The Hemoglobin, Albumin, Lymphocyte, and Platelet Score as a Prognostic Indicator for Dogs with Congestive Heart Failure Secondary to Myxomatous Mitral Valve Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. HALP Score Calculation
2.3. Outcome Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Population
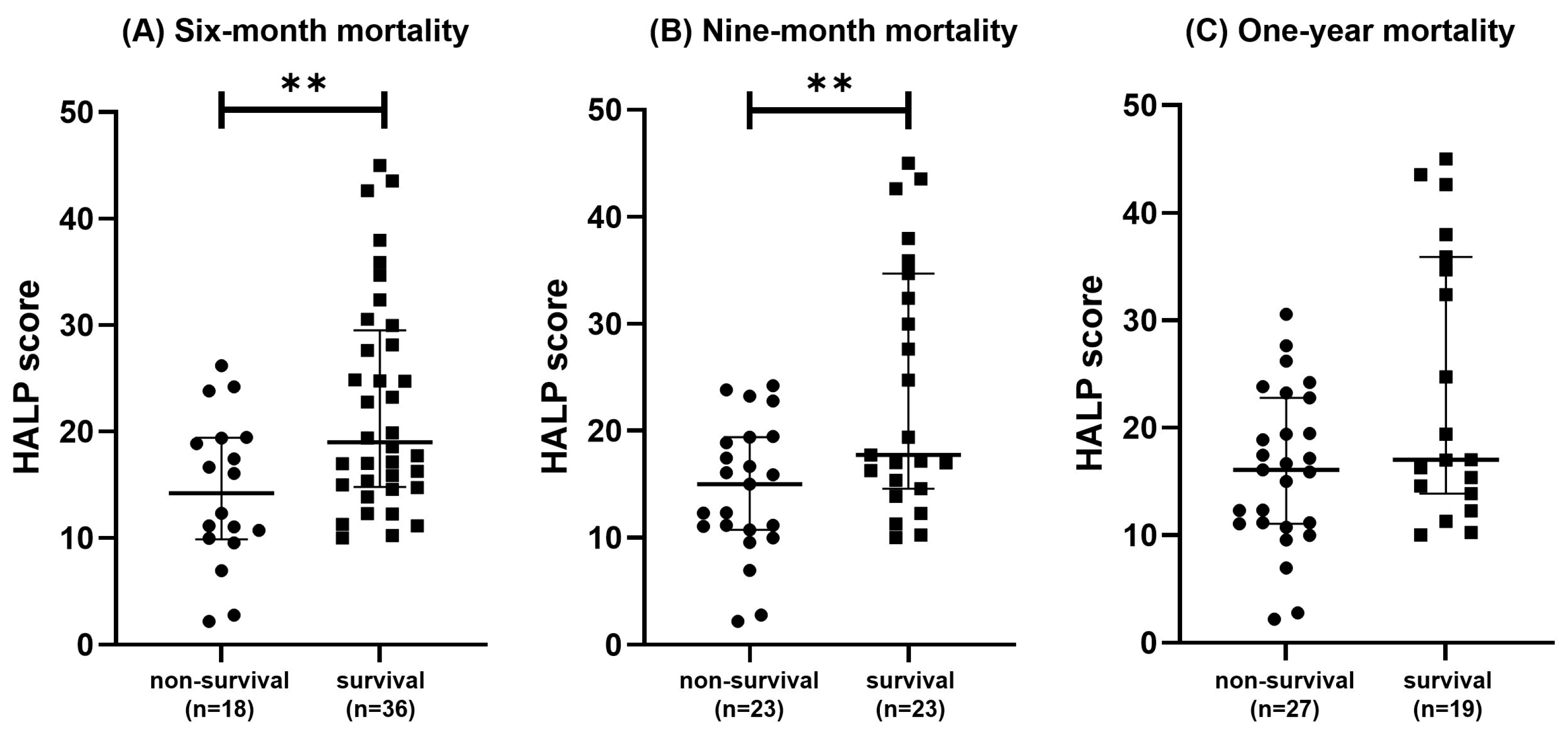
3.2. Comparesion of HALP Score
3.3. ROC Curve of the HALP Score for Short-Term Mortality
3.4. Kaplan–Meier Survival Analysis Based on HALP Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, P.R. Pathology of myxomatous mitral valve disease in the dog. J. Vet. Cardiol. 2012, 14, 103–126. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Ljungvall, I.; Höglund, K.; Tidholm, A.; Olsen, L.H.; Borgarelli, M.; Venge, P.; Häggström, P. Cardiac troponin I is associated with severity of myxomatous mitral valve disease, age, and C-reactive protein in dogs. J. Vet. Intern. Med. 2010, 24, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.R.S.; Monteith, G.; Raheb, S.; Colpitts, M.; Fonfara, S. Echocardiographic parameters for the assessment of congestive heart failure in dogs with myxomatous mitral valve disease and moderate to severe mitral regurgitation. Vet. J. 2020, 263, 105518. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.P.; Saril, A.; Kocaturk, M.; Tanaka, R.; Koch, J.; Ceron, J.J.; Yilmaz, Z. Changes of inflammatory and oxidative stress biomarkers in dogs with different stages of heart failure. BMC Vet. Res. 2020, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Hori, Y.; Kanno, N.; Iwasa, N.; Toyofuku, T.; Isayama, N.; Yoshikawa, A.; Akabane, R.; Sakatani, A.; Miyakawa, H.; et al. Comparison of N-terminal pro-atrial natriuretic peptide and three cardiac biomarkers for discriminatory ability of clinical stage in dogs with myxomatous mitral valve disease. J. Vet. Med. Sci. 2021, 83, 705–715. [Google Scholar] [CrossRef]
- Saril, A.; Kocaturk, M.; Shimada, K.; Uemura, A.; Akgün, E.; Levent, P.; Baykal, A.T.; Prieto, A.M.; Agudelo, C.F.; Tanaka, R.; et al. Serum Proteomic Changes in Dogs with Different Stages of Chronic Heart Failure. Animals 2022, 12, 490. [Google Scholar] [CrossRef]
- DeProspero, D.J.; Hess, R.S.; Silverstein, D.C. Neutrophil-to-lymphocyte ratio is increased in dogs with acute congestive heart failure secondary to myxomatous mitral valve disease compared to both dogs with heart murmurs and healthy controls. J. Am. Vet. Med. Assoc. 2023, 261, 1–8. [Google Scholar] [CrossRef]
