Effects of Canthoplasty in Preventing Secondary Ocular Surface Lesions in Brachycephalic Dogs
Simple Summary
Abstract
1. Introduction
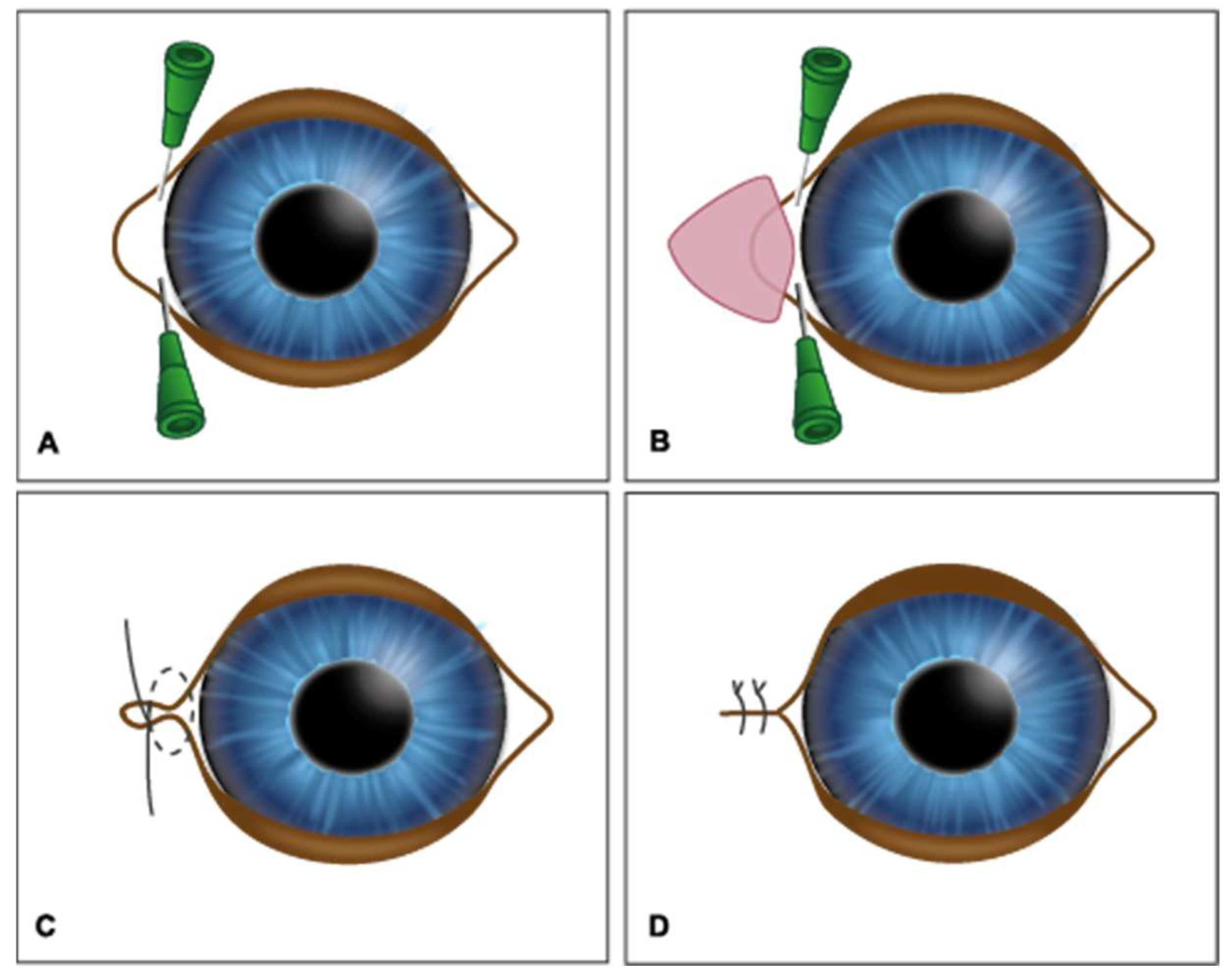
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCT | Tear crystallization test |
| TFBUT | Tear film break-up time |
References
- Nicholas, F.W.; Wade, C.M.; Williamson, P. Disorders in pedigree dogs: Assembling the evidence. Vet. J. 2010, 183, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, K.K.; Lima, T.B.; Padua, I.R.M.; Sobrinho, A.A.F.d.B.; Marinho, F.d.A.; Ortêncio, K.P.; Laus, J.L. Ophthalmic parameters in adult Shih Tzu dogs. Cienc. Rural 2015, 45, 1280–1285. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Hendricks, A.; Tivers, M.S.; Burn, C.C.; Ambrósio, C.E. Impact of facial conformation on canine health: Brachycephalic obstructive airway syndrome. PLoS ONE 2015, 10, e0137496. [Google Scholar] [CrossRef]
- Gelatt, K.N.; Gelatt, J.P. Surgery of the cornea and sclera. In Veterinary Ophthalmic Surgery, 1st ed.; Gelatt, K.N., Gelatt, J.P., Eds.; Elsevier Health Sciences: St. Louis, MO, USA, 2011. [Google Scholar]
- Andrews, A.L.M.M.; Youngman, K.L.; Packer, R.M.A.; O’neill, D.G.; Kafarnik, C. A review of clinical outcomes, owner understanding and satisfaction following medial canthoplasty in brachycephalic dogs in a UK referral setting (2016–2021). Animals 2023, 13, 2032. [Google Scholar] [CrossRef] [PubMed]
- Gelatt, K.N.; Gelatt, J.P. Veterinary Ophthalmic Surgery; Saunders: St. Louis, MO, USA, 1994. [Google Scholar]
- Rolando, M. Tear mucus ferning test in normal and keratoconjunctivitis sicca eyes. Chibret Int. J. Ophthalmol. 1984, 2, 32–41. [Google Scholar]
- Pe’er, O.; Ofri, R.; Sebbag, L. Schirmer tear test-1 with open or closed eyelids: An evaluation in brachycephalic and nonbrachycephalic dogs. Vet. Ophthalmol. 2025, 28, 21–27. [Google Scholar] [CrossRef]
- Gelatt, K.N. Essentials of Veterinary Ophthalmology, 2nd ed.; Blackwell Publishing Professional: Ames, IA, USA, 2008; p. 81. [Google Scholar]
