Genomic Characteristics of a Novel Recombinant Bluetongue Virus Serotype 1 in Yunnan, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Strains and Cells
2.3. Experimental Infection of BHK-21 Cells and OLMECs with BTV-1/YNDH/103/2013
2.4. Preparation and Detection of Viral Genomic dsRNA
2.5. Library Construction, and Next-Generation Sequencing
2.6. Genome Sequence, Assembly, Annotation, and Phylogenetic Tree Construction
2.7. Analysis of Recombination Events in the Complete Genome
3. Results
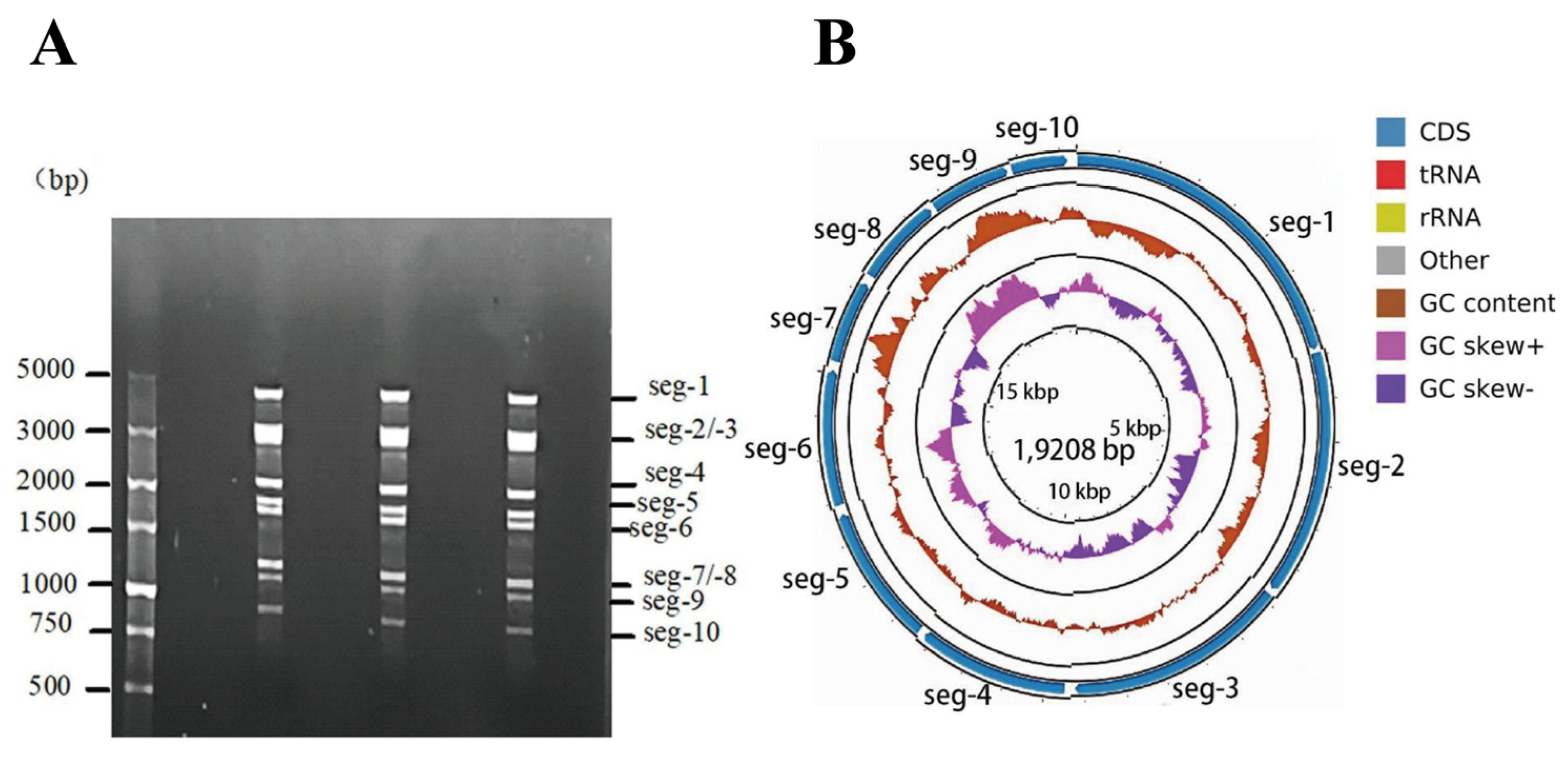
3.1. Identification of the dsRNA Genome Structure and NGS Analysis
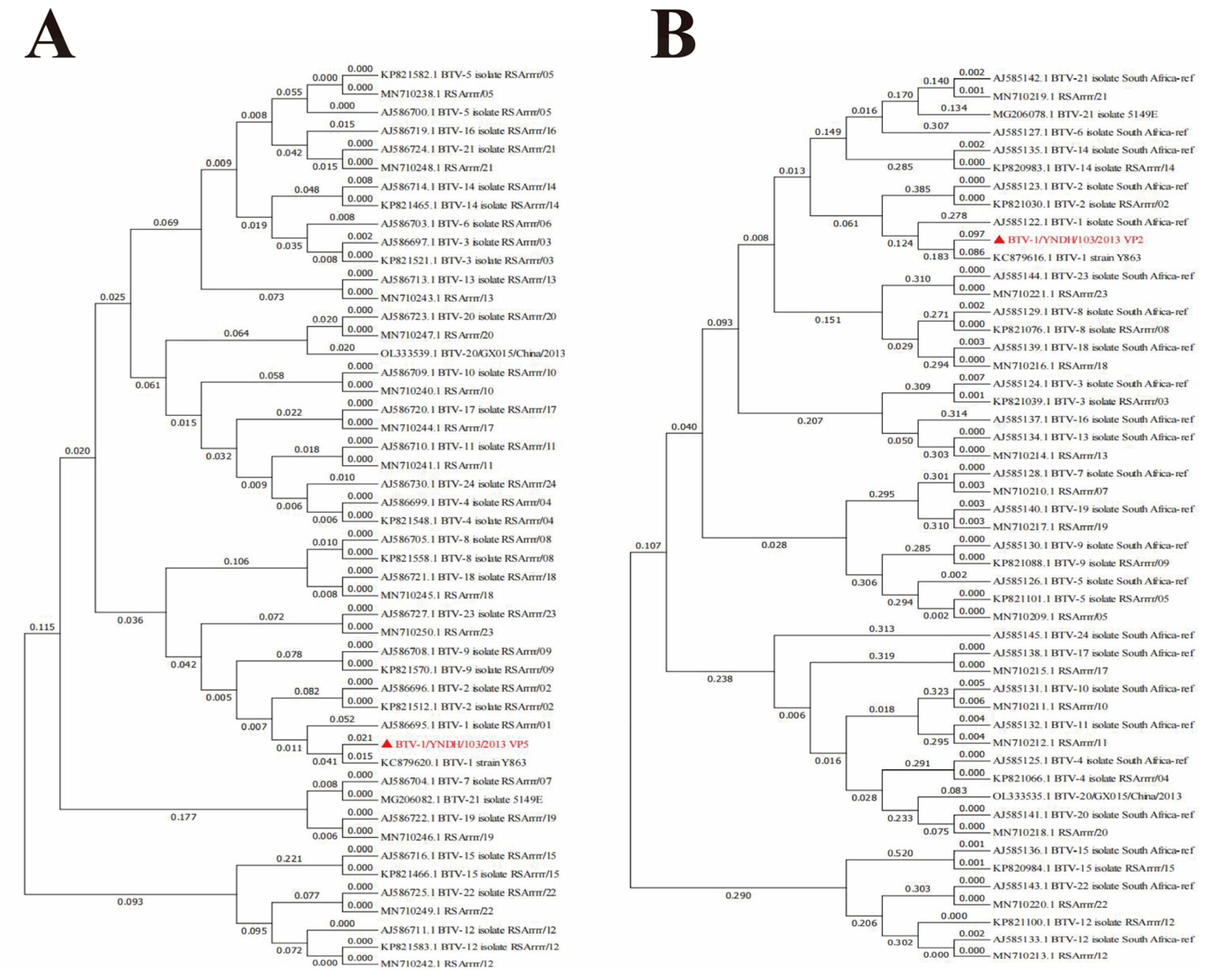
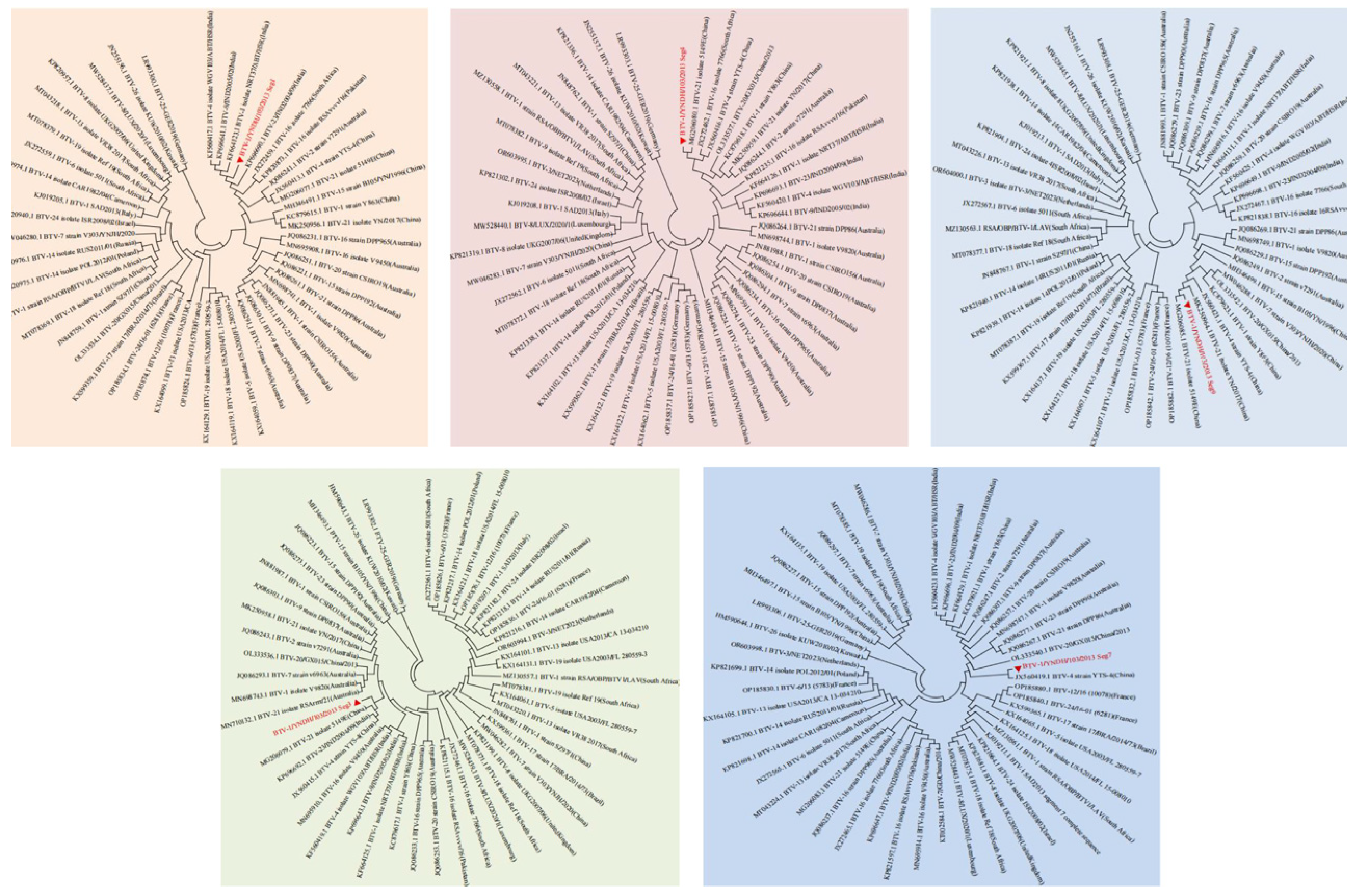
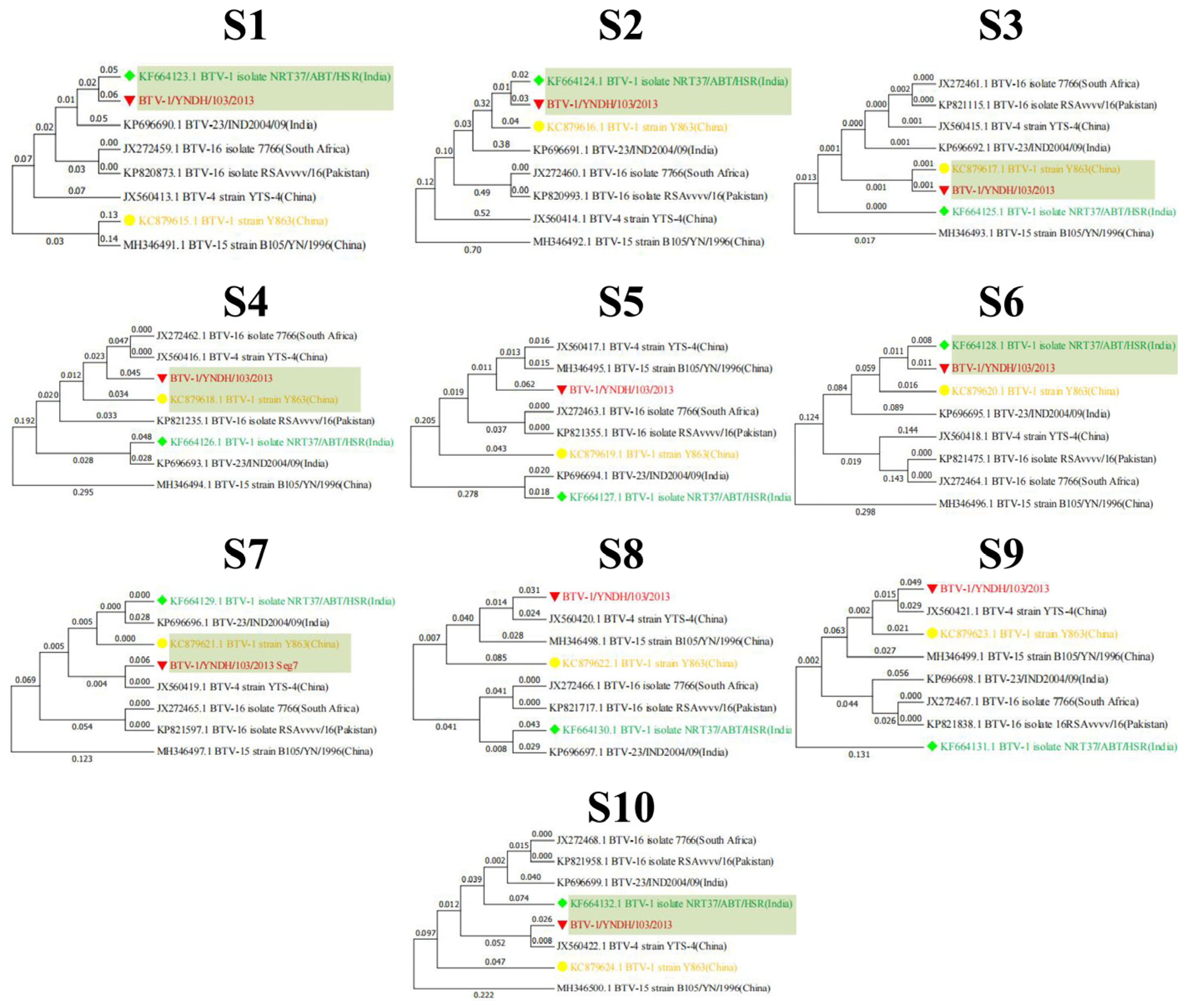
3.2. Phylogenetic Analysis of BTV-1/YNDH/103/2013
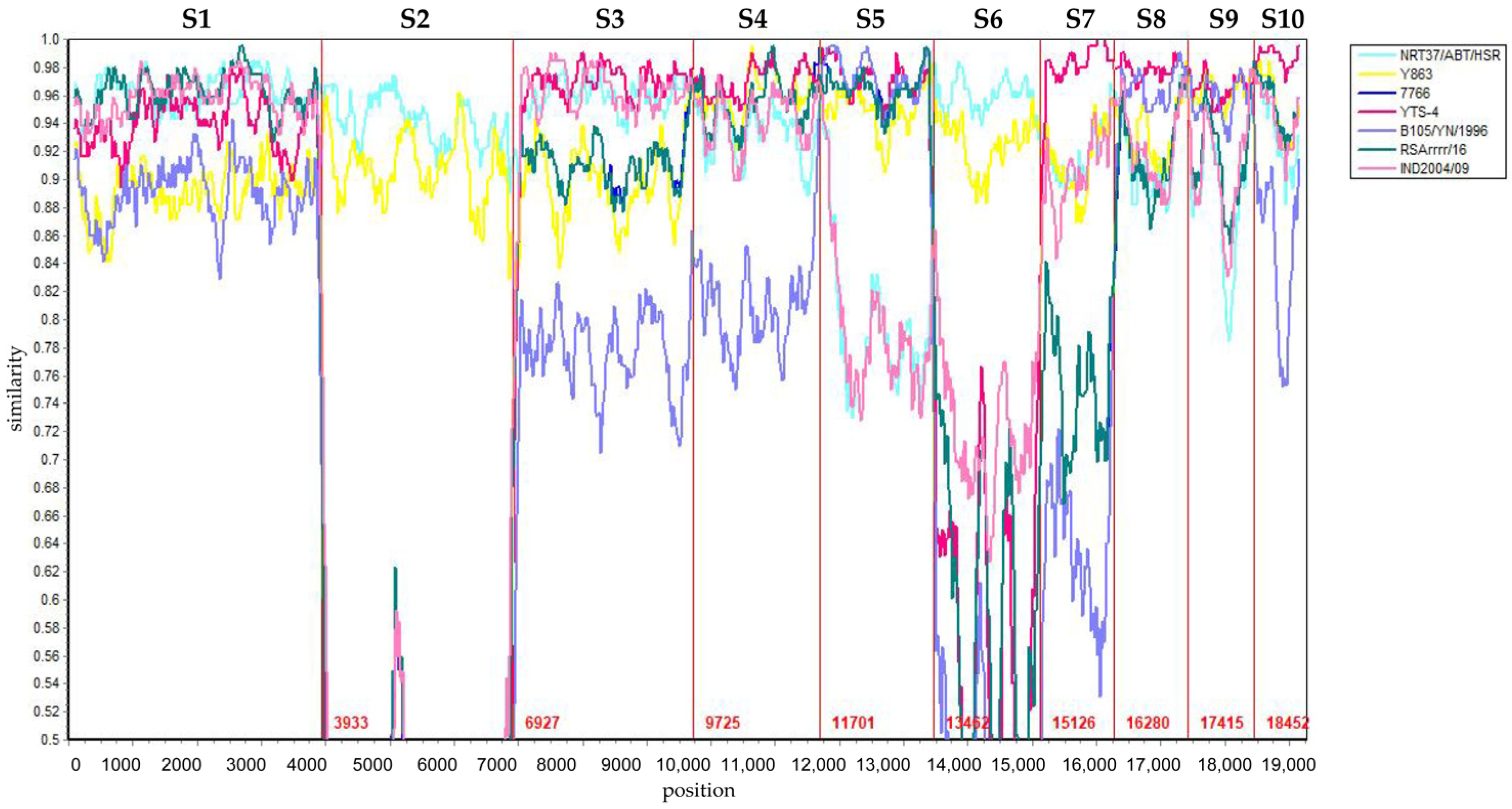
3.3. Reassortment Analysis of BTV-1/YNDH/103/2013
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Meng, J.; He, Y.; Wang, W.; Wang, J. Potential Roles of Culicoides spp. (Culicoides imicola, Culicoides oxystoma) as Biological Vectors of Bluetongue Virus in Yuanyang of Yunnan, P.R. China. Front. Cell. Infect. Microbiol. 2023, 13, 1283216. [Google Scholar] [CrossRef]