- Grosso, G.; Vezzosi, T.; Domenech, O.; Tognetti, R. Prognostic relevance of left cardiac enlargement in dogs with preclinical myxomatous mitral valve disease. J. Vet. Cardiol. 2023, 45, 50–58. [Google Scholar] [CrossRef]
- Ku, D.; Chae, Y.; Kim, C.; Koo, Y.; Lee, D.; Yun, T.; Chang, D.; Kang, B.T.; Yang, M.P.; Kim, H. Severity of myxomatous mitral valve disease in dogs may be predicted using neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratio. Am. J. Vet. Res. 2023, 84, ajvr.23.01.0012. [Google Scholar] [CrossRef]
- Beaumier, A.; Rush, J.E.; Yang, V.K.; Freeman, L.M. Clinical findings and survival time in dogs with advanced heart failure. J. Vet. Intern. Med. 2018, 32, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Güler, İ.; Ustaalioğlu, İ. Predictive power of HALP score in estimating short-term mortality in patients with acute pancreatitis. Ulus. Travma Ve Acil Cerrahi Derg. 2023, 29, 1098–1102. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, S.; Cao, W.; Geng, N.; Feng, C. HALP score based on hemoglobin, albumin, lymphocyte and platelet can predict the prognosis of tongue squamous cell carcinoma patients. Heliyon 2023, 9, e20126. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, L. Prognostic significance of HALP score and combination of peripheral blood multiple indicators in patients with early breast cancer. Front. Oncol. 2023, 13, 1253895. [Google Scholar] [CrossRef]
- Liu, L.; Gong, B.; Wang, W.; Xu, K.; Wang, K.; Song, G. Association between haemoglobin, albumin, lymphocytes, and platelets and mortality in patients with heart failure. ESC Heart Fail. 2024, 11, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, R.; Toprak, K.; Yilmaz, M.; Karagoz, A.; Öz, E. Investigation of the Usefulness of HALP Score in Predicting Short-Term Mortality in Patients with Acute Decompensated Heart Failure in a Coronary Care Unit. Medicina 2024, 60, 1385. [Google Scholar] [CrossRef] [PubMed]
- Duzkopru, Y.; Kocanoglu, A.; Dogan, O.; Sahinli, H.; Cilbir, E.; Altinbas, M. Hemoglobin, albumin, lymphocyte, and platelet score as a predictor of prognosis in metastatic gastric cancer. World. J. Gastrointest. Oncol. 2023, 15, 1626–1635. [Google Scholar] [CrossRef]