- Barachetti, L.; Rampazzo, A.; Mortellaro, C.M.; Scevola, S.; Gilger, B.C. Use of episcleral cyclosporine implants in dogs with keratoconjunctivitis sicca: Pilot study. Vet. Ophthalmol. 2015, 18, 234–241. [Google Scholar] [CrossRef]
- Santos, T.G.S. Incidência de Ceratite Ulcerativa em Cães–Estudo Comparativo em Braquicefálicos e Não Braquicefálicos. Bachelor’s Thesis, CEPLAC–Faculdade de Medicina Veterinária, Itabuna, Brazil, 2020. [Google Scholar]
- Masmali, A.M.; Al-Qhtani, S.; Al-Gasham, T.M.; El-Hiti, G.A.; Purslow, C.; Murphy, P.J. Application of a new grading scale for tear ferning in non-dry eye and dry eye subjects. Cont. Lens Anterior Eye 2015, 38, 39–43. [Google Scholar] [CrossRef]
- Belknap, E.; Genetics Committee of the American College of Veterinary Ophthalmologists. Ocular disorders, proven or presumed to be inherited in purebred dogs. In The Blue Book: Ocular Disorders Presumed to Be Inherited in Purebred Dogs, 13th ed.; ACVO, Ed.; American College of Veterinary Ophthalmologists: Meridian, ID, USA, 2015. [Google Scholar]
- Costa, J.V. Síndrome Braquicefálica Ocular. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2019. [Google Scholar]
- Gipson, I.K. The ocular surface: The challenge to enable and protect vision. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4391–4398. [Google Scholar] [CrossRef]
- Christmas, R. Common ocular problems of Shih Tzu dogs. Can. Vet. J. 1992, 33, 390–393. [Google Scholar]
- Galley, A.P.; Beltran, E.; Tetas Pont, R. Neurogenic keratoconjunctivitis sicca in 34 dogs: A case series. Vet. Ophthalmol. 2022, 25, 140–152. [Google Scholar] [CrossRef]
- Cooper, S. Keratoconjunctivitis sicca in the dog. Companion Anim. 2012, 17, 37–42. [Google Scholar] [CrossRef]
- Allgoewer, I. Simplified medial canthoplasty: Technique and early postoperative complications in 601 dogs (1180 eyes). Vet. Ophthalmol. 2025, 28, 497–505. [Google Scholar] [CrossRef]