- Maclachlan, N.J. Bluetongue: History, Global Epidemiology, and Pathogenesis. Prev. Vet. Med. 2011, 102, 107–111. [Google Scholar] [CrossRef]
- Roy, P. Bluetongue Virus Structure and Assembly. Curr. Opin. Virol. 2017, 24, 115–123. [Google Scholar] [CrossRef]
- Xia, X.; Sung, P.-Y.; Martynowycz, M.W.; Gonen, T.; Roy, P.; Zhou, Z.H. RNA Genome Packaging and Capsid Assembly of Bluetongue Virus Visualized in Host Cells. Cell 2024, 187, 2236–2249.e17. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gong, Q.-L.; Zhang, R.; Chen, Z.-Y.; Wang, Q.; Sun, Y.-H.; Sheng, C.-Y.; Ma, B.-Y.; Li, J.-M.; Shi, K.; et al. Prevalence and Risk Factors of Bluetongue Virus Infection in Sheep and Goats in China: A Systematic Review and Meta-Analysis. Microb. Pathog. 2021, 161, 105170. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, E.A.; Pinheiro, M.A.; Lucchesi, V.S.; Oliveira, A.G.G.; Galinari, G.C.F.; Tinoco, H.P.; Coelho, C.M.; Lobato, Z.I.P. The Study of Bluetongue Virus (BTV) and Epizootic Hemorrhagic Disease Virus (EHDV) Circulation and Vectors at the Municipal Parks and Zoobotanical Foundation of Belo Horizonte, Minas Gerais, Brazil (FPMZB-BH). Viruses 2024, 16, 293. [Google Scholar] [CrossRef]
