Comparative Study of DTMUV and LPS on Duck Liver Disease
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Experimental Animals
2.2. Preparation of Injection
2.3. Sample Collection
2.4. HE Staining
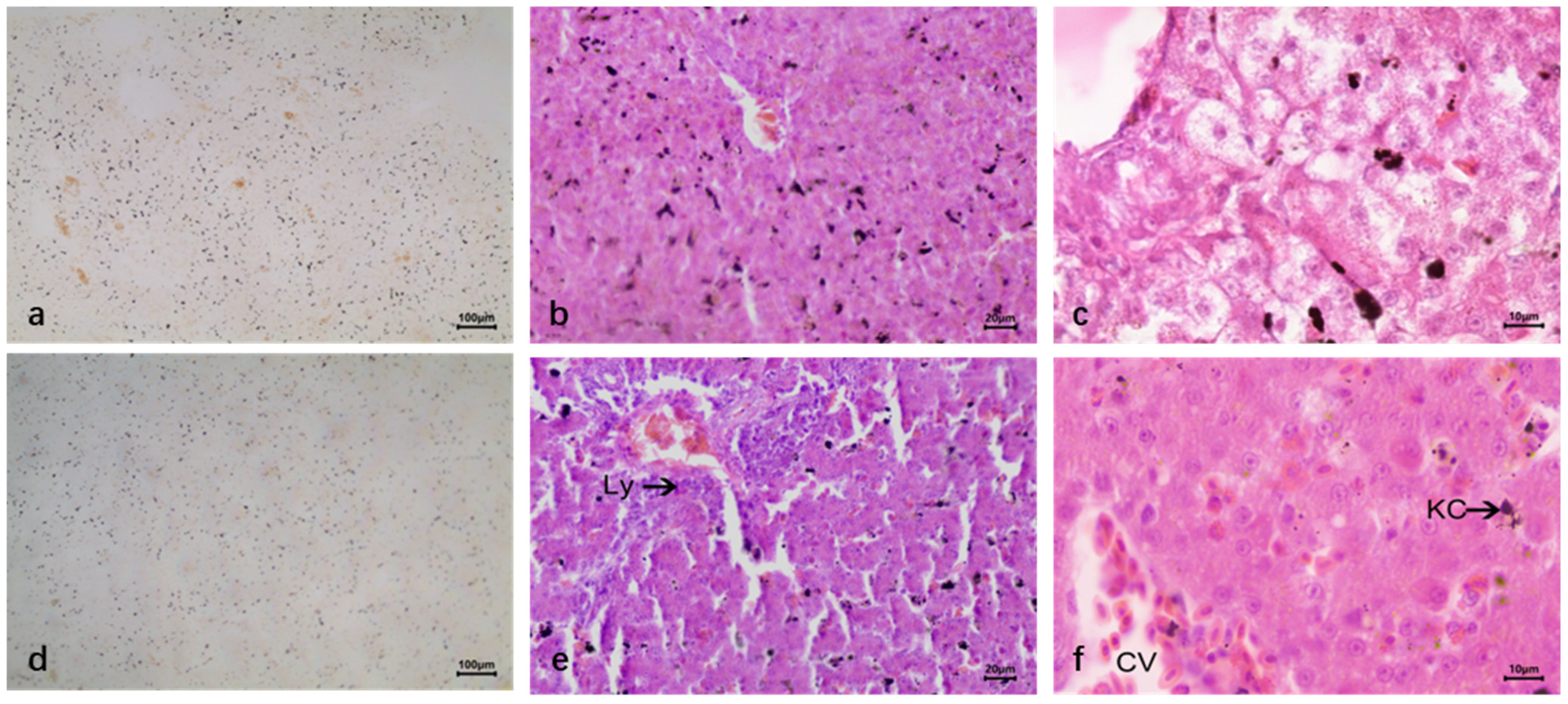
2.5. Reticulate Fiber Staining
2.6. Trichrome Staining with Masson Trichrome Staining Kit
3. Results
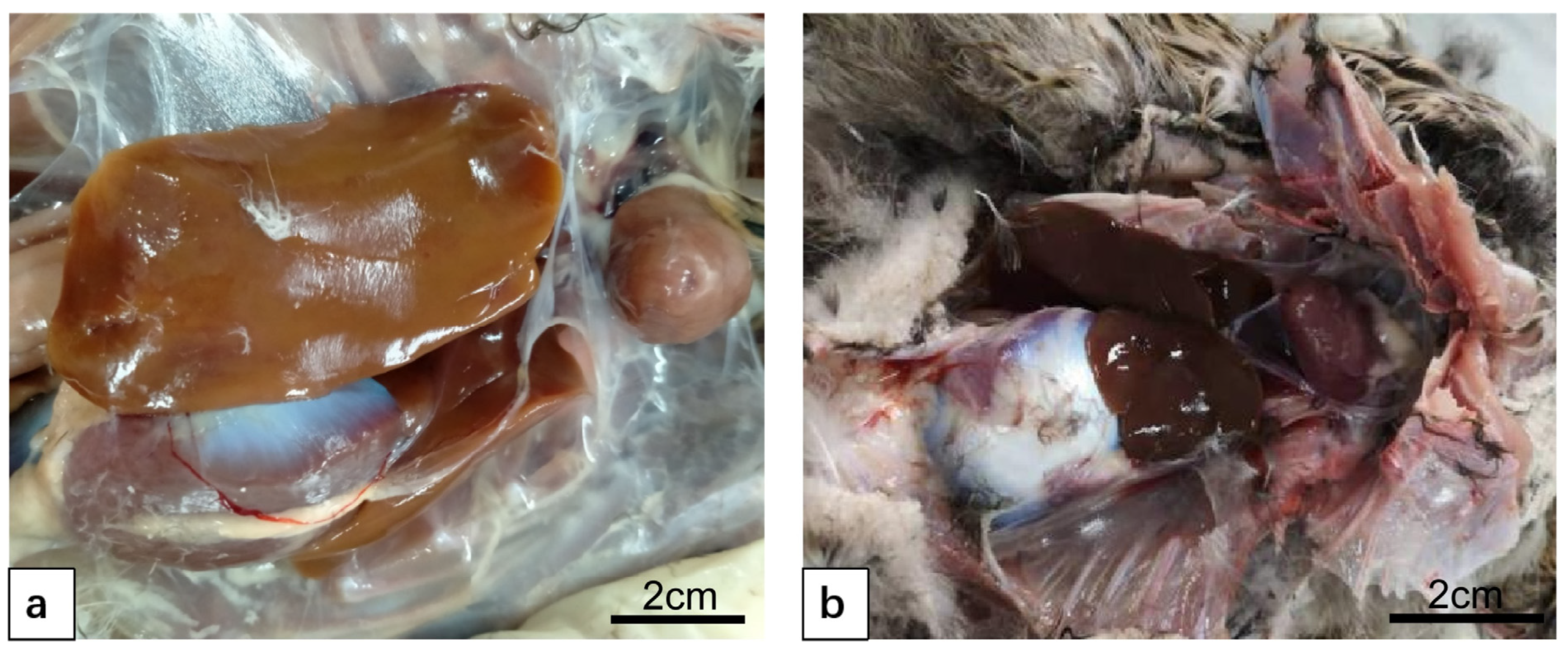
3.1. Anatomy and Structure of Duck Liver
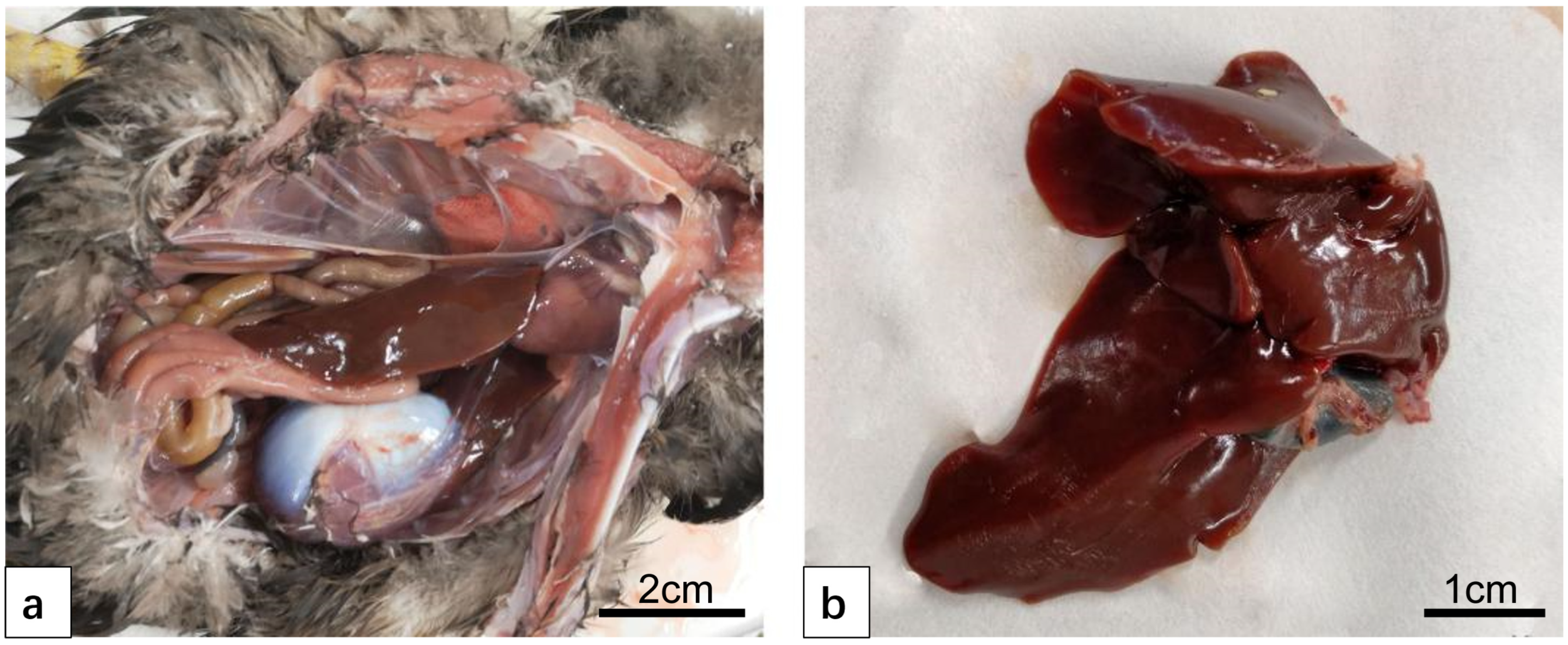
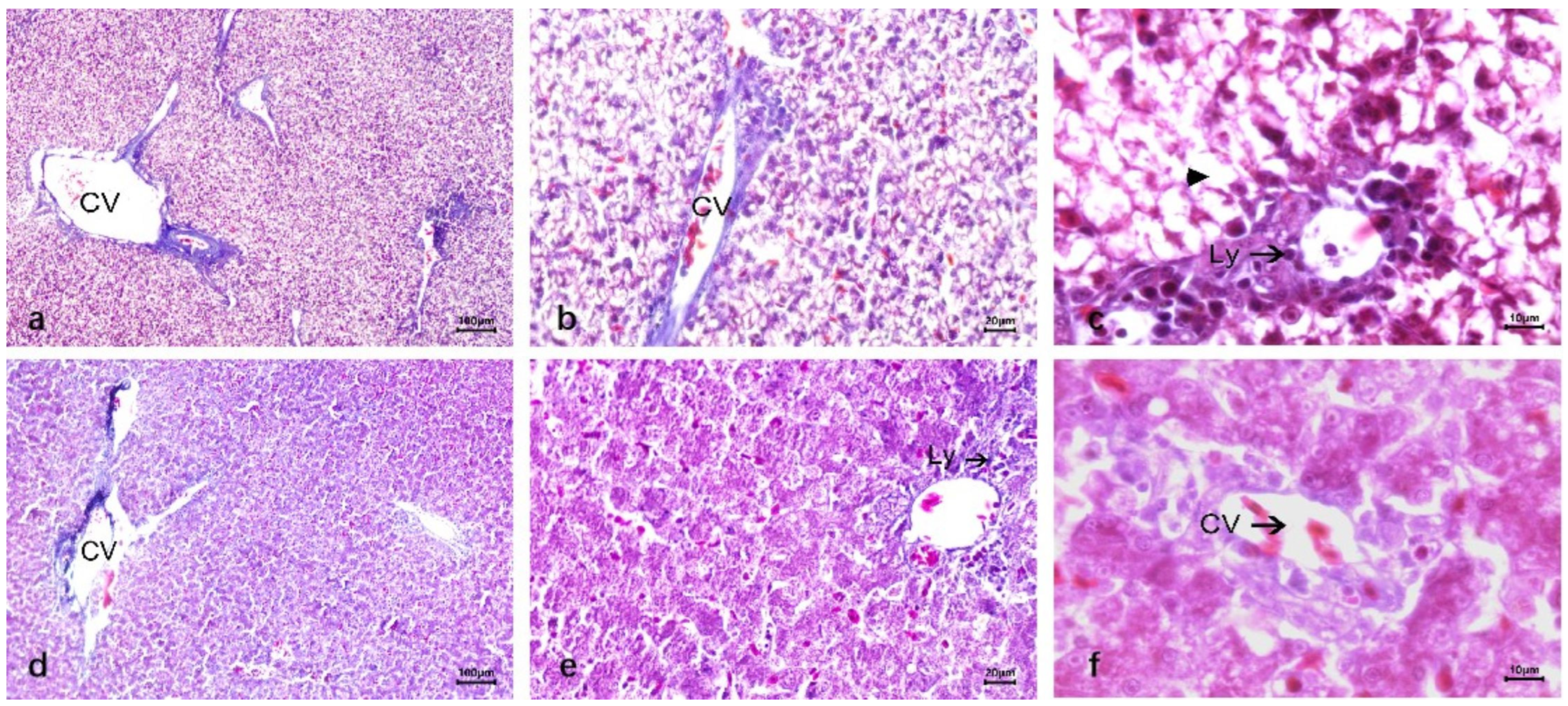
3.2. Liver Lesions After Infection DTMUV and Inoculation LPS
3.2.1. Anatomical Structure of the Liver After Infection DTMUV and Inoculation LPS
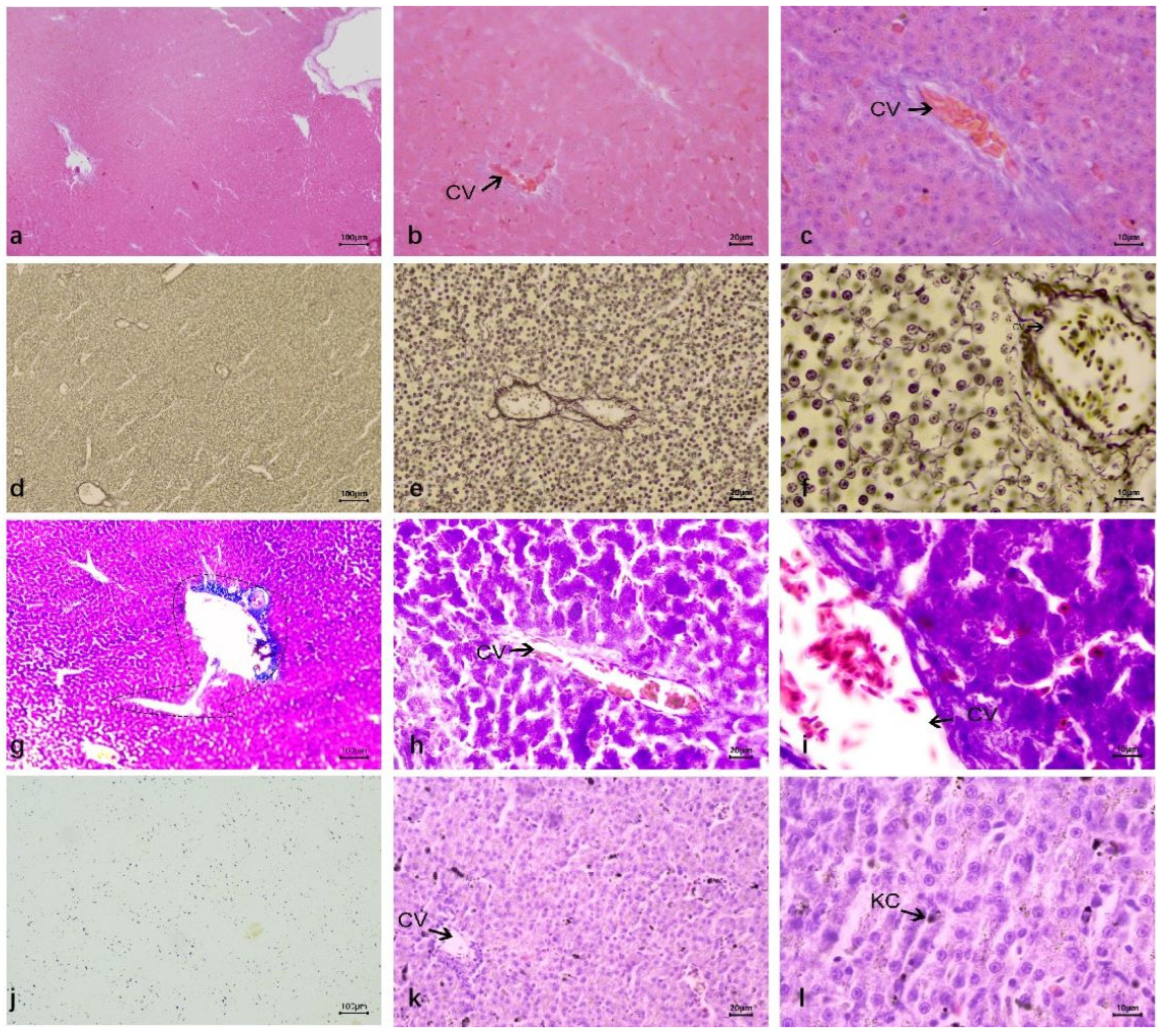
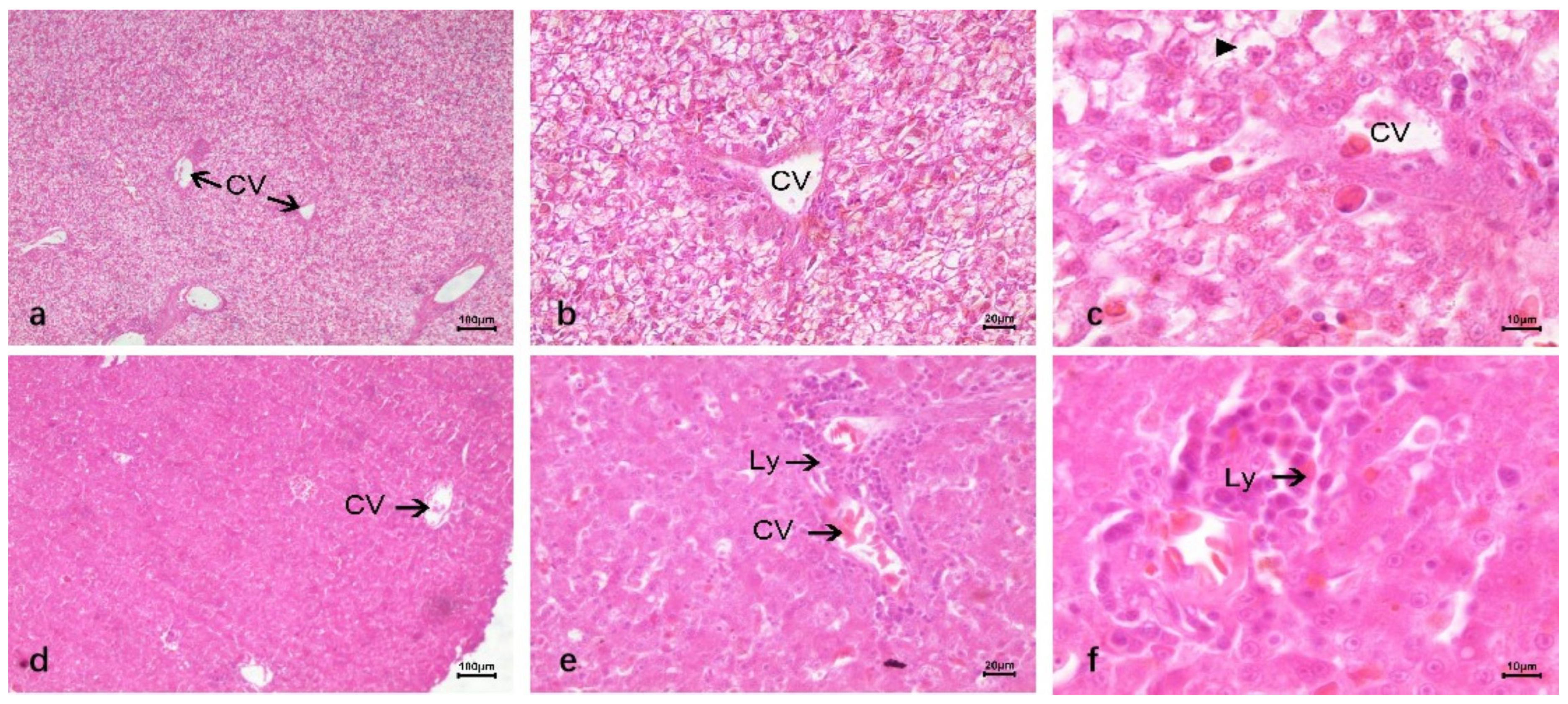
3.2.2. Duck Liver After Infection DTMUV and Inoculation LPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DTMUV | duck Tembusu virus |
| LPS | lipopolysaccharide |
| CV | Central vein |
| Ly | Lymphocyte |
| KC | Kupffer cells |
References
- Liao, J.-Y.; Tang, H.; Xiong, W.-J.; Liu, T.-N.; Li, J.-Y.; Xia, J.-Y.; Xiao, C.-T. Isolation and characterization of a novel goose ovaritis-associated cluster 3 Tembusu virus. Poult. Sci. 2023, 102, 102867. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Chen, Y.; Liu, C.; Chen, S.; Yin, X.; Li, G.; Zhang, Y. Adapted Tembusu-like virus in chickens and geese in China. J. Clin. Microbiol. 2012, 50, 2807–2809. [Google Scholar] [CrossRef]
- Li, N.; Zhao, J.; Yang, Y.; Zeng, Y.; Liu, S. Innate immune responses to duck Tembusu virus infection. Vet. Res. 2020, 51, 87. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, W.; Liu, E.; Huang, H.; Wang, T.; Wang, X.; Shi, Y.; Yang, P.; Chen, Q. In Vivo cellular and molecular study on duck spleen infected by duck Tembusu virus. Vet. Microbiol. 2019, 230, 32–44. [Google Scholar]
- Sun, X.; Liu, E.; Iqbal, A.; Wang, T.; Wang, X.; Haseeb, A.; Ahmed, N.; Yang, P.; Chen, Q. The dynamic distribution of duck Tembusu virus in the spleen of infected shelducks. BMC Vet. Res. 2019, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cai, W.; Cheng, A.; Wang, M.; Chen, S.; Huang, J.; Yang, Q.; Wu, Y.; Sun, D.; Mao, S.; et al. Duck Tembusu virus infection induces mitochondrial-mediated and death receptor-mediated apoptosis in duck embryo fibroblasts. Vet. Res. 2022, 53, 53. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, Q.; Huang, X.; Liu, X.; Han, K.; Liu, Y.; Yang, J.; Zhao, D.; Bi, K.; Sun, W. Effect of experimental infections of various Tembusu virus strains isolated from geese, ducks and chickens on ducklings. Pol. J. Vet. Sci. 2018, 21, 389–396. [Google Scholar] [CrossRef]
- Yang, H.; Yu, C.; Yin, Z.; Guan, P.; Jin, S.; Wang, Y.; Feng, X. Curcumin: A potential exogenous additive for the prevention of LPS-induced duck ileitis by the alleviation of inflammation and oxidative stress. J. Sci. Food Agric. 2023, 103, 1550–1560. [Google Scholar] [CrossRef]
- Romano, K.; Hung, D. Targeting LPS biosynthesis and transport in gram-negative bacteria in the era of multi-drug resistance. BBA Mol. Cell Res. 2023, 1870, 119407. [Google Scholar] [CrossRef]
- Deng, B.; Yu, T.; Liu, W.; Ye, S.; Wang, L.; Yang, Y.; Gong, P.; Ran, Z.; Huang, H.; Wen, J. Identification of genes and pathways related to lipopolysaccharide signaling in duckling spleens. Genet. Mol. Res. 2015, 14, 17312–17321. [Google Scholar] [CrossRef]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef]