- Appelboam, H. Pug appeal: Brachycephalic ocular health. Companion Anim. 2016, 1, 36. [Google Scholar] [CrossRef]
- Milner, M.S.; Beckman, K.A.; Luchs, J.I.; Allen, Q.B.; Awdeh, R.M.; Berdahl, J.; John, B.; Boland, T.S.; Buznego, C.; Gira, J.P.; et al. Dysfunctional tear syndrome: Dry eye disease and associated tear film disorders. Curr. Opin. Ophthalmol. 2017, 28, 3–47. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefe’s Arch Clin Exp Ophthalmol. 2006, 244, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.L.; Ihle, S.L.; Webb, A.A. Keratoconjunctival effects of diabetes mellitus in dogs. Vet. Ophthalmol. 2005, 8, 215–224. [Google Scholar] [CrossRef]
- Gelatt, K.N.; Gilger, B.C.; Kern, T.J. Veterinary Ophthalmology, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Pigatto, J.A.T.; Pereira, F.Q.; Almeida, A.C.V.R.; Redaeli, R.; Faganello, C.S.; Franzen, A.A. Keratoconjunctivitis in dogs and cats. Acta Sci. Vet. 2007, 35, 250–251. [Google Scholar]
- Peng, C.-C.; Cerretani, C.; Braun, R.J.; Radke, C. Evaporation-driven instability of the precorneal tear film. Adv. Colloid Interface Sci. 2014, 206, 250–264. [Google Scholar] [CrossRef]
- Murube, J. Tear osmolarity. Ocul. Surf. 2008, 4, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of hyperosmolarity in dry eye disease. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Mota, L.P.A. Evaluation of the Ocular Surface of Dogs with Protrusion of the Third Eyelid Gland. Ph.D. Thesis, Universidade de Brasília, Brasília, Brazil, 2014. [Google Scholar]
- Silva, L.R.; Gouveia, A.F.; de Fátima, C.J.; Oliveira, L.B.; Reis, J.L., Jr.; Ferreira, R.F.; Pimentel, C.M.; Galera, P.D. Tear ferning test in horses and its correlation with ocular surface evaluation. Vet. Ophthalmol. 2016, 19, 117–123. [Google Scholar] [CrossRef]
- Avancini, J.B. Tear Crystallization Test in Healthy Shih Tzu Dogs. Bachelor’s Thesis, Faculdade de Medicina Veterinária da FAV, Brasília, Brazil, 2016. [Google Scholar]