- Monsion, B.; Mohd Jaafar, F.; Mertens, P.P.C.; Attoui, H. Uncovering the Underlying Mechanisms Blocking Replication of Bluetongue Virus Serotype 26 (BTV-26) in Culicoides Cells. Biomolecules 2023, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Attoui, H.; Monsion, B.; Klonjkowski, B.; Zientara, S.; Mertens, P.P.C.; Mohd Jaafar, F. Identification of the Genome Segments of Bluetongue Virus Type 26/Type 1 Reassortants Influencing Horizontal Transmission in a Mouse Model. Viruses 2021, 13, 2208. [Google Scholar] [CrossRef]
- Ries, C.; Sharav, T.; Tseren-Ochir, E.-O.; Beer, M.; Hoffmann, B. Putative Novel Serotypes “33” and “35” in Clinically Healthy Small Ruminants in Mongolia Expand the Group of Atypical BTV. Viruses 2020, 13, 42. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, H.; Li, H.; Xiao, L.; Wang, J.; Li, N.; Zhang, N. Full-Genome Sequence of Bluetongue Virus Serotype 1 (BTV-1) Strain Y863, the First BTV-1 Isolate of Eastern Origin Found in China. Genome Announc. 2013, 1, e00403-13. [Google Scholar] [CrossRef]
- Qin, S.; Lin, J.; Li, L.; Zhang, Y.; Xiao, L.; Cao, Y.; Ren, P.; Li, H.; Wu, J. Seroprevalence and Potential Risk Factors of Bluetongue Virus Infection in Domestic Cattle and Goats in Guangxi Province, Southern China. Vector Borne Zoonotic Dis. 2020, 20, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.