison of paste and suspension formulations of omeprazole in the healing of gastric ulcers in racehorses in active training. J. Am. Vet. Med. Assoc. 2002, 221, 1139–1143. [Google Scholar] [CrossRef]
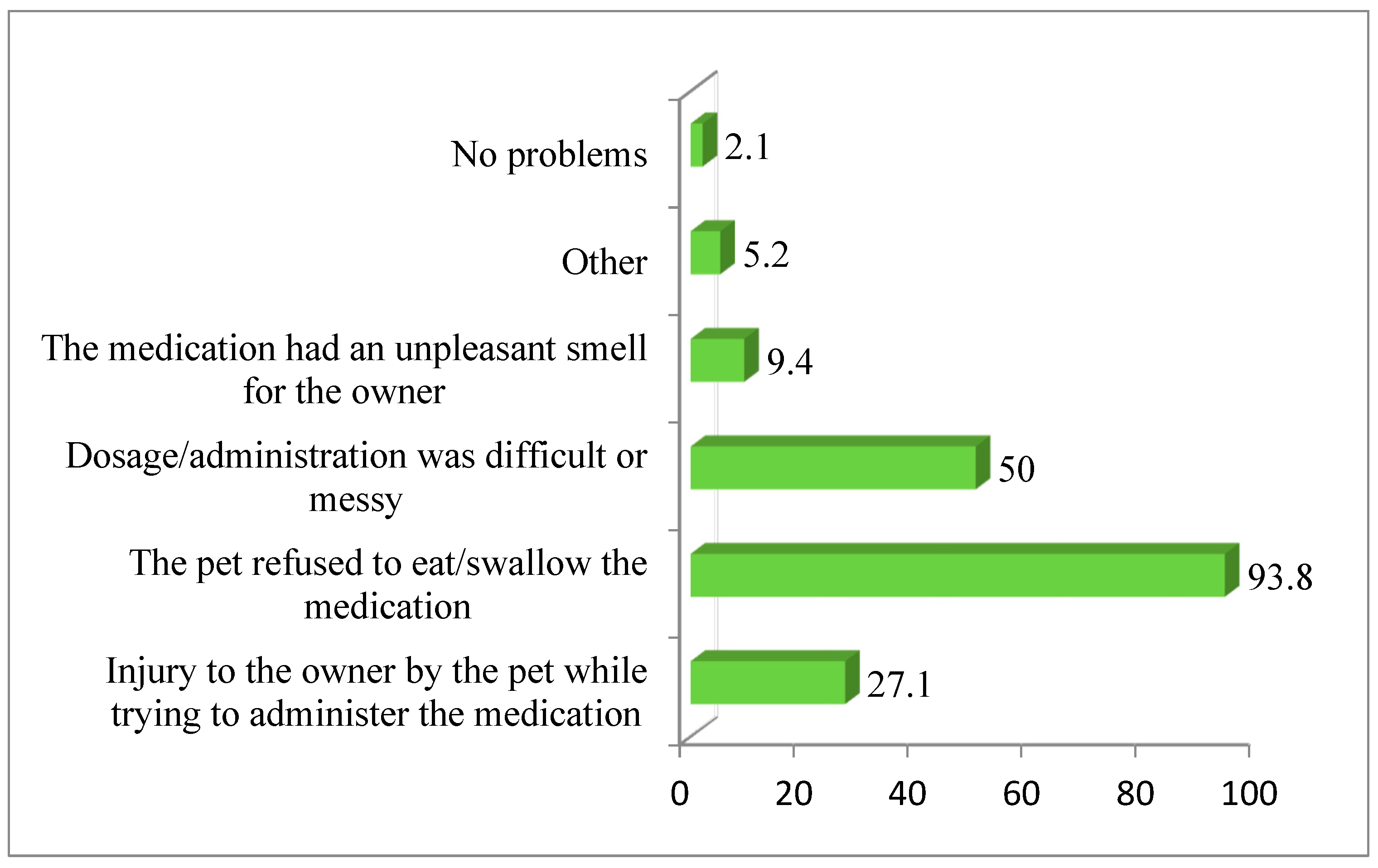
| Variable | Response | n | % |
|---|---|---|---|
| Gender | Female | 42 | 43.8 |
| Male | 54 | 56.2 | |
| Age group | ≤30 | 18 | 18.8 |
| 31–40 | 42 | 43.8 | |
| 41–50 | 22 | 22.9 | |
| 51–60 | 12 | 12.5 | |
| 61+ | 2 | 2.1 | |
| Work sector | State institution Private institution | 26 | 27.1 |
| 70 | 72.9 | ||
| Level of education | Doctor of veterinary medicine Master of veterinary medicine Doctor of medical sciences—veterinary medicine Doctor of veterinary medicine—specialist | 73 | 76 |
| 3 | 3.1 | ||
| 12 | 12.5 | ||
| 8 | 8.3 | ||
| Number of years working in practice | 0–5 | 26 | 27.1 |
| 6–15 | 48 | 50 | |
| 16+ | 22 | 22.9 | |
| Type of practice | Small practice | 58 | 60.4 |
| Small and large practice | 38 | 39.6 | |
| Number of monthly cases | 0–100 | 50 | 52.1 |
| 101–200 | 21 | 21.9 | |
| 201–300 | 15 | 15.6 | |
| 301+ | 10 | 10.4 |
| Variable | Response | n | % |
|---|---|---|---|
| Do you face challenges regarding the availability of appropriate medications for animals? | Yes | 82 | 85.4 |
| No | 14 | 14.6 | |
| How often do you prescribe human medicinal products? | Often | 44 | 45.8 |
| Moderately | 39 | 40.6 | |
| Rarely or never | 13 | 13.5 | |
| What are the reasons for prescribing human medicinal products? | Financial situation of pet owners | 10 | 10.4 |
| Prescribing practices | 0 | 0 | |
| Availability of medication | 40 | 41.7 | |
| Pressure from owners | 1 | 1 | |
| The financial situation of pet owners and the availability of medications | 34 | 35.4 | |
| The financial situation of pet owners, the availability of medications, and prescribing practices | 6 | 6.3 | |
| The financial situation of pet owners, the availability of medications, and pressure from owners | 3 | 3.1 | |
| Prescribing practices and the availability of medications | 2 | 2.1 |
| Variable | Response | n | % |
|---|---|---|---|
