Identification of Risk Factors Associated with Treatment for BRD in Beef Calves Within the First 60 Days After Arrival at Fattening Operations in Northwestern Italy Beef Calves
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Selection
2.2. Population Sample
2.3. Clinical Examination
2.4. Blood Sample Collection and Management
2.5. Laboratory Analysis
2.6. Pharmacological Treatment
2.7. Statistical Analysis
3. Results
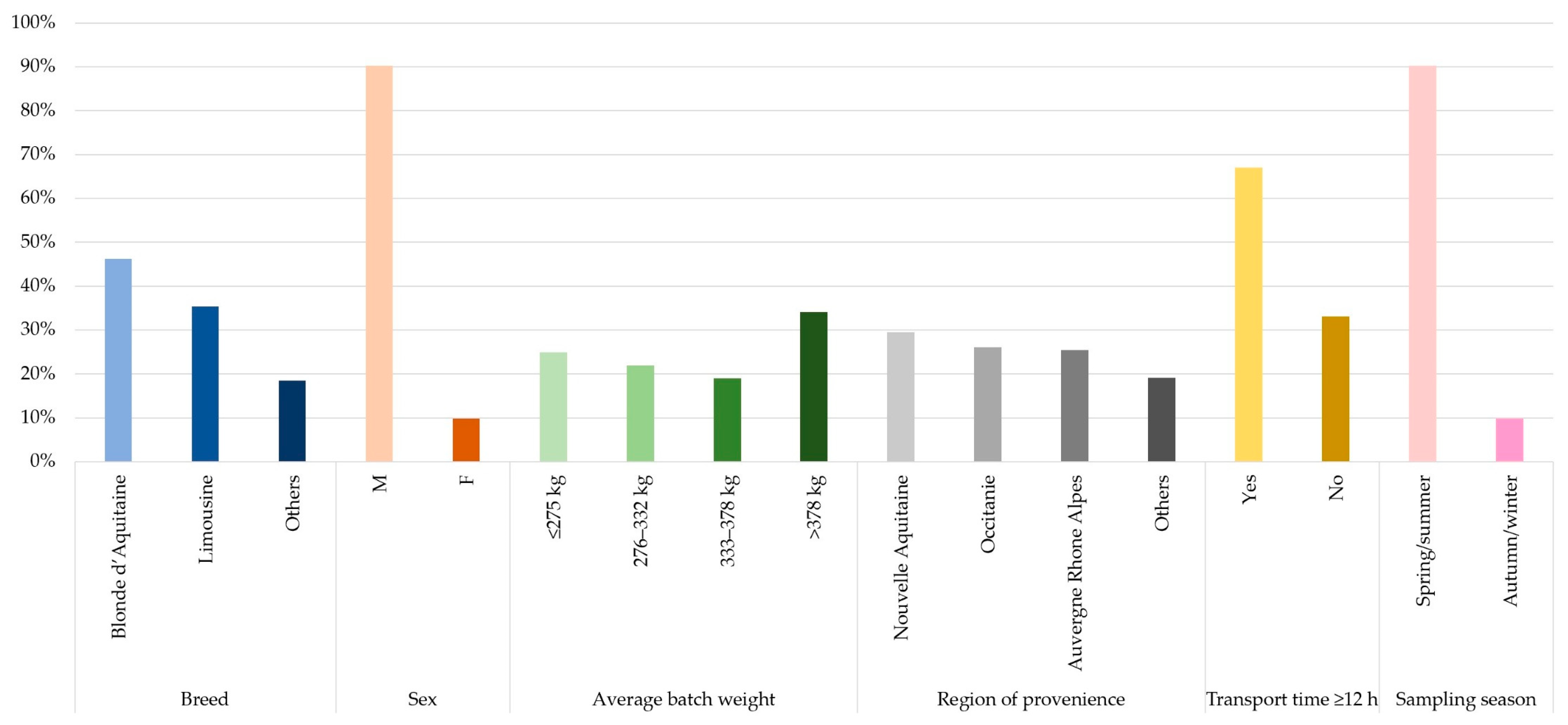
3.1. Farm Selection
3.2. Population Sample
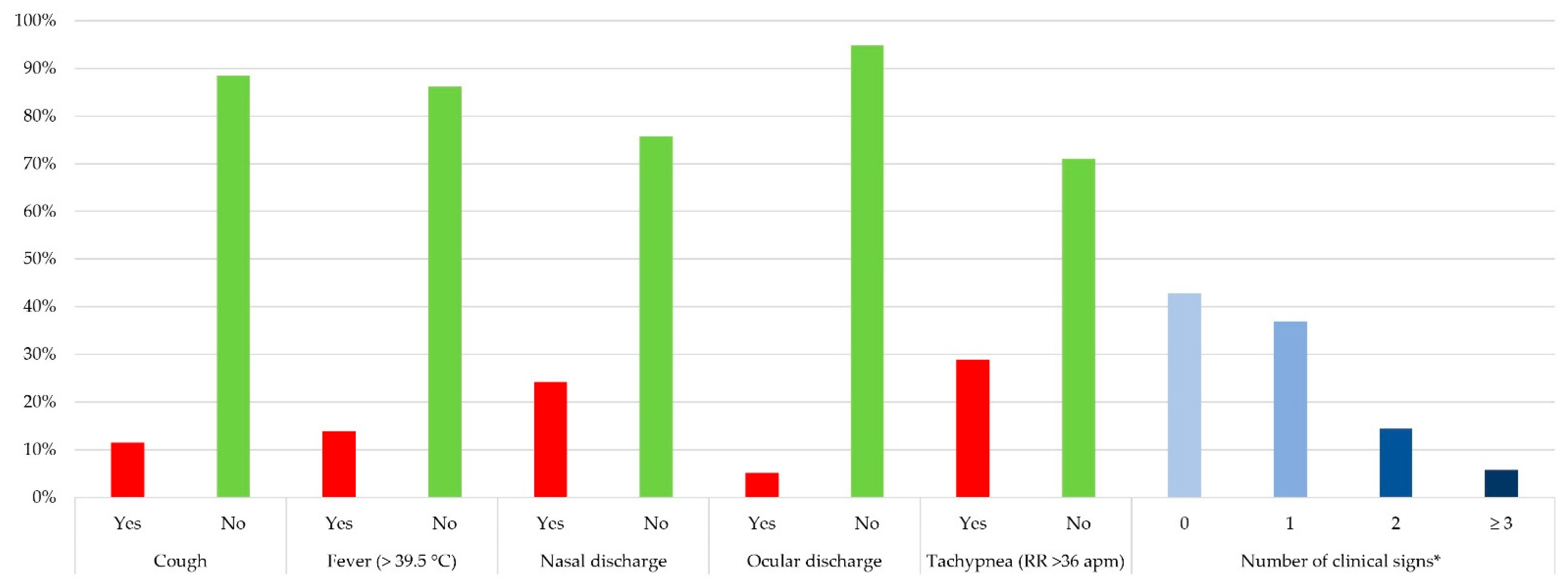
3.3. Clinical Examination
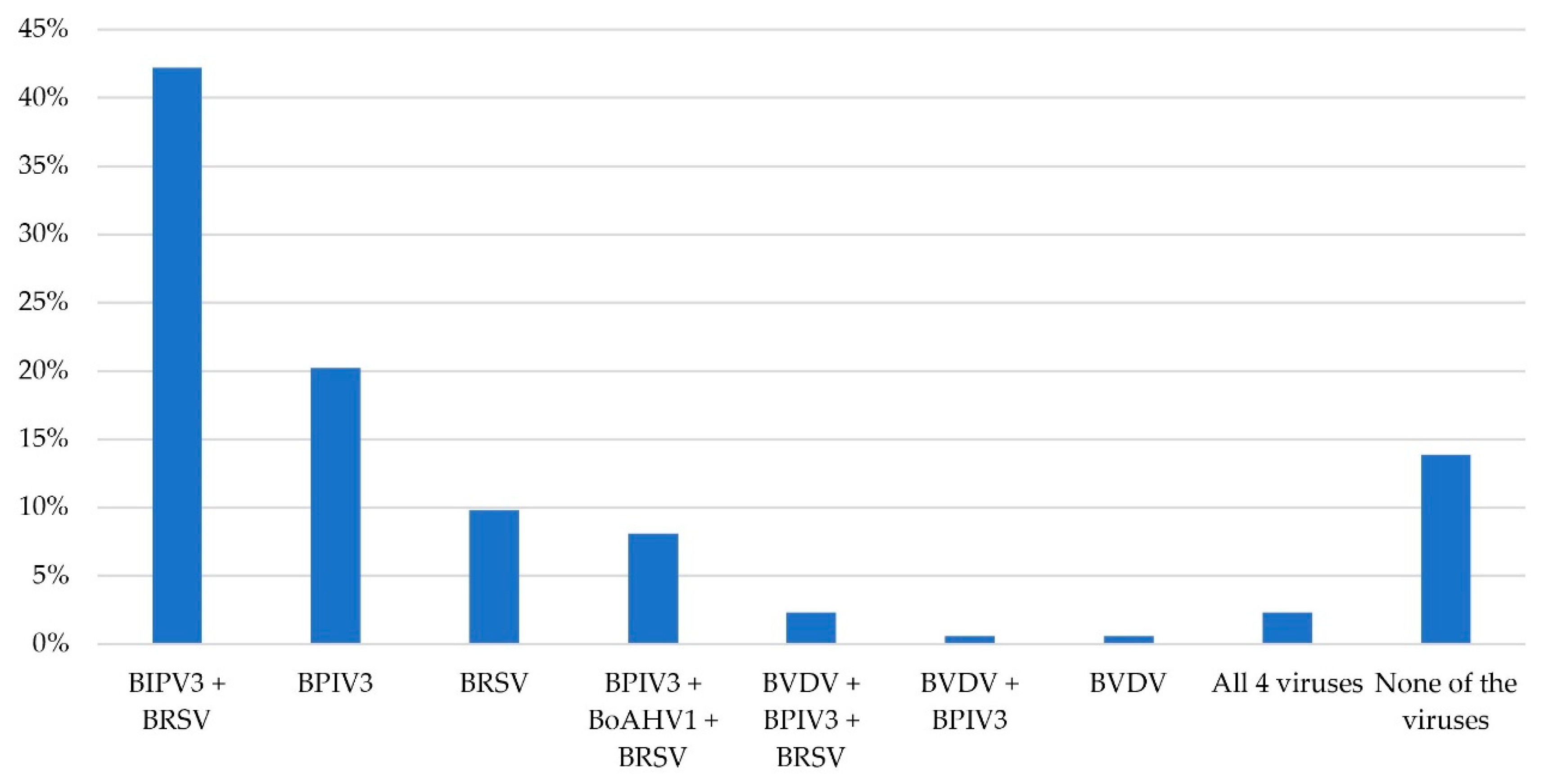
3.4. Laboratory Analysis
3.5. Pharmacological Treatment
3.6. Reactive Oxygen Metabolites and Haptoglobin
3.7. Univariate and Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BRD | Bovine respiratory disease |
| BRSV | Bovine respiratory syncytial virus |
| BPIV3 | Parainfluenza-3 virus |
| BoAHV1 | Bovine herpesvirus 1 |
| BVDV | Bovine viral diarrhea virus |
| PCR | Polymerase chain reaction |
| HP | Haptoglobin |
| ROM | Reactive oxygen metabolites |
References
- Edwards, T.A. Control Methods for Bovine Respiratory Disease for Feedlot Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 273–284. [Google Scholar] [CrossRef]
- Deepak; Aly, S.S.; Love, W.J.; Blanchard, P.C.; Crossley, B.; Van Eenennaam, A.L.; Lehenbauer, T.W. Etiology and Risk Factors for Bovine Respiratory Disease in Pre-Weaned Calves on California Dairies and Calf Ranches. Prev. Vet. Med. 2021, 197, 105506. [Google Scholar] [CrossRef]
- Step, D.L.; Krehbiel, C.R.; DePra, H.A.; Cranston, J.J.; Fulton, R.W.; Kirkpatrick, J.G.; Gill, D.R.; Payton, M.E.; Montelongo, M.A.; Confer, A.W. Effects of Commingling Beef Calves from Different Sources and Weaning Protocols during a Forty-Two-Day Receiving Period on Performance and Bovine Respiratory Disease. J. Anim. Sci. 2008, 86, 3146–3158. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The Epidemiology of Bovine Respiratory Disease: What Is the Evidence for Predisposing Factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar] [PubMed]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial Pathogens of the Bovine Respiratory Disease Complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Blakebrough-Hall, C.; McMeniman, J.P.; González, L.A. An Evaluation of the Economic Effects of Bovine Respiratory Disease on Animal Performance, Carcass Traits, and Economic Outcomes in Feedlot Cattle Defined Using Four BRD Diagnosis Methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef]