- Chen, S.-N.; Tan, Y.; Xiao, X.-C.; Li, Q.; Wu, Q.; Peng, Y.-Y.; Ren, J.; Dong, M.-L. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol. Sin. 2021, 42, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hong, L.; Ke, J.; Zhong, Y.; Cao, N.; Li, W.; Xu, D.; Tian, Y.; Huang, Y.; Chen, W.; et al. Polysaccharide of Atractylodes macrocephala Koidz alleviate lipopolysaccharide-induced liver injury in goslings via the p53 and FOXO pathways. Poult. Sci. 2023, 102, 102480. [Google Scholar] [CrossRef]
- El-Wahab, A.A.; Chuppava, B.; Radko, D.; Visscher, C. Hepatic lipidosis in fattening turkeys: A review. Ger. J. Vet. Res. 2021, 1, 48–66. [Google Scholar] [CrossRef]
- Boltjes, A.; Movita, D.; Boonstra, A.; Woltman, A.M. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J. Hepatol. 2014, 61, 660–671. [Google Scholar] [CrossRef]
- Kong, X. The histopathologic changes of the liver from the ducks naturally infected with Tembusu virus. Anim. Husb. Vet. Med. 2020, 52, 110–115. [Google Scholar]
- Ma, Z. Animal Anatomy, Histology and Embryology; China Agriculture Press: Beijing, China, 2002. [Google Scholar]
