Statistical Triage Model for Feline Infectious Diseases in a Veterinary Isolation Unit: The Case of Feline Immunodeficiency and Leukemia Viruses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Participants and Data Collection
2.3. Sample Testing
2.4. Variables
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Basic Characteristics of the FIV and FeLV-Infected Cats and Their Controls
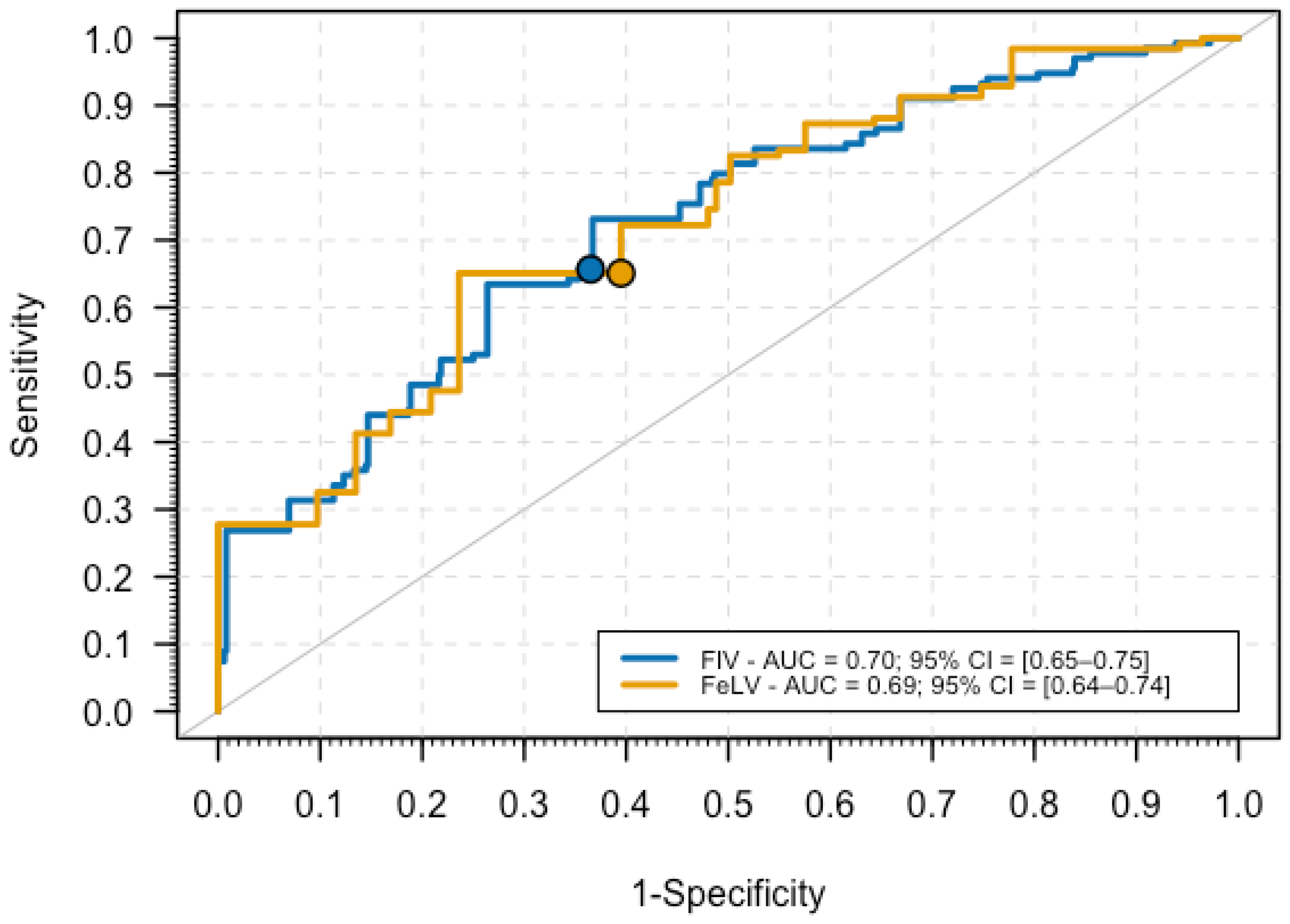
3.2. Prediction of FIV Status Infections Using Logistic Regression
3.3. Prediction of FeLV Infections Using Logistic Regression
4. Discussion
4.1. FIV Infections
4.2. FeLV Infections
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BICU | Biological Isolation and Containment Unit |
| CI | Confidence Interval |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FeLV | Feline Leukemia Virus |
| FIV | Feline Immunodeficiency Virus |
| FMV-ULisboa | Faculty of Veterinary Medicine, University of Lisbon |
| HEPA | High-Efficiency Particulate Air |
| VTH-FMV | Veterinary Teaching Hospital of the Faculty of Veterinary Medicine |
| IFA | Indirect Fluorescent Antibody |
| LOOCV | Leave One Out Cross-Validation |
| ML | Machine Learning |
| NPV | Negative Predictive Value |
| PCR | Polymerase Chain Reaction |
| PPV | Positive Predictive Value |
| ROC | Receiver Operating Characteristic |
| SVM | Support Vector Machines |
| TH | Teaching Hospital |
| VGG | Vaccination Guidelines Group |
| VIL | Virology and Immunology Laboratory |
References
- Stull, J.W.; Bjorvik, E.; Bub, J.; Dvorak, G.; Petersen, C.; Troyer, H.L. 2018 AAHA infection control, prevention, and biosecurity guidelines. J. Am. Anim. Hosp. Assoc. 2018, 54, 297–326. [Google Scholar] [CrossRef]
- Möstl, K.; Egberink, H.; Addie, D.; Frymus, T.; Boucraut-Baralon, C.; Truyen, U.; Hartmann, K.; Lutz, H.; Gruffydd-Jones, T.; Radford, A.D.; et al. Prevention of infectious diseases in cat shelters: ABCD guidelines. J. Feline Med. Surg. 2013, 15, 546–554. [Google Scholar] [CrossRef]
- Machado, I.C.; Nunes, T.; Maximino, M.; Malato, J.; Tavares, L.; Almeida, V.; Sepúlveda, N.; Gil, S. Epidemiologic factors supporting triage of infected dog patients admitted to a veterinary hospital biological isolation and containment unit. Vet. Sci. 2023, 10, 186. [Google Scholar] [CrossRef]