| Are you familiar with the concept of compounding medications? | Yes | 56 | 58.3 |
| No | 21 | 21.9 | |
| I have educated myself independently | 19 | 19.8 | |
| How often do you use compounded medications in your daily practice? | Often | 0 | 0 |
| Moderately | 56 | 58.3 | |
| Never | 40 | 41.7 | |
| What do you consider to be the main advantages of compounded medications? | Dose adjustment for the patient | 42 | 43.8 |
| Customization of the pharmaceutical dosage form for the patient | 26 | 27.1 | |
| Customization of the taste for the patient | 1 | 1 | |
| Controlled quality | 1 | 1 | |
| Easier preparation | 7 | 7.3 | |
| Fast delivery | 0 | 0 | |
| All of the above | 19 | 19.8 | |
| Do you think that compounded medications contribute to better treatment outcomes for certain patients? | Yes | 63 | 65.6 |
| No | 6 | 6.3 | |
| Unsure | 27 | 28.1 | |
| Where would you recommend pet owners procure compounded medications for their pets? | Veterinary clinic | 25 | 26 |
| Pharmacy | 64 | 66.7 | |
| By post office | 2 | 2.1 | |
| Other | 5 | 5.2 | |
| Would you like more education about compounded medications to better understand their capabilities and limitations? | Yes | 84 | 87.5 |
| No | 4 | 4.2 | |
| Unsure | 8 | 8.3 | |
| Do you think the compounding of veterinary medications is adequately regulated in your field of work? | Yes | 8 | 8.3 |
| No | 43 | 44.8 | |
| Unsure | 45 | 46.9 |
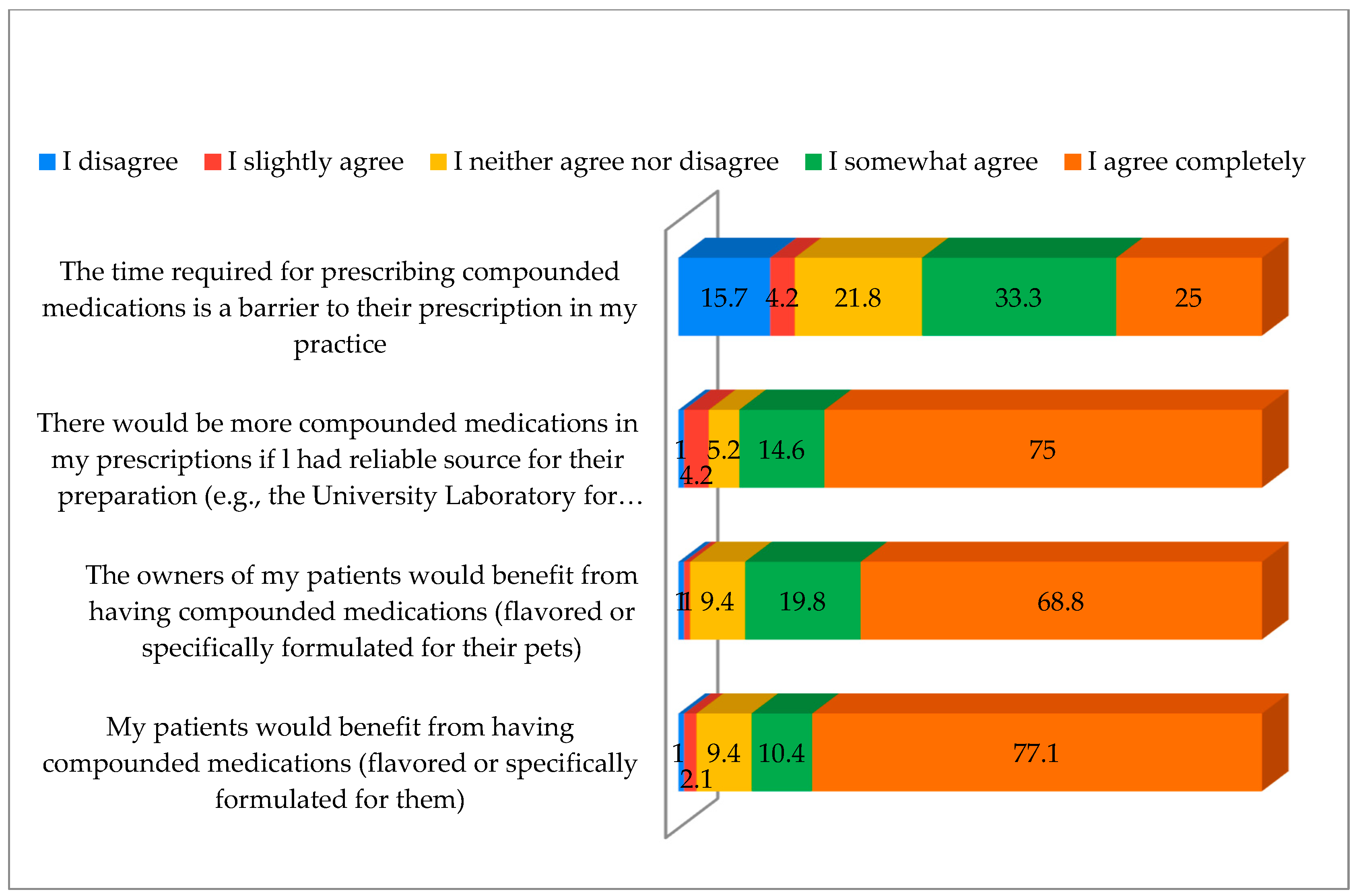
| My Patients Would Benefit from Having Their Medications Compounded (with Flavoring or Formulated Specifically for Them) | The Owners of My Patients Would Benefit from Having Their Medications Compounded (with Flavoring or Formulated Specifically for Their Pet) | There Would Be More Compounded Medications in My Prescriptions If I Had a Reliable Source for Their Preparation (e.g., a University Compounding Laboratory in the Department of Veterinary Medicine) | The Time Required to Prescribe Compounded Medications Is a Barrier to Their Prescription in My Practice | ||
|---|---|---|---|---|---|
| ± SD | ± SD | ± SD | ± SD | ||