- Bastien Assié, S.; Bareille, N.; Beaudeau, F.; Seegers, H. Management-and Housing-Related Risk Factors of Respiratory Disorders in Non-Weaned French Charolais Calves. Prev. Vet. Med. 2009, 91, 218–225. [Google Scholar] [CrossRef]
- Sanderson, M.W.; Dargatz, D.A.; Wagner, B.A. Risk Factors for Initial Respiratory Disease in United States’ Feedlots Based on Producer-Collected Daily Morbidity Counts. Can. Vet. J. 2008, 49, 373. [Google Scholar]
- Hay, K.E.; Morton, J.M.; Clements, A.C.A.; Mahony, T.J.; Barnes, T.S. Population-Level Effects of Risk Factors for Bovine Respiratory Disease in Australian Feedlot Cattle. Prev. Vet. Med. 2017, 140, 78–86. [Google Scholar] [CrossRef]
- Muggli-Cockett, N.E.; Cundiff, L.V.; Gregory, K.E. Genetic Analysis of Bovine Respiratory Disease in Beef Calves during the First Year of Life. J. Anim. Sci. 1992, 70, 2013–2019. [Google Scholar] [CrossRef]
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Bovine Respiratory Disease in Feedlot Cattle: Environmental, Genetic, and Economic Factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef]
- Burdick, N.C.; Randel, R.D.; Carroll, J.A.; Welsh, T.H. Interactions between Temperament, Stress, and Immune Function in Cattle. Int. J. Zool. 2011, 2011, 373197. [Google Scholar] [CrossRef]
- Kim, M.H.; Yang, J.Y.; Upadhaya, S.D.; Lee, H.J.; Yun, C.H.; Ha, J.K. The Stress of Weaning Influences Serum Levels of Acute-Phase Proteins, Iron-Binding Proteins, Inflammatory Cytokines, Cortisol, and Leukocyte Subsets in Holstein Calves. J. Vet. Sci. 2011, 12, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Odore, R.; D’Angelo, A.; Badino, P.; Bellino, C.; Pagliasso, S.; Re, G. Road Transportation Affects Blood Hormone Levels and Lymphocyte Glucocorticoid and β-Adrenergic Receptor Concentrations in Calves. Vet. J. 2004, 168, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Panciera, R.J.; Confer, A.W. Pathogenesis and Pathology of Bovine Pneumonia. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 191. [Google Scholar] [CrossRef]