- Kalita, K.; Rajkhowa, J.; Sarma, K.; Talukdar, M.; Sinha, S. Gross Morphological, Histological and Histochemical Studies on Liver of Pati Ducks (Anas platyrhynchos domesticus) of Assam. Indian J. Anim. Res. 2023, 7, 1–20. [Google Scholar] [CrossRef]
- Lin, D.C. Anatomy of Beijing Duck; Beijing Agricultural University Press: Beijing, China, 1994. [Google Scholar]
- Evans, H. Miller’s Anatomy of the Dog; Saunders: Philadelphia, PA, USA, 1993. [Google Scholar]
- Li, J. Color Atlas of Duck Anatomical Tissue; Chemical Industry Press: Beijing, China, 2016. [Google Scholar]
- Ali Alshammary, H.K. Histomorphological Study of the Liver in Muscovy Duck (Cairina moschata domestica). Tikrit J. Agric. Sci. 2021, 21, 112–118. [Google Scholar] [CrossRef]
- Wei, Y.-Y.; Zhang, Y.-Z.; Song, D.; Li, J.; Xu, Z.-R. Alkaline phosphatase-regulated in situ formation of chromogenic probes for multicolor visual sensing of biomarkers. Talanta 2021, 228, 122222. [Google Scholar] [CrossRef]
- Tang, Y.; Diao, Y.; Gao, X.; Yu, C.; Chen, L.; Zhang, D. Analysis of the complete genome of Tembusu virus, a flavivirus isolated from ducks in China. Transbound. Emerg. Dis. 2012, 59, 336–343. [Google Scholar] [CrossRef]