| Gender | Male Female | 4.762 ± 0.753 | 4.690 ± 0.795 | 4.854 ± 0.905 | 3.341 ± 1.285 |
| 4.596 ± 0.697 | 4.538 ± 0.517 | 4.538 ± 0.786 | 3.500 ± 1.356 | ||
| U = 962.000; p = 0.166 | U = 1028.0000; p = 0.544 | U = 860.000; p = 0.029 | U = 1133.000; p = 0.591 | ||
| Age group | 30 31–40 41–50 51–60 61+ | 4.333 ± 0.970 | 4.500 ± 0.786 | 5.000 ± 0.010 | 3.167 ± 1.543 |
| 4.762 ± 0.670 | 4.714 ± 0.564 | 4.643 ± 0.905 | 3.643 ± 1.224 | ||
| 4.905 ± 0.338 | 4.762 ± 0.542 | 4.667 ± 0.961 | 3.619 ± 1.358 | ||
| 4.455 ± 0.535 | 4.091 ± 0.900 | 4.300 ± 0.756 | 2.900 ± 0.900 | ||
| 4.500 ± 0.957 | 4.500 ± 1.000 | 4.000 ± 1.069 | 2.000 ± 0.787 | ||
| H = 9.071; p = 0.059 | H = 6.687; p = 0.153 | H = 11.083; p = 0.026 | H = 7.963; p = 0.093 | ||
| Work sector | State institution Private institution | 4.640 ± 0.700 | 4.560 ± 0.860 | 4.760 ± 0.905 | 3.000 ± 1.356 |
| 4.681 ± 0.071 | 4.623 ± 0.623 | 4.632 ± 0.843 | 3.588 ± 1.269 | ||
| U = 893.000; p = 0.715 | U = 837.500; p = 0.790 | U = 845.500; p = 0.957 | U = 1261.000; p = 0.048 | ||
| Level of education | Doctor of veterinary medicine Master of veterinary medicine Doctor of medical sciences—veterinary medicine Doctor of veterinary medicine—specialist | 4.667 ± 0.736 | 5.000 ± 0.637 | 4.818 ± 0.788 | 4.375 ± 1.385 |
| 4.639 ± 0.000 | 4.667 ± 0.577 | 4.818 ± 0.577 | 4.000 ± 0.577 | ||
| 4.690 ± 0.603 | 4.667 ± 0.788 | 4.818 ± 1.267 | 4.250 ± 1.379 | ||
| 3.479 ± 0.518 | 3.667 ± 0.926 | 3.091 ± 0.707 | 3.375 ± 0.518 | ||
| H = 7.238; p = 0.065 | H = 7.151; p = 0.067 | H = 7.693; p = 0.053 | H = 1.579; p = 0.664 | ||
| Number of years working in practice | 0–5 6–15 16+ | 4.538 ± 0.859 | 4.754 ± 0.706 | 4.667 ± 0.392 | 4.670 ± 1.419 |
| 4.538 ± 0.642 | 4.702 ± 0.595 | 4.476 ± 0.986 | 4.606 ± 1.320 | ||
| 4923 ± 0.587 | 4.574 ± 0.854 | 4.550 ± 0.854 | 4.667 ± 1.167 | ||
| H = 1.142; p = 0.565 | H = 1.283; p = 0.526 | H = 6.713; p = 0.035 | H = 7.963; p = 0.093 | ||
| Type of practice | Small practice Small and large practice | 4.649 ± 0.767 | 4.684 ± 0.602 | 4.807 ± 0.755 | 3.632 ± 1.175 |