- O’Connor, A.; Martin, S.W.; Nagy, É.; Menzies, P.; Harland, R. The Relationship between the Occurrence of Undifferentiated Bovine Respiratory Disease and Titer Changes to Bovine Coronavirus and Bovine Viral Diarrhea Virus in 3 Ontario Feedlots. Can. J. Vet. Res. 2001, 65, 137. [Google Scholar]
- Hay, K.E.; Barnes, T.S.; Morton, J.M.; Gravel, J.L.; Commins, M.A.; Horwood, P.F.; Ambrose, R.C.; Clements, A.C.A.; Mahony, T.J. Associations between Exposure to Viruses and Bovine Respiratory Disease in Australian Feedlot Cattle. Prev. Vet. Med. 2016, 127, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute Phase Proteins in Ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Carter, J.N.; Meredith, G.L.; Montelongo, M.; Gill, D.R.; Krehbiel, C.R.; Payton, M.E.; Confer, A.W. Relationship of Vitamin E Supplementation and Antimicrobial Treatment with Acute-Phase Protein Responses in Cattle Affected by Naturally Acquired Respiratory Tract Disease. Am. J. Vet. Res. 2002, 63, 1111–1117. [Google Scholar] [CrossRef]
- Humblet, M.F.; Coghe, J.; Lekeux, P.; Godeau, J.M. Acute Phase Proteins Assessment for an Early Selection of Treatment in Growing Calves Suffering from Bronchopneumonia under Field Conditions. Res. Vet. Sci. 2004, 77, 41–47. [Google Scholar] [CrossRef]
- Gånheim, C.; Alenius, S.; Persson Waller, K. Acute Phase Proteins as Indicators of Calf Herd Health. Vet. J. 2007, 173, 645. [Google Scholar] [CrossRef]
- Chirase, N.K.; Greene, L.W.; Purdy, C.W.; Loan, R.W.; Auvermann, B.W.; Parker, D.B.; Walborg, E.F.; Stevenson, D.E.; Xu, Y.; Klaunig, J.E. Effect of Transport Stress on Respiratory Disease, Serum Antioxidant Status, and Serum Concentrations of Lipid Peroxidation Biomarkers in Beef Cattle. Am. J. Vet. Res. 2004, 65, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative Stress, Antioxidants, and Animal Function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Dohoo, I.R.; Martin, S.W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Charlottetown, PE, Canada, 2009. [Google Scholar]