- Xu, S.S.; Li, S.; Xu, H.X.; Li, H.; Wu, C.T.; Wang, W.Q.; Gao, H.L.; Jiang, W.; Zhang, W.H.; Li, T.J.; et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World. J. Gastroenterol. 2020, 26, 828–838. [Google Scholar] [CrossRef]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B. Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N. Pathophysiology of acute heart failure syndrome: A knowledge gap. Heart Fail. Rev. 2017, 22, 621–639. [Google Scholar] [CrossRef]
- Piantedosi, D.; Musco, N.; Palatucci, A.T.; Carriero, F.; Rubino, V.; Pizzo, F.; Nasir, S.; Molinaro, G.; Ruggiero, G.; Terrazzano, G.; et al. Pro-Inflammatory and Immunological Profile of Dogs with Myxomatous Mitral Valve Disease. Vet. Sci. 2022, 9, 326. [Google Scholar] [CrossRef]
- Kocaoglu, S.; Alatli, T. The Efficiency of the HALP Score and the Modified HALP Score in Predicting Mortality in Patients with Acute Heart Failure Presenting to the Emergency Department. J. Coll. Physicians Surg. Pak. 2022, 32, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Yaman, M.; Orak, M.; Durgun, H.M.; Tekin, V.; Ülgüt, Ş.G.; Belek, S.; Günel, B.T.; Üstündağ, M.; Güloğlu, C.; Gündüz, E. The prognostic value of HALP score and sPESI in predicting in-hospital mortality in patients with pulmonary thromboembolism. Postgrad. Med. J. 2024, 101, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Agarwal, A.K. Anemia associated with chronic heart failure: Current concepts. Clin. Interv. Aging 2013, 8, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.W.; Ashok, T.; Patni, N.; Fatima, M.; Lamis, A.; Anne, K.K. Anemia and Heart Failure: A Narrative Review. Cureus 2022, 14, e27167. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Zhang, J.; Pellicori, P.; Dicken, B.; Dierckx, R.; Shoaib, A.; Wong, K.; Rigby, A.; Goode, K.; Clark, A.L. Prevalence and Outcomes of Anemia and Hematinic Deficiencies in Patients with Chronic Heart Failure. JAMA Cardiol. 2016, 1, 539–547. [Google Scholar] [CrossRef]
- Ballmer, P.E. Causes and mechanisms of hypoalbuminaemia. Clin. Nutr. 2001, 20, 271–273. [Google Scholar] [CrossRef]
- Chao, P.; Cui, X.; Wang, S.; Zhang, L.; Ma, Q.; Zhang, X. Serum albumin and the short-term mortality in individuals with congestive heart failure in intensive care unit: An analysis of MIMIC. Sci. Rep. 2022, 12, 16251. [Google Scholar] [CrossRef]
- Yücel, H.; Refiker Ege, M.; Zorlu, A.; Kaya, H.; Beton, O.; Güngör, H.; Acar, G.; Temizhan, A.; Çavuşoğlu, Y.; Zoghi, M.; et al. Lymphocytopenia is associated with poor NYHA functional class in chronic heart failure patients with reduced ejection fraction. Turk Kardiyol. Dern. Ars. 2015, 43, 427–433. [Google Scholar] [CrossRef]