- de Barros, V.E.D.; Saggioro, F.P.; Neder, L.; França, R.F.d.O.; Mariguela, V.; Chávez, J.H.; Penharvel, S.; Forjaz, J.; da Fonseca, B.A.L.; Figueiredo, L.T.M. An experimental model of meningoencephalomyelitis by Rocio flavivirus in BALB/c mice: Inflammatory response, cytokine production, and histopathology. Am. J. Trop. Med. Hyg. 2011, 85, 363–373. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Li, Y.; Yu, K.; Yang, J.; Xu, H.; Zhang, Y.; Yu, K.; Liao, M.; Qin, Z. Rapid detection of newly isolated Tembusu-related Flavivirus by reverse-transcription loop-mediated isothermal amplification assay. Virol. J. 2011, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.C.; Qin, J.; Zoghbi, H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Sugi, Y.; Hosono, A.; Kaminogawa, S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J. Immunol. 2009, 183, 6522–6529. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.-L.; Chen, S.-R.; Wu, Z.-T.; Hu, L.-H.; Zhang, J.-Y.; Chang, H.-J.; Zhou, H.; Liu, Y. LPS-TLR4/MD-2-TNF-alpha signaling mediates alcohol-induced liver fibrosis in rats. J. Toxicol. Pathol. 2022, 35, 193–203. [Google Scholar] [CrossRef]