- USDA. Feedlot 2011 PArt IV: Health and HEalth MAnagementon US Feedlots with A Capacity of 1000 or More Head; USDA-APHIS-VS-CEAHNAHMS: Washington, DC, USA, 2013. [Google Scholar]
- Assié, S.; Seegers, H.; Makoschey, B.; Désirébousquié, L.; Bareille, N. Exposure to Pathogens and Incidence of Respiratory Disease in Young Bulls on Their Arrival at Fattening Operations in France. Vet. Rec. 2009, 165, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.J.; Tait, R.G.; Busby, W.D.; Reecy, J.M. An Evaluation of Bovine Respiratory Disease Complex in Feedlot Cattle: Impact on Performance and Carcass Traits Using Treatment Records and Lung Lesion Scores. J. Anim. Sci. 2009, 87, 1821–1827. [Google Scholar] [CrossRef]
- Byrne, A.; Rojas, H.A.; White, B.J.; Amrine, D.E.; Larson, R.L. Predicting Bovine Respiratory Disease Risk in Feedlot Cattle in the First 45 Days Post Arrival. Pathogens 2022, 11, 442. [Google Scholar] [CrossRef]
- Gummow, B.; Mapham, P.H. A Stochastic Partial-Budget Analysis of an Experimental Pasteurella Haemolytica Feedlot Vaccine Trial. Prev. Vet. Med. 2000, 43, 29–42. [Google Scholar] [CrossRef]
- De Irala, J.; Navajas, R.F.C.; Del Castillo, A.S. Abnormally Wide Confidence Intervals in Logistic Regression: Interpretation of Statistical Program Results. Rev. Panam. Salud Publica/Pan Am. J. Public Health 1997, 2, 268–271. [Google Scholar] [CrossRef]
- Pinchak, W.E.; Tolleson, D.R.; McCloy, M.; Hunt, L.J.; Gill, R.J.; Ansley, R.J.; Bevers, S.J. Morbidity Effects on Productivity and Profitability of Stocker Cattle Grazing in the Southern Plains. J. Anim. Sci. 2004, 82, 2773–2779. [Google Scholar] [CrossRef] [PubMed]
- Cirone, F.; Padalino, B.; Tullio, D.; Capozza, P.; Surdo, M.L.; Lanave, G.; Pratelli, A. Prevalence of Pathogens Related to Bovine Respiratory Disease Before and After Transportation in Beef Steers: Preliminary Results. Animals 2019, 9, 1093. [Google Scholar] [CrossRef]
- Hay, K.E.; Morton, J.M.; Schibrowski, M.L.; Clements, A.C.A.; Mahony, T.J.; Barnes, T.S. Associations between Prior Management of Cattle and Risk of Bovine Respiratory Disease in Feedlot Cattle. Prev. Vet. Med. 2016, 127, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Riondato, F.; D’Angelo, A.; Miniscalco, B.; Bellino, C.; Guglielmino, R. Effects of Road Transportation on Lymphocyte Subsets in Calves. Vet. J. 2008, 175, 364–368. [Google Scholar] [CrossRef]