- Peschel, T.; Schönauer, M.; Thiele, H.; Anker, S.D.; Schuler, D.; Niebauer, J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur. J. Heart Fail. 2003, 5, 609–614. [Google Scholar] [CrossRef]
- Krack, A.; Sharma, R.; Figulla, H.R.; Anker, S.D. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur. Heart J. 2005, 26, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Ye, G.L.; Chen, Q.; Chen, X.; Liu, Y.Y.; Yin, T.T.; Meng, Q.H.; Liu, Y.C.; Wei, H.Q.; Zhou, Q.H. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: A cohort study. Sci. Rep. 2019, 9, 10639. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget 2023, 14, 153–172. [Google Scholar] [CrossRef]
- Sica, D.A. Angiotensin-converting enzyme inhibitors side effects--physiologic and non-physiologic considerations. J. Clin. Hypertens. 2004, 6, 410–416. [Google Scholar] [CrossRef]


| Dogs with CHF (n = 54) | |
|---|---|
| Age (years) | 13.79 ± 3.43 |
| Sex | |
| Male | 26 (48.1%) |
| Female | 28 (51.9%) |
| Breeds | |
| Maltese | 23 (39.7%) |
| Shih Tzu | 11 (20.3%) |
| Miniature or Toy Poodle | 7 (12.9%) |
| Pomeranian | 5 (7.4%) |
| Chihuahua | 3 (5.5%) |
| Yorkshire Terrier | 3 (5.5%) |
| Mixed breeds | 3 (5.5%) |
| Spitz | 2 (3.7%) |
| Miniature Schnauzer | 1 (1.8%) |
| Non-Survival (n = 18) | Survival (n = 36) | p-Value | ||
|---|---|---|---|---|
| Age (years) | 12.5 (9.9–14.0) | 12.5 (10.6–14.7) | 0.6725 | |
| Body weight (kg) | 3.08 (2.61–4.52) | 3.76 (2.95–5.07) | 0.2442 | |
| Sex (number) | Male | 8 | 18 | 0.7773 |
| Female | 10 | 18 | ||
| Hemoglobin (g/L) (RI, 131–205) | 151.7 ± 23.41 | 157.9 ± 26.46 | 0.3953 | |
| Albumin (g/L) (RI, 26–33) | 25.50 (22.75–36.50) | 28.00 (25.00–32.00) | 0.9239 | |
| Lymphocyte (109/L) (RI, 1.05–5.10) | 1.485 (1.265–1.788) | 1.745 (1.206–2.470) | 0.1206 | |
| Platelet (109/L) (RI, 148–484) | 467.0 ± 145.7 | 378.8 ± 106.8 | 0.0133 * | |
| Neutrophil (109/L) (RI, 2.95–11.64) | 10.31 (8.048–12.41) | 8.280 (6.536–10.70) | 0.1618 | |
| HALP score | 14.20 (9.883–19.41) | 18.99 (14.78–29.50) | 0.0067 ** | |
| Non-Survival (n = 23) | Survival (n = 23) | p-Value | ||
|---|---|---|---|---|
| Age (years) | 13.00 (9.723–14.44) | 12.79 (10.76–14.11) | 0.7892 | |
| Body weight (kg) | 4.06 (2.98–5.72) | 3.34(2.93–5.07) | 0.3417 | |
| Sex (number) | Male | 10 | 11 | 0.7672 |
| Female | 13 | 12 | ||
| Hemoglobin (g/L) (RI, 131–205) | 151.8 ± 23.48 | 158.0 ± 28.57 | 0.4273 | |
| Albumin (g/L) (RI, 26–33) | 27.00 (23.00–36.00) | 28.00 (25.00–31.20) | 0.9176 | |