| 4.703 ± 0.577 | 4.486 ± 0.760 | 4.444 ± 0.942 | 3.111 ± 1.467 | ||
| U = 1027.500; p = 0.770 | U = 907,500; p = 0.157 | U = 793,500; p = 0.012 | U = 844.000; p = 0.137 | ||
| Number of monthly cases | 0–100 101–200 201–300 301+ | 4.551 ± 0.739 | 4.857 ± 0.710 | 4.714 ± 0.819 | 4.670 ± 1.384 |
| 4.429 ± 0.529 | 4.810 ± 0.332 | 4.786 ± 0.529 | 4.606 ± 1.173 | ||
| 4.646 ± 0.816 | 4.857 ± 0.907 | 4.571 ± 1.231 | 4.667 ± 1.227 | ||
| 3.497 ± 0.647 | 2.952 ± 0.647 | 3.500 ± 0.647 | 3.430 ± 0.674 | ||
| H = 3.722; p = 0.293 | H = 8.282; p = 0.041 | H = 2.649; p = 0.449 | H = 6.746; p = 0.080 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, Z.; Gračner, G.G.; Tomanić, D.; Vlahović, K.; Šarić, L.; Novaković, D.; Galić, I.; Pajić, K.; Marić, D.; Samardžija, M.; et al. Compounding and Use of Human Medicinal Products in Small Animal Practice: What Are the Perspectives of Veterinarians?—A Pilot Study. Vet. Sci. 2025, 12, 914. https://doi.org/10.3390/vetsci12090914
Kovačević Z, Gračner GG, Tomanić D, Vlahović K, Šarić L, Novaković D, Galić I, Pajić K, Marić D, Samardžija M, et al. Compounding and Use of Human Medicinal Products in Small Animal Practice: What Are the Perspectives of Veterinarians?—A Pilot Study. Veterinary Sciences. 2025; 12(9):914. https://doi.org/10.3390/vetsci12090914
Chicago/Turabian StyleKovačević, Zorana, Gordana Gregurić Gračner, Dragana Tomanić, Ksenija Vlahović, Ljubiša Šarić, Dragana Novaković, Ivan Galić, Katarina Pajić, Dragoljub Marić, Marko Samardžija, and et al. 2025. "Compounding and Use of Human Medicinal Products in Small Animal Practice: What Are the Perspectives of Veterinarians?—A Pilot Study" Veterinary Sciences 12, no. 9: 914. https://doi.org/10.3390/vetsci12090914
APA StyleKovačević, Z., Gračner, G. G., Tomanić, D., Vlahović, K., Šarić, L., Novaković, D., Galić, I., Pajić, K., Marić, D., Samardžija, M., & Sič, O. (2025). Compounding and Use of Human Medicinal Products in Small Animal Practice: What Are the Perspectives of Veterinarians?—A Pilot Study. Veterinary Sciences, 12(9), 914. https://doi.org/10.3390/vetsci12090914