- McGinnigle, S.; Naroo, S.A.; Eperjesi, F. Evaluation of dry eye. Surv. Ophthalmol. 2012, 57, 293–316. [Google Scholar] [CrossRef]
- Julio, G.; Lluch, S.; Pujol, P.; Merindano, M.D. Effects of tear hyperosmolarity on conjunctival cells. Ophthalmic Physiol. Opt. 2012, 32, 317–323. [Google Scholar] [CrossRef]
- Sebbag, L.; Sanchez, R.F. The pandemic of ocular surface disease in brachycephalic dogs: The brachycephalic ocular syndrome. Vet. Ophthalmol. 2023, 26, 31–46. [Google Scholar] [CrossRef]
- Lima, A.M.V. Tear Production and Goblet Cell Density in Shih-Tzu. Ph.D. Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2008. [Google Scholar]
- Iwashita, H.; Sebbag, L.; Leonard, B.C.; Saito, A. A review of diagnostic tests for qualitative and quantitative tear film deficiency in dogs. Vet. Ophthalmol. 2023, 26, 5–15. [Google Scholar] [CrossRef]
- Kim, J.Y.; Won, H.J.; Jeong, S.W. A retrospective study of ulcerative keratitis in 32 dogs. Int. J. Appl. Res. Vet. Med. 2009, 7, 27–31. [Google Scholar]
- Bedford, P.G.C.; Jones, R.G. Abnormal appearance. In Small Animal Ophthalmology: A Problem-Oriented Approach; Peiffer, R.L., Jr., Petersen-Jones, S.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2001; pp. 94–95. [Google Scholar]
- Voitena, J.N.; Brito, F.L.C.; Marinho, T.O.C.; Montiani-Ferreira, F.; Cremonini, D.N.; Chiurciu, J.L.V.; Jesus, N.S.; Leonard, B.C.; da Cunha, O. Application of OSA-VET® and qualiquantitative tear tests in brachycephalic dogs with and without keratoconjunctivitis sicca. Vet. Res. Commun. 2025, 49, 40. [Google Scholar] [CrossRef] [PubMed]
- Imcharoon, K.; Pinyosnit, N.; Srilert, P.; Ngampongsai, T.; Hunprasit, V.; Tuntivanich, N.; Jaturakan, O. Comparative study of healing time of canine non-infectious deep ulcerative keratitis between medical therapy alone and combined treatment with medical therapy and a nictitating membrane flap: A retrospective study. Open Vet. J. 2022, 12, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Carrington, S.D.; Bedford, P.G.; Guillon, J.P.; Woodward, E.G. Biomicroscopy of the tear film of the Pekingese dog. Vet. Rec. 1989, 124, 323–328. [Google Scholar] [CrossRef]