- Awaysheh, A.; Wilcke, J.; Elvinger, F.; Rees, L.; Fan, W.; Zimmerman, K.L. Review of medical decision support and machine-learning methods. Vet. Pathol. 2019, 56, 512–525. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Pantelis, G.; Lescure, F.X.; Birgand, G.; Holmes, A.H. Machine learning for clinical decision support in infectious diseases: A narrative review of current applications. Clin. Microbiol. Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Marati, L.; Pugliese, A.; Levante, M.; Ferrara, G.; de Carlo, E.; Amoroso, M.G.; Montagnaro, S. Prevalence of feline leukemia virus and feline immunodeficiency virus in cats from southern Italy: A 10-year cross-sectional study. Front. Vet. Sci. 2023, 10, 1260081. [Google Scholar] [CrossRef] [PubMed]
- Paulo, C.; Machado, I.; Carvalho, H.; Gomes, J.; Mota, A.D.; Tavares, L.; Almeida, V.; Gil, S. A 5-year retrospective study of canine and feline patients referred to an isolation unit for infectious diseases. Vet. Rec. Open 2021, 8, e5. [Google Scholar] [CrossRef]
- Sykes, J.E. Feline immunodeficiency virus infection. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 209–223. [Google Scholar]
- Bęczkowski, P.M.; Beatty, J.A. Feline immunodeficiency virus. Adv. Small Anim. Care 2022, 3, 145–159. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Hartmann, K. Feline leukaemia virus infection: A practical approach to diagnosis. J. Feline Med. Surg. 2020, 22, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Bande, F.; Arshad, S.S.; Hassan, L.; Zakaria, Z.; Sapian, N.A.; Rahman, N.A.; Alazawy, A. Prevalence and risk factors of feline leukaemia virus and feline immunodeficiency virus in Peninsular Malaysia. BMC Vet. Res. 2012, 8, 33. [Google Scholar] [CrossRef]
- Hartmann, K.; Hofmann-Lehmann, R. What’s new in feline leukemia virus infection. Vet. Clin. North Am. Small Anim. Pract. 2020, 50, 1013–1036. [Google Scholar] [CrossRef]
- Studer, N.; Lutz, H.; Saegerman, C.; Gönczi, E.; Meli, M.L.; Boo, G.; Hartmann, K.; Hosie, M.J.; Moestl, K.; Tasker, S.; et al. Pan-European study on the prevalence of the feline leukaemia virus infection—Reported by the European Advisory Board on Cat Diseases (ABCD Europe). Viruses 2019, 11, 993. [Google Scholar] [CrossRef]
- Burling, A.N.; Levy, J.K.; Scott, H.M.; Crandall, M.M.; Tucker, S.J.; Wood, E.G.; Foster, J.D. Seroprevalences of feline leukemia virus and feline immunodeficiency virus infection in cats in the United States and Canada and risk factors for seropositivity. J. Am. Vet. Med. Assoc. 2017, 251, 187–194. [Google Scholar] [CrossRef]