-L.; Wang, Q.; Yang, X.-Y.; Li, D.-L.; Zhao, B.; Ge, G.-Y.; Zong, Y.; Li, J.-M.; Leng, X.; Shi, K.; et al. Seroprevalence and Risk Factors of the Bluetongue Virus in Cattle in China from 1988 to 2019: A Comprehensive Literature Review and Meta-Analysis. Front. Vet. Sci. 2020, 7, 550381. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, K.R.; Mullens, B.A.; MacLachlan, N.J. Occurrence of Genetic Drift and Founder Effect during Quasispecies Evolution of the VP2 and NS3/NS3A Genes of Bluetongue Virus upon Passage between Sheep, Cattle, and Culicoides sonorensis. J. Virol. 2001, 75, 8298–8305. [Google Scholar] [CrossRef]
- Mayo, C.; McDermott, E.; Kopanke, J.; Stenglein, M.; Lee, J.; Mathiason, C.; Carpenter, M.; Reed, K.; Perkins, T.A. Ecological Dynamics Impacting Bluetongue Virus Transmission in North America. Front. Vet. Sci. 2020, 7, 186. [Google Scholar] [CrossRef]
- Vijaykrishna, D.; Mukerji, R.; Smith, G.J.D. RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion. PLoS Pathog. 2015, 11, e1004902. [Google Scholar] [CrossRef]