| Lymphocyte (109/L) (RI, 1.05–5.10) | 1.370 (0.9890–1.700) | 1.870 (1.560–2.410) | 0.0048 ** | |
| Platelet (109/L) (RI, 148–484) | 406.0 (348.0–492.0) | 373.0 (270.0–486.0) | 0.2358 | |
| Neutrophil (109/L) (RI, 2.95–11.64) | 9.950 (5.580–12.39) | 8.953 (7.170–10.79) | 0.9826 | |
| HALP score | 14.98 (10.72–19.40) | 17.73 (14.55–34.67) | 0.0080 ** | |
| Non-Survival (n = 27) | Survival (n = 19) | p-Value | ||
|---|---|---|---|---|
| Age (years) | 12.80 (11.00–14.93) | 11.52 (9.96–13.4) | 0.0829 | |
| Body weight (kg) | 3.06 (2.50–4.80) | 3.80(2.96–7.00) | 0.1148 | |
| Sex (number) | Male | 12 | 8 | 0.9035 |
| Female | 15 | 11 | ||
| Hemoglobin (g/L) (RI, 131–205) | 151.8 ± 23.01 | 161.6 ± 30.01 | 0.2156 | |
| Albumin (g/L) (RI, 26–33) | 29.00 (23.00–36.00) | 38.50 (25.00–31.00) | 0.3546 | |
| Lymphocyte (109/L) (RI, 1.05–5.10) | 1.410 (1.100–1.980) | 1.760 (1.281–1.950) | 0.1404 | |
| Platelet (109/L) (RI, 148–484) | 447.4 ± 136 | 374.4 ± 115.4 | 0.0633 | |
| Neutrophil (109/L) (RI, 2.95–11.64) | 9.160 (6.290–12.1100) | 8.440 (7.010–10.06) | 0.7572 | |
| HALP score | 16.08 (11.06–22.78) | 17.01 (13.86–35.9) | 0.0741 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Chae, Y.; Lee, S.; Koo, Y.; Kim, H.; Kang, B.-T.; Yun, T. The Hemoglobin, Albumin, Lymphocyte, and Platelet Score as a Prognostic Indicator for Dogs with Congestive Heart Failure Secondary to Myxomatous Mitral Valve Disease. Vet. Sci. 2025, 12, 908. https://doi.org/10.3390/vetsci12090908
Park J, Chae Y, Lee S, Koo Y, Kim H, Kang B-T, Yun T. The Hemoglobin, Albumin, Lymphocyte, and Platelet Score as a Prognostic Indicator for Dogs with Congestive Heart Failure Secondary to Myxomatous Mitral Valve Disease. Veterinary Sciences. 2025; 12(9):908. https://doi.org/10.3390/vetsci12090908
Chicago/Turabian StylePark, Jayeon, Yeon Chae, Sungjae Lee, Yoonhoi Koo, Hakhyun Kim, Byeong-Teck Kang, and Taesik Yun. 2025. "The Hemoglobin, Albumin, Lymphocyte, and Platelet Score as a Prognostic Indicator for Dogs with Congestive Heart Failure Secondary to Myxomatous Mitral Valve Disease" Veterinary Sciences 12, no. 9: 908. https://doi.org/10.3390/vetsci12090908
APA StylePark, J., Chae, Y., Lee, S., Koo, Y., Kim, H., Kang, B.-T., & Yun, T. (2025). The Hemoglobin, Albumin, Lymphocyte, and Platelet Score as a Prognostic Indicator for Dogs with Congestive Heart Failure Secondary to Myxomatous Mitral Valve Disease. Veterinary Sciences, 12(9), 908. https://doi.org/10.3390/vetsci12090908