- Little, S.; Levy, J.; Hartmann, K.; Hofmann-Lehmann, R.; Hosie, M.; Olah, G.; Denis, K.S. 2020 AAFP feline retrovirus testing and management guidelines. J. Feline Med. Surg. 2020, 22, 5–30. [Google Scholar] [CrossRef]
- Dunbar, D.; Babayan, S.A.; Krumrie, S.; Haining, H.; Hosie, M.J.; Weir, W. Assessing the feasibility of applying machine learning to diagnosing non-effusive feline infectious peritonitis. Sci. Rep. 2024, 14, 2517. [Google Scholar] [CrossRef] [PubMed]
- Macieira, D.B.; de Menezes, R.D.C.A.; Damico, C.B.; Almosny, N.R.P.; McLane, H.L.; Daggy, J.K.; Messick, J.B. Prevalence and risk factors for hemoplasmas in domestic cats naturally infected with feline immunodeficiency virus and/or feline leukemia virus in Rio de Janeiro—Brazil. J. Feline Med. Surg. 2008, 10, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.K.; Roberts, M.A.; Skillings, E.; Morrow, L.D.; Gruffydd-Jones, T.J. Risk factors for feline immunodeficiency virus antibody test status in Cats Protection adoption centres (2004). J. Feline Med. Surg. 2009, 11, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Griessmayr, P.; Schulz, B.; Greene, C.E.; Vidyashankar, A.N.; Jarrett, O.; Egberink, H.F. Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. J. Feline Med. Surg. 2007, 9, 439–445. [Google Scholar] [CrossRef]
- NovaTec Immundiagnostica GmbH. VetLine Feline Immunodeficiency Virus (FIV) ELISA—Performance Characteristics; NovaTec Immundiagnostica GmbH: Dietzenbach, Germany, 2020. [Google Scholar]
- Zoetis Inc. Diagnostic-Rapid-Test-Accuracy-(Sensitivity-Specificity); Zoetis Inc.: Parsippany, NJ, USA, 2020. [Google Scholar]
- Finka, L.R.; Foreman-Worsley, R. Are multi-cat homes more stressful? A critical review of the evidence associated with cat group size and wellbeing. J. Feline Med. Surg. 2022, 24, 65–76. [Google Scholar] [CrossRef]
- Quimby, J.; Gowland, S.; Carney, H.C.; DePorter, T.; Plummer, P.; Westropp, J.L. 2021 AAHA/AAFP feline life stage guidelines. J. Feline Med. Surg. 2021, 23, 211–233. [Google Scholar] [CrossRef]
- Stone, A.E.S.; Brummet, G.O.; Carozza, E.M.; Kass, P.H.; Petersen, E.P.; Sykes, J.; Westman, M.E. 2020 AAHA/AAFP feline vaccination guidelines. J. Feline Med. Surg. 2020, 22, 813–830. [Google Scholar] [CrossRef]
- MSD. Hematology (Complete Blood Count) Reference Ranges. Available online: https://www.msdvetmanual.com/multimedia/table/hematology-complete-blood-count-reference-ranges (accessed on 10 September 2023).