- Yi, C.K.; Bansal, O.B.; Hong, M.L.; Chatterjee, S.; Roy, P. Sequences within the VP6 Molecule of Bluetongue Virus That Determine Cytoplasmic and Nuclear Targeting of the Protein. J. Virol. 1996, 70, 4778–4782. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Prjibelsky, A.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling Genomes and Mini-Metagenomes from Highly Chimeric Reads. In Proceedings of the Research in Computational Molecular Biology; Deng, M., Jiang, R., Sun, F., Zhang, X., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 158–170. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Singh, K.P.; Belaganahalli, M.N.; Guimera, M.; Pullinger, G.; Nomikou, K.; Mertens, P.P.C. Complete Genome Sequence Analysis of a Reference Strain of Bluetongue Virus Serotype 16. J. Virol. 2012, 86, 10255–10256. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and Analysis of Recombination Patterns in Virus Genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-Length Human Immunodeficiency Virus Type 1 Genomes from Subtype C-Infected Seroconverters in India, with Evidence of Intersubtype Recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [CrossRef]
- White, J.R.; Eaton, B.T. Conformation of the VP2 protein of bluetongue virus (BTV) determines the involvement in virus neutralization of highly conserved epitopes within the BTV serogroup. J. Gen. Virol. 1990, 71 Pt 6, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Mertens, P.P.; Pedley, S.; Cowley, J.; Burroughs, J.N.; Corteyn, A.H.; Jeggo, M.H.; Jennings, D.M.; Gorman, B.M. Analysis of the roles of bluetongue virus outer capsid proteins VP2 and VP5 in determination of virus serotype. Virology 1989, 170, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lv, M.; Sun, M.; Lin, L.; Kou, M.; Gao, L.; Liao, D.; Xiong, H.; He, Y.; Li, H. Complete genome sequence of the first bluetongue virus serotype 7 isolate from China: Evidence for entry of African-lineage strains and reassortment between the introduced and native strains. Arch. Virol. 2016, 161, 223–227. [Google Scholar] [CrossRef]
- Golender, N.; Eldar, A.; Ehrlich, M.; Khinich, Y.; Kenigswald, G.; Varsano, J.S.; Ertracht, S.; Abramovitz, I.; Assis, I.; Shlamovitz, I.; et al. Emergence of a Novel Reassortant Strain of Bluetongue Serotype 6 in Israel, 2017: Clinical Manifestations of the Disease and Molecular Characterization. Viruses 2019, 11, 633. [Google Scholar] [CrossRef]