- Startup, F.G. Corneal ulceration in the dog. J. Small Anim. Pract. 1984, 25, 737–752. [Google Scholar] [CrossRef]
| Groups | Time | Mean ± S.D. Overtime | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T7 | T30 | T3M | T6M | T9M | T12M | T15M | T18M | ||
| Control group | 14.36 ± 7.91 aA | 14.72 ± 7.72 aA | 16.6 ± 5.88 aA | 15.14 ± 5.47 aA | 14.68 ± 5.65 aA | 14.41 ± 4.67 aA | 11.00 ± 4.90 aA | 11.14 ± 4.83 aA | 11.43 ± 4.89 aA | 14.07 ± 6.23 A |
| Treated group | 15.74 ± 8.85 aA | 20.66 ± 7.05 bB | 17.98 ± 7.09 abA | 18.00 ± 5.63 abA | 18.22 ± 5.59 abB | 19.59 ± 5.71 bB | 20.15 ± 5.17 bcB | 20.86 ± 4.41 bcB | 23.24 ± 2.54 cB | 18.92 ± 6.57 B |
| Groups | Tear Film Break-Up Time (TFBUT) (s) | Mean ±S.D. Overtime | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T7 | T30 | T3M | T6M | T9M | T12M | T15M | T18M | ||
| Control group | 6.73 aA ± 4.32 | 6.91 aA ± 4.44 | 7.00 aA ± 3.52 | 6.45 aA ± 3.69 | 5.90 aA ± 3.66 | 5.27 aA ± 3.08 | 3.43 aA ± 1.65 | 3.29 aA ± 1.64 | 3.29 aA ± 1.64 | 5.64 A ± 3.63 |
| Treated group | 6.38 aA ± 3.87 | 6.27 aA ± 3.74 | 6.17 aA ± 3.48 | 7.32 abA ± 2.98 | 7.80 abB ± 3.02 | 9.34 bcB ± 4.26 | 9.81 cB ± 3.42 | 10.14 cB ± 2.69 | 11.00 cB ± 3.41 | 7.78 B ± 3.79 |
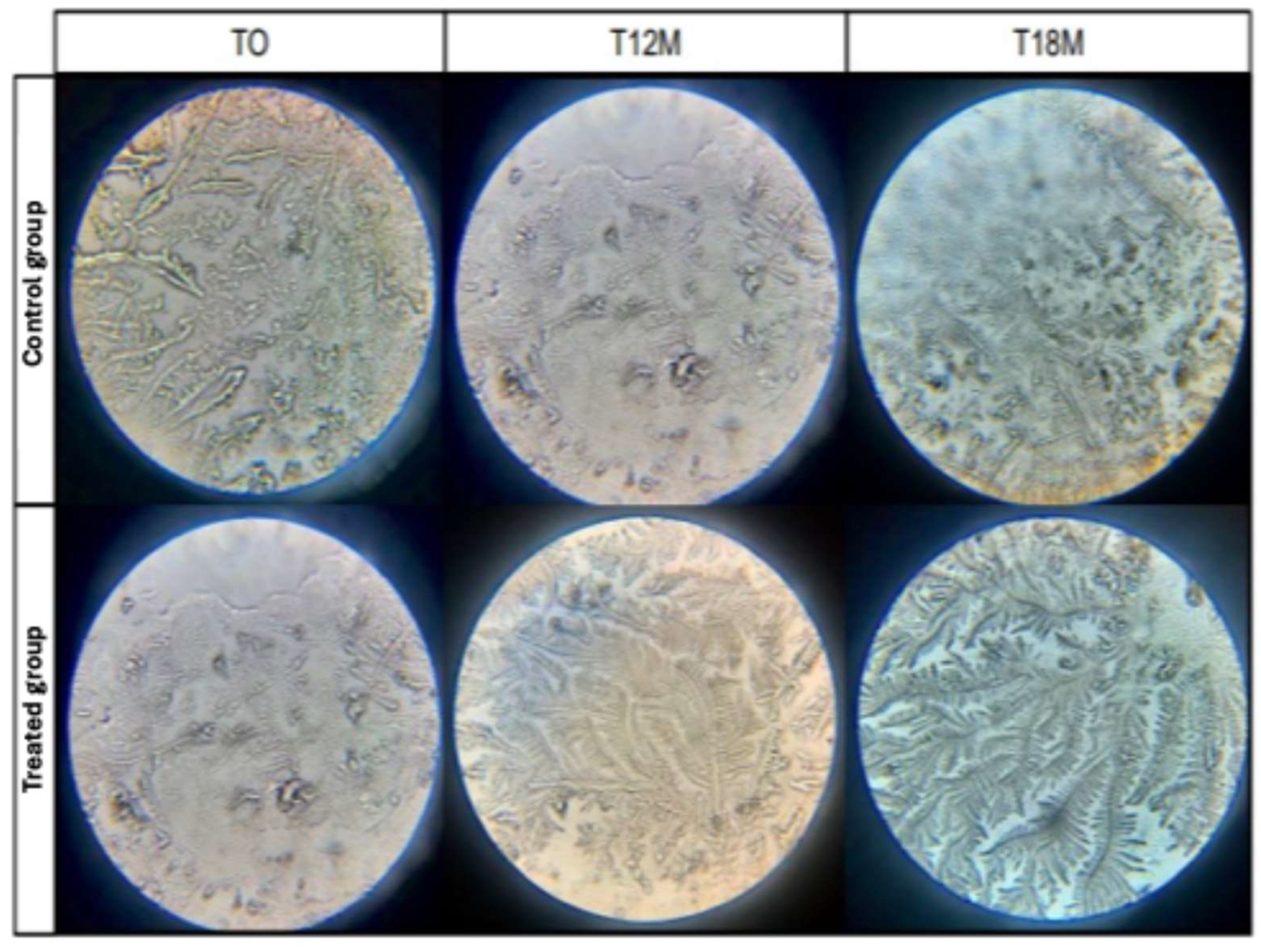
| Groups | Tear Crystallization Test (TCT) | Mean ± S.D. Overtime | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T7 | T30 | T3M | T6M | T9M | T12M | T15M | T18M | ||
| Control group | 2.82 aA ± 0.73 | 2.91 aA ± 0.68 | 2.91 aA ± 0.52 | 3.00 aA ± 0.62 | 3.00 aA ± 0.62 | 3.09 aA ± 0.52 | 3.29 aA ± 0.47 | 3.29 aA ± 0.47 | 3.29 aA ± 0.47 | 3.03 A ± 0.61 |
| Treated group | 2.90 aA ± 0.88 | 2.95 aA ± 0.84 | 2.94 aA ± 0.85 | 2.78 aA ± 0.69 | 2.78 aA ± 0.65 | 2.72 aA ± 0.75 | 2.56 bB ± 0.75 | 2.47 bB ± 0.75 | 2.29 bB ± 0.77 | 2.77 B ± 0.79 |
| Groups | Presence or Absence of Corneal Ulcers | T7 | T30 | T3M | T6M | T9M | T12M | T15M | T18M |