- Dohoo, I.R.; Nielsen, C.R.; Emanuelson, U. Multiple imputation in veterinary epidemiological studies: A case study and simulation. Prev. Vet. Med. 2016, 129, 35–47. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Model-building strategies and methods for logistic regression. In Applied Logistic Regression; Balding, D.J., Cressie, N.A.C., Fitzmaurice, G.M., Goldstein, H., Johnstone, I.M., Molenberghs, G., Scott, D.W., Smith, A.F.M., Tsay, R.S., Weisberg, S., et al., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000; pp. 89–151. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. PROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- López-Ratón, M.; Rodríguez-Álvarez, M.X.; Cadarso-Suárez, C.; Gude-Sampedro, F. Optimalcutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests; American Statistical Association: Alexandria, VA, USA, 2021. [Google Scholar]
- Biezus, G.; Machado, G.; Ferian, P.E.; da Costa, U.M.; da Silva Pereira, L.H.H.; Withoeft, J.A.; Nunes, I.A.C.; Muller, T.R.; de Cristo, T.G.; Casagrande, R.A. Prevalence of and factors associated with feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) in cats of the state of Santa Catarina, Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2019, 63, 17–21. [Google Scholar] [CrossRef]
- Gleich, S.E.; Krieger, S.; Hartmann, K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. J. Feline Med. Surg. 2009, 11, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Goldkamp, C.E.; Levy, J.K.; Edinboro, C.H.; Lachtara, J.L. Seroprevalences of feline leukemia virus and feline immunodeficiency virus in cats: Guidelines for retrovirus testing. J. Am. Vet. Med. Assoc. 2008, 232, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Sears, W.; Lachtara, J.; Bienzle, D. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in Canada. Can. Vet. J. 2009, 50, 644–648. [Google Scholar] [PubMed] [PubMed Central]
- Litster, A.L. Transmission of feline immunodeficiency virus (FIV) among cohabiting cats in two cat rescue shelters. Vet. J. 2014, 201, 184–188. [Google Scholar] [CrossRef]
- Sykes, J.E.; Hartmann, K. Feline leukemia virus infection. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 224–238. ISBN 9781437707953. [Google Scholar]
- Hosie, M.J.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Lloret, A.; Lutz, H.; et al. Feline immunodeficiency ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 575–584. [Google Scholar] [CrossRef]
- Westman, M.E.; Coggins, S.J.; van Dorsselaer, M.; Norris, J.M.; Squires, R.A.; Thompson, M.; Malik, R. Feline immunodeficiency virus (FIV) infection in domestic pet cats in Australia and New Zealand: Guidelines for diagnosis, prevention and management. Aust. Vet. J. 2022, 100, 345–359. [Google Scholar] [CrossRef]
- Sellon, R.K.; Hartmann, K. Feline immunodeficiency virus infection. In Infectious Diseases of the Dog and Cat; Greene, C.E., Stringer, S., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 136–149. [Google Scholar]