- Shafiq, M.; Minakshi, P.; Bhateja, A.; Ranjan, K.; Prasad, G. Evidence of genetic reassortment between Indian isolate of bluetongue virus serotype 21 (BTV-21) and bluetongue virus serotype 16 (BTV-16). Virus Res. 2013, 173, 336–343. [Google Scholar] [CrossRef]
- Shaw, A.E.; Ratinier, M.; Nunes, S.F.; Nomikou, K.; Caporale, M.; Golder, M.; Allan, K.; Hamers, C.; Hudelet, P.; Zientara, S.; et al. Reassortment between two serologically unrelated bluetongue virus strains is flexible and can involve any genome segment. J. Virol. 2013, 87, 543–557. [Google Scholar] [CrossRef]
- Yang, T.; Liu, N.; Xu, Q.; Sun, E.; Qin, Y.; Zhao, J.; Wu, D. Complete genomic sequence of bluetongue virus serotype 16 from China. J. Virol. 2011, 85, 13472. [Google Scholar] [CrossRef] [PubMed]

| Number of Recombinant Events | Recombinant Sequences | Major Parents | Minor Parents | Breakpoint Position | Detection Methods | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | G | B | M | C | S | T | |||||
| 1 | YNDH/103/2013 | NRT37/ABT/HSR | Y863 | 10,465–13,413 nt | + | + | + | + | + | + | + |
| 2 | YNDH/103/2013 | NRT37/ABT/HSR | RSArrrr/16 | 11,376–13,312 nt | + | + | + | + | + | + | + |
| 3 | YNDH/103/2013 | NRT37/ABT/HSR | YTS-4 | 15,117–16,277 nt | + | - | + | + | + | + | + |