- Valadez-Noriega, M.; Estévez-Moreno, L.X.; Galindo, F.; Pérez-Martínez, F.; Villarroel, M.; Miranda-de la Lama, G.C. Consequences of Long-Distance Transport on the Behavior and Health of Young-Bulls That May Affect Their Fitness to Adapt to Feedlots. Livest. Sci. 2022, 265, 105083. [Google Scholar] [CrossRef]
- Alexander, B.H.; MacVean, D.W.; Salman, M.D. Risk Factors for Lower Respiratory Tract Disease in a Cohort of Feedlot Cattle. J. Am. Vet. Med. Assoc. 1989, 195, 207–211. [Google Scholar] [CrossRef]
- MacVean, D.W.; Franzen, D.K.; Keefe, T.J.; Bennett, B.W. Airborne Particle Concentration and Meteorologic Conditions Associated with Pneumonia Incidence in Feedlot Cattle. Am. J. Vet. Res. 1986, 47, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Ribble, C.S.; Meek, A.H.; Shewen, P.E.; Jim, G.K.; Guichon, P.T. Effect of Transportation on Fatal Fibrinous Pneumonia and Shrinkage in Calves Arriving at a Large Feedlot. J. Am. Vet. Med. Assoc. 1995, 207, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Cernicchiaro, N.; Renter, D.G.; White, B.J.; Babcock, A.H.; Fox, J.T. Associations between Weather Conditions during the First 45 Days after Feedlot Arrival and Daily Respiratory Disease Risks in Autumn-Placed Feeder Cattle in the United States. J. Anim. Sci. 2012, 90, 1328–1337. [Google Scholar] [CrossRef]
- Padalino, B.; Cirone, F.; Zappaterra, M.; Tullio, D.; Ficco, G.; Giustino, A.; Ndiana, L.A.; Pratelli, A. Factors Affecting the Development of Bovine Respiratory Disease: A Cross-Sectional Study in Beef Steers Shipped from France to Italy. Front. Vet. Sci. 2021, 8, 627894. [Google Scholar] [CrossRef]
- Smith, K.J.; Amrine, D.E.; Larson, R.L.; Theurer, M.E.; Szaz, J.I.; Bryant, T.; White, B.J. Risk Factors for Mid- and Late-Feeding-Stage Bovine Respiratory Morbidity and Mortality Based on Individual Animal Treatments of Beef Feedlot Cattle. Appl. Anim. Sci. 2022, 38, 360–372. [Google Scholar] [CrossRef]