- Levy, J.K.; Scott, H.M.; Lachtara, J.L.; Crawford, C.P. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J. Am. Vet. Med. Assoc. 2006, 228, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Muchaamba, F.; Mutiringindi, T.H.; Tivapasi, M.T.; Dhliwayo, S.; Matope, G. A survey of feline leukaemia virus infection of domestic cats from selected areas in Harare, Zimbabwe. J. S. Afr. Vet. Assoc. 2014, 85, 1126. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline leukaemia ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Thrall, M.A.; Weiser, G.; Allison, R.W.; Campbell, T.W. Hematologia e Bioquímica Clínica Veterinária, 2nd ed.; Editora Roca: São Paulo, Brazil, 2015. [Google Scholar]
- Westman, M.E.; Giselbrecht, J.; Norris, J.M.; Malik, R.; Green, J.; Burton-Bradley, E.; Cheang, A.; Meili, T.; Meli, M.L.; Hartmann, K.; et al. Field performance of a rapid test to detect progressive, regressive, and abortive feline leukemia virus infections in domestic cats in Australia and Germany. Viruses 2023, 15, 491. [Google Scholar] [CrossRef]
| Characteristics | Categories | FIV | Controls | FeLV |
|---|---|---|---|---|
| Case, n = 134 Cats (%) | n = 504 Cats (%) | Case, n = 126 Cats (%) | ||
| Breed | Purebred | 5 (3.7%) | 47 (9.3%) | 4 (3.2%) |
| Mixed-breed | 129 (96.3%) | 457 (90.7%) | 122 (96.8%) | |
| Sex and Neuter Status | Neutered Male | 71 (53.0%) | 199 (39.5%) | 39 (31.0%) |
| Intact Male | 20 (14.9%) | 72 (14.3%) | 23 (18.3%) | |
| Neutered Female | 29 (21.6%) | 152 (30.2%) | 39 (31.0%) | |
| Intact Female | 14 (10.4%) | 81 (16.1%) | 25 (19.8%) | |
| Number of cats in the household | Single Cat | 58 (43.3%) | 210 (41.7%) | 57 (45.2%) |
| ≥2 Cats | 76 (56.7%) | 294 (58.3%) | 69 (54.8%) | |
| Lifestyle | Indoor | 52 (38.8%) | 314 (62.3%) | 72 (57.1%) |
| Outdoor | 82 (61.2%) | 190 (37.7%) | 54 (42.9%) | |
| Concomitant Disorders/Diseases | No | 23 (17.2%) | 156 (31.0%) | 27 (21.4%) |
| Yes | 111 (82.8%) | 348 (69.0%) | 99 (78.6%) | |
| Age Groups | <2 | 13 (9.7%) | 132 (26.2%) | 25 (19.8%) |
| (Years) | ≥2 and <10 | 82 (61.2%) | 231 (45.8%) | 81 (64.3%) |
| ≥10 | 39 (29.1%) | 141 (28.0%) | 20 (15.9%) | |
| Leukocytes Classification | High 1 | 28 (20.9%) | 120 (23.8%) | 25 (19.8%) |
| Normal 2 | 79 (59.0%) | 285 (56.5%) | 69 (54.8%) | |
| Low 3 | 27 (20.1%) | 99 (19.6%) | 32 (25.4%) | |
| Hematocrit Classification | High 4 | 16 (11.9%) | 65 (12.9%) | 12 (9.5%) |
| Normal 5 | 77 (57.5%) | 276 (54.8%) | 45 (35.7%) | |
| Low 6 | 41 (30.6%) | 163 (32.3%) | 69 (54.8%) |
| Characteristics | Categories | Simple Logistic Regression | Multiple Logistic Regression | ||||
|---|---|---|---|---|---|---|---|
| Estimate | Std Error | p-Value | Estimate | Std Error | p-Value | ||
| Breed | Purebred (reference) | — | — | NA | — | — | NA |
| Mixed-breed | 0.976 | 0.481 | 0.042 | 0.627 | 0.497 | 0.207 | |
| Sex and Neuter Status | Intact Female (reference) | — | — | NA | — | — | NA |
| Intact Male | 0.474 | 0.384 | 0.217 | 0.606 | 0.413 | 0.142 | |
| Neutered Female | 0.098 | 0.353 | 0.779 | −0.306 | 0.372 | 0.411 | |
| Neutered Male | 0.724 | 0.321 | 0.024 | 0.272 | 0.341 | 0.425 | |
| Number of cats in the household | 1 (reference) | — | — | NA | — | — | NA |
| >1 | −0.066 | 0.196 | 0.736 | — | — | NA | |
| Lifestyle | Indoor (reference) | — | — | NA | — | — | NA |
| Outdoor | 0.958 | 0.2 | <0.001 | 0.864 | 0.208 | <0.001 | |
| Concomitant Disorders/Diseases | No (reference) | — | — | NA | — | — | NA |
| Yes | 0.772 | 0.246 | 0.002 | 0.674 | 0.269 | 0.012 | |
| Age Groups (Years) | <2 (reference) | — | — | NA | — | — | NA |
| ≥2 and <10 | 1.282 | 0.318 | <0.001 | 1.185 | 0.351 | <0.001 | |