|---|---|---|---|---|---|---|---|---|---|
| Control group | Presence | 7 10.7% | 0 0% | 0 0% | 3 4.8% | 4 6.4% | 3 4.8% | 2 3.2% | 4 6.4% |
| Absent | 15 23.0% | 22 34.4% | 22 34.4% | 18 28.6% | 17 27% | 18 28.9% | 19 30.2% | 17 27% | |
| Treated group | Present | 14 21.5% | 0 0% | 0 0% | 0 0% | 0 0% | 1 1.6% | 1 1.6% | 0 0% |
| Absent | 29 44.6% | 42 65.65 | 42 65.6% | 42 66.7% | 42 66.7% | 41 65.1% | 41 65.1% | 42 66.7% | |
| Tests | Q-square (p value) | 0.95 | - | - | - | - | - | - | - |
| Fisher test (p value) | - | 1.00 | 1.00 | 0.03 | 0.01 | 0.09 | 0.21 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.R.; Ribeiro, T.A.; Silva, D.M.C.; Júnior, J.R.d.S.; Lima, T.B.; Gil da Costa, R.M.; Faustino-Rocha, A.I.; Oliveira, P.A.; Abreu-Silva, A.L. Effects of Canthoplasty in Preventing Secondary Ocular Surface Lesions in Brachycephalic Dogs. Vet. Sci. 2025, 12, 889. https://doi.org/10.3390/vetsci12090889
Costa AR, Ribeiro TA, Silva DMC, Júnior JRdS, Lima TB, Gil da Costa RM, Faustino-Rocha AI, Oliveira PA, Abreu-Silva AL. Effects of Canthoplasty in Preventing Secondary Ocular Surface Lesions in Brachycephalic Dogs. Veterinary Sciences. 2025; 12(9):889. https://doi.org/10.3390/vetsci12090889
Chicago/Turabian StyleCosta, Alcyjara Rêgo, Tatiane Avelar Ribeiro, Diego Marques C. Silva, José Ribamar da Silva Júnior, Tiago Barbalho Lima, Rui M. Gil da Costa, Ana I. Faustino-Rocha, Paula A. Oliveira, and Ana Lúcia Abreu-Silva. 2025. "Effects of Canthoplasty in Preventing Secondary Ocular Surface Lesions in Brachycephalic Dogs" Veterinary Sciences 12, no. 9: 889. https://doi.org/10.3390/vetsci12090889
APA StyleCosta, A. R., Ribeiro, T. A., Silva, D. M. C., Júnior, J. R. d. S., Lima, T. B., Gil da Costa, R. M., Faustino-Rocha, A. I., Oliveira, P. A., & Abreu-Silva, A. L. (2025). Effects of Canthoplasty in Preventing Secondary Ocular Surface Lesions in Brachycephalic Dogs. Veterinary Sciences, 12(9), 889. https://doi.org/10.3390/vetsci12090889