- Vasudevan, S.O.; Russo, A.J.; Kumari, P.; Vanaja, S.K.; Rathinam, V.A. A TLR4-independent critical role for CD14 in intracellular LPS sensing. Cell Rep. 2022, 39, 110755. [Google Scholar] [CrossRef]
- Fredericksen, B.L.; Keller, B.C.; Fornek, J.; Katze, M.G.; Gale, M. Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 2008, 82, 609–616. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Ansari, A.R.; Huang, H.-B.; Zhao, X.; Li, N.-Y.; Sun, Z.-J.; Peng, K.-M.; Zhong, J.; Liu, H.-Z. Lipopolysaccharide mediates immuno-pathological alterations in young chicken liver through TLR4 signaling. BMC Immunol. 2017, 18, 12. [Google Scholar] [CrossRef]
- Han, K.; Zhao, D.; Liu, Q.; Liu, Y.; Huang, X.; Yang, J.; Zhang, L.; Li, Y. Transcriptome analysis reveals new insight of duck Tembusu virus (DTMUV)-infected DF-1 cells. Res. Vet. Sci. 2021, 137, 150–158. [Google Scholar] [CrossRef]
- Li, N. Host Innate Immune Response Induced by Duck Tembusu Virus and the Interaction Between the Virus and RLR-Mediated Signaling Pathway. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2017. [Google Scholar]