- Hay, K.E.; Barnes, T.S.; Morton, J.M.; Clements, A.C.A.; Mahony, T.J. Risk Factors for Bovine Respiratory Disease in Australian Feedlot Cattle: Use of a Causal Diagram-Informed Approach to Estimate Effects of Animal Mixing and Movements before Feedlot Entry. Prev. Vet. Med. 2014, 117, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, S.; Hjort, M.; Graham, D.A.; Öhagen, P.; Törnquist, M.; Alenius, S. A Six-Year Study on Respiratory Viral Infections in a Bull Testing Facility. Vet. J. 2007, 173, 585. [Google Scholar] [CrossRef]
- Diana, A.; Penasa, M.; Santinello, M.; Scali, F.; Magni, E.; Alborali, G.L.; Bertocchi, L.; De Marchi, M. Exploring Potential Risk Factors of Antimicrobial Use in Beef Cattle. Animal 2021, 15, 100091. [Google Scholar] [CrossRef]
- Gay, E.; Barnouin, J. A Nation-Wide Epidemiological Study of Acute Bovine Respiratory Disease in France. Prev. Vet. Med. 2009, 89, 265–271. [Google Scholar] [CrossRef]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Willard, S.T.; Vann, R.C.; Welsh, T.H.; Randel, R.D. Relationships between Temperament and Transportation with Rectal Temperature and Serum Concentrations of Cortisol and Epinephrine in Bulls. Livest. Sci. 2010, 129, 166–172. [Google Scholar] [CrossRef]
- Timsit, E.; Dendukuri, N.; Schiller, I.; Buczinski, S. Diagnostic Accuracy of Clinical Illness for Bovine Respiratory Disease (BRD) Diagnosis in Beef Cattle Placed in Feedlots: A Systematic Literature Review and Hierarchical Bayesian Latent-Class Meta-Analysis. Prev. Vet. Med. 2016, 135, 67–73. [Google Scholar] [CrossRef]
- Buczinski, S.; O’Connor, A.M. Specific Challenges in Conducting and Reporting Studies on the Diagnostic Accuracy of Ultrasonography in Bovine Medicine. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.E.; Morton, J.M.; Mahony, T.J.; Clements, A.C.A.; Barnes, T.S. Associations between Animal Characteristic and Environmental Risk Factors and Bovine Respiratory Disease in Australian Feedlot Cattle. Prev. Vet. Med. 2016, 125, 66–74. [Google Scholar] [CrossRef]
- Durham, P.J.K.; Hassard, L.E.; Donkersgoed, J. Van Serological Studies of Infectious Bovine Rhinotracheitis, Parainfluenza 3, Bovine Viral Diarrhea, and Bovine Respiratory Syncytial Viruses in Calves Following Entry to a Bull Test Station. Can. Vet. J. 1991, 32, 427. [Google Scholar]
- Ngwa-Mbot, D.; Mémeteau, S.; Gache, K.; Azéma, P.; Valas, S. Un Dispositif Réglementaire de Lutte Renforcé. 2015.