| ≥10 | 1.033 | 0.342 | 0.003 | 0.974 | 0.386 | 0.011 | |
| Classification of Leukocyte | High 1 (reference) | — | — | NA | — | — | NA |
| Normal 2 | 0.172 | 0.245 | 0.483 | — | — | NA | |
| Low 3 | 0.156 | 0.302 | 0.605 | — | — | NA | |
| Classification of Hematocrit | High 4 (reference) | — | — | NA | — | — | NA |
| Normal 5 | 0.125 | 0.307 | 0.684 | — | — | NA | |
| Low 6 | 0.022 | 0.329 | 0.948 | — | — | NA | |
| Characteristic | Categories | Simple Logistic Regression | Multiple Logistic Regression | ||||
|---|---|---|---|---|---|---|---|
| Estimate | Std Error | p-Value | Estimate | Std Error | p-Value | ||
| Breed | Purebred (reference) | — | — | NA | — | — | NA |
| Mixed-breed | 1.143 | 0.531 | 0.031 | 1.221 | 0.541 | 0.024 | |
| Sex and Neuter Status | Intact Female (reference) | — | — | NA | — | — | NA |
| Intact Male | 0.034 | 0.331 | 0.917 | — | — | NA | |
| Neutered Female | −0.185 | 0.291 | 0.525 | — | — | NA | |
| Neutered Male | −0.454 | 0.288 | 0.115 | — | — | NA | |
| Number of cats in the household | 1 (reference) | — | — | NA | — | — | NA |
| >1 | −0.145 | 0.201 | 0.468 | — | — | NA | |
| Lifestyle | Indoor (reference) | — | — | — | — | — | NA |
| Outdoor | 0.215 | 0.202 | 0.288 | — | — | NA | |
| Concomitant Disorders/Diseases | No (reference) | — | — | NA | — | — | NA |
| Yes | 0.497 | 0.238 | 0.036 | 0.522 | 0.254 | 0.04 | |
| Age Groups (Years) | <2 (reference) | — | — | NA | — | — | NA |
| ≥2 and <10 | 0.616 | 0.254 | 0.016 | 0.408 | 0.266 | 0.125 | |
| ≥10 | −0.289 | 0.324 | 0.372 | −0.617 | 0.344 | 0.073 | |
| Classification of Leukocyte | High 1 (reference) | — | — | NA | — | — | NA |
| Normal 2 | 0.15 | 0.258 | 0.56 | — | — | NA | |
| Low 3 | 0.439 | 0.299 | 0.142 | — | — | NA | |
| Classification of Hematocrit | High 4 (reference) | — | — | NA | — | — | NA |
| Normal 5 | −0.124 | 0.353 | 0.725 | −0.050 | 0.358 | 0.888 | |
| Low 6 | 0.886 | 0.372 | 0.017 | 0.897 | 0.352 | 0.011 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maximino, M.M.; Machado, I.C.; Nunes, T.P.; Tavares, L.M.; Almeida, V.S.; Gil, S.A.; Sepúlveda, N. Statistical Triage Model for Feline Infectious Diseases in a Veterinary Isolation Unit: The Case of Feline Immunodeficiency and Leukemia Viruses. Vet. Sci. 2025, 12, 902. https://doi.org/10.3390/vetsci12090902
Maximino MM, Machado IC, Nunes TP, Tavares LM, Almeida VS, Gil SA, Sepúlveda N. Statistical Triage Model for Feline Infectious Diseases in a Veterinary Isolation Unit: The Case of Feline Immunodeficiency and Leukemia Viruses. Veterinary Sciences. 2025; 12(9):902. https://doi.org/10.3390/vetsci12090902
Chicago/Turabian StyleMaximino, Miguel M., Inês C. Machado, Telmo P. Nunes, Luís M. Tavares, Virgílio S. Almeida, Solange A. Gil, and Nuno Sepúlveda. 2025. "Statistical Triage Model for Feline Infectious Diseases in a Veterinary Isolation Unit: The Case of Feline Immunodeficiency and Leukemia Viruses" Veterinary Sciences 12, no. 9: 902. https://doi.org/10.3390/vetsci12090902
APA StyleMaximino, M. M., Machado, I. C., Nunes, T. P., Tavares, L. M., Almeida, V. S., Gil, S. A., & Sepúlveda, N. (2025). Statistical Triage Model for Feline Infectious Diseases in a Veterinary Isolation Unit: The Case of Feline Immunodeficiency and Leukemia Viruses. Veterinary Sciences, 12(9), 902. https://doi.org/10.3390/vetsci12090902