- Wei, L.; Cui, J.; Song, Y.; Zhang, S.; Han, F.; Yuan, R.; Gong, L.; Jiao, P.; Liao, M. Duck MDA5 functions in innate immunity against H5N1 highly pathogenic avian influenza virus infections. Vet. Res. 2014, 45, 66. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Liu, S.; Wu, Y.; Lei, C.; Zhou, J.; Zhang, S. Diet-induced bacterial immunogens in the gastrointestinal tract of dairy cows: Impacts on immunity and metabolism. Acta Vet. Scand. 2011, 53, 48. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Z.; Sun, Z.; Wang, Y.; Li, H.; Sun, X. Comparative Study of DTMUV and LPS on Duck Liver Disease. Vet. Sci. 2025, 12, 900. https://doi.org/10.3390/vetsci12090900
Lan Z, Sun Z, Wang Y, Li H, Sun X. Comparative Study of DTMUV and LPS on Duck Liver Disease. Veterinary Sciences. 2025; 12(9):900. https://doi.org/10.3390/vetsci12090900
Chicago/Turabian StyleLan, Zhenghui, Zhigang Sun, Yi Wang, Huatao Li, and Xuejing Sun. 2025. "Comparative Study of DTMUV and LPS on Duck Liver Disease" Veterinary Sciences 12, no. 9: 900. https://doi.org/10.3390/vetsci12090900
APA StyleLan, Z., Sun, Z., Wang, Y., Li, H., & Sun, X. (2025). Comparative Study of DTMUV and LPS on Duck Liver Disease. Veterinary Sciences, 12(9), 900. https://doi.org/10.3390/vetsci12090900