- Groupements de Defénse Sanitaire Hauts de France. BVD, Contrôle Des Risques. Available online: https://www.gds64.fr/maladies-actions-sanitaires/bovins/ibr/caracteristiques-de-libr/ (accessed on 30 June 2025).
- BVD, le plan d’éradication de GDS Bretagne. Available online: https://www.gds-bretagne.fr/mes-besoins/bvd-plan-deradication-de-gds-bretagne/ (accessed on 30 June 2025).
- Booker, C.W.; Guichon, P.T.; Jim, G.K.; Schunicht, O.C.; Harland, R.J.; Morley, P.S. Seroepidemiology of Undifferentiated Fever in Feedlot Calves in Western Canada. Can. Vet. J. 1999, 40, 40–48. [Google Scholar] [PubMed]
- Orro, T.; Pohjanvirta, T.; Rikula, U.; Huovilainen, A.; Alasuutari, S.; Sihvonen, L.; Pelkonen, S.; Soveri, T. Acute Phase Protein Changes in Calves during an Outbreak of Respiratory Disease Caused by Bovine Respiratory Syncytial Virus. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 23–29. [Google Scholar] [CrossRef]
- El-Deeb, W.; Elsohaby, I.; Fayez, M.; Mkrtchyan, H.V.; El-Etriby, D.; ElGioushy, M. Use of Procalcitonin, Neopterin, Haptoglobin, Serum Amyloid A and Proinflammatory Cytokines in Diagnosis and Prognosis of Bovine Respiratory Disease in Feedlot Calves under Field Conditions. Acta Trop. 2020, 204, 105336. [Google Scholar] [CrossRef] [PubMed]
- Tsukano, K.; Fukuda, T.; Ikeda, K.; Sato, K.; Suzuki, K. Serum Iron Concentration Is Candidate Inflammatory Marker for Respiratory Diseases in Beef Cows. J. Vet. Med. Sci. 2021, 83, 824. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of Body Condition Score on Relationships between Metabolic Status and Oxidative Stress in Periparturient Dairy Cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Bedard, K.; Attar, H.; Bonnefont, J.; Jaquet, V.; Borel, C.; Plastre, O.; Stasia, M.J.; Antonarakis, S.E.; Krause, K.H. Three Common Polymorphisms in the CYBA Gene Form a Haplotype Associated with Decreased ROS Generation. Hum. Mutat. 2009, 30, 1123–1133. [Google Scholar] [CrossRef]
| Parameter | Category | ROM (U/CARR) | HP (mg/dL) |
|---|---|---|---|
| Treatment within 7 days | Yes | 62 (40, 192) | 33 (15, 78) |
| No | 92 (40, 228) | 31 (11, 280) | |
| Treatment within 60 days | Yes | 70 (40, 192) | 32.5 (11, 280) |
| No | 95 (40, 228) | 30 (11, 150) |
| Variable | Category | OR | 95% CI | p Value |
|---|---|---|---|---|
| Breed | Blonde d’Aquitaine | Referent | - | - |
| Limousine | 0.21 | 0.01–1.25 | 0.148 | |
| Others * | 2.85 | 0.82–9.87 | 0.092 | |
| Average weight on arrival | ≤275 kg | Referent | - | - |
| 276–332 kg | 0 | NA–∞ | 0.995 | |
| 333–378 kg | 0 | NA–∞ | 0.996 | |
| >378 kg | 10.72 | 1.98–199.49 | 0.026 | |
| Positivity to at least three viruses | No | Referent | - | - |
| Yes | 3.51 | 0.8–12.02 | 0.054 | |
| Province of origin | Auvergne-Rhone-Alpes | Referent | - | - |
| Nouvelle Aquitaine | 0.42 | 0.02–4.53 | 0.485 | |
| Occitanie | 3.23 | 0.7–22.96 | 0.166 | |
| Others ** | 2.9 | 0.53–21.9 | 0.237 | |