| 4 | YNDH/103/2013 | NRT37/ABT/HSR | YTS-4 | 18,511–19,080 nt | + | + | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Luo, S.; Lei, N.; Ye, Z.; Ma, X.; Yang, S.; Jia, H.; Qi, G.; Wang, G.; Yi, H. Genomic Characteristics of a Novel Recombinant Bluetongue Virus Serotype 1 in Yunnan, China. Vet. Sci. 2025, 12, 886. https://doi.org/10.3390/vetsci12090886
Chen Y, Luo S, Lei N, Ye Z, Ma X, Yang S, Jia H, Qi G, Wang G, Yi H. Genomic Characteristics of a Novel Recombinant Bluetongue Virus Serotype 1 in Yunnan, China. Veterinary Sciences. 2025; 12(9):886. https://doi.org/10.3390/vetsci12090886
Chicago/Turabian StyleChen, Yunyi, Shimei Luo, Nijing Lei, Zhenghao Ye, Xianping Ma, Shaoyu Yang, Huaijie Jia, Guangyu Qi, Guanghua Wang, and Huashan Yi. 2025. "Genomic Characteristics of a Novel Recombinant Bluetongue Virus Serotype 1 in Yunnan, China" Veterinary Sciences 12, no. 9: 886. https://doi.org/10.3390/vetsci12090886
APA StyleChen, Y., Luo, S., Lei, N., Ye, Z., Ma, X., Yang, S., Jia, H., Qi, G., Wang, G., & Yi, H. (2025). Genomic Characteristics of a Novel Recombinant Bluetongue Virus Serotype 1 in Yunnan, China. Veterinary Sciences, 12(9), 886. https://doi.org/10.3390/vetsci12090886