| ROMs | - | 0.97 | 0.94–0.99 | 0.01 |
| Variable | Category | OR | 95% CI | p Value |
|---|---|---|---|---|
| Breed | Blonde d’Aquitaine | Referent | - | - |
| Limousine | 0.29 | 0.1–0.72 | 0.012 | |
| Others * | 0.61 | 0.2–1.6 | 0.337 | |
| Average weight on arrival | ≤275 kg | Referent | - | - |
| 276–332 kg | 0.28 | 0.06–1.02 | 0.072 | |
| 333–378 kg | 0.33 | 0.07–1.2 | 0.116 | |
| >378 kg | 1.45 | 0.6–3.66 | 0.419 | |
| Transportation time ≥ 12 h | No | Referent | - | - |
| Yes | 3.47 | 1.36–10.68 | 0.016 | |
| Province of origin | Auvergne-Rhone-Alpes | Referent | - | - |
| Nouvelle Aquitaine | 0.84 | 0.26–2.67 | 0.765 | |
| Occitanie | 2.64 | 0.98–7.70 | 0.061 | |
| Others ** | 0.94 | 0.26–3.27 | 0.928 | |
| ROMs | - | 0.98 | 0.96–0.99 | 0.002 |
| Treatment Within 7 Days | Treatment Within 60 Days | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | OR | 95% CI | p Value | OR | 95% CI | p-Value |
| Average weight on arrival | ≤275 kg | Referent | - | - | Referent | - | - |
| 276–332 kg | 0 | NA–∞ | 0.995 | 0.22 | 0.05–0.83 | 0.036 | |
| 333–378 kg | 0 | NA–∞ | 0.996 | 0.43 | 0.09–1.67 | 0.252 | |
| >378 kg | 11.54 | 2.07–216.9 | 0.023 | 3.09 | 1.09–9.4 | 0.039 | |
| Transportation time ≥ 12 h | No | - | - | - | Referent | - | - |
| Yes | - | - | - | 6.65 | 2.13–24.65 | 0.002 | |
| ROMs | - | 0.98 | 0.96–0.99 | 0.023 | 0.99 | 0.97–1 | 0.056 |
| AIC | - | 71.291 | 148.62 | ||||
| R2 | - | 0.236 | 0.213 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicola, I.; Borriello, G.; Ramacciotti, E.; Gallina, G.; Beltramo, M.; Bellino, C. Identification of Risk Factors Associated with Treatment for BRD in Beef Calves Within the First 60 Days After Arrival at Fattening Operations in Northwestern Italy Beef Calves. Vet. Sci. 2025, 12, 898. https://doi.org/10.3390/vetsci12090898
Nicola I, Borriello G, Ramacciotti E, Gallina G, Beltramo M, Bellino C. Identification of Risk Factors Associated with Treatment for BRD in Beef Calves Within the First 60 Days After Arrival at Fattening Operations in Northwestern Italy Beef Calves. Veterinary Sciences. 2025; 12(9):898. https://doi.org/10.3390/vetsci12090898
Chicago/Turabian StyleNicola, Isabella, Giuliano Borriello, Edoardo Ramacciotti, Giovanni Gallina, Maurizio Beltramo, and Claudio Bellino. 2025. "Identification of Risk Factors Associated with Treatment for BRD in Beef Calves Within the First 60 Days After Arrival at Fattening Operations in Northwestern Italy Beef Calves" Veterinary Sciences 12, no. 9: 898. https://doi.org/10.3390/vetsci12090898
APA StyleNicola, I., Borriello, G., Ramacciotti, E., Gallina, G., Beltramo, M., & Bellino, C. (2025). Identification of Risk Factors Associated with Treatment for BRD in Beef Calves Within the First 60 Days After Arrival at Fattening Operations in Northwestern Italy Beef Calves. Veterinary Sciences, 12(9), 898. https://doi.org/10.3390/vetsci12090898






